Abstract
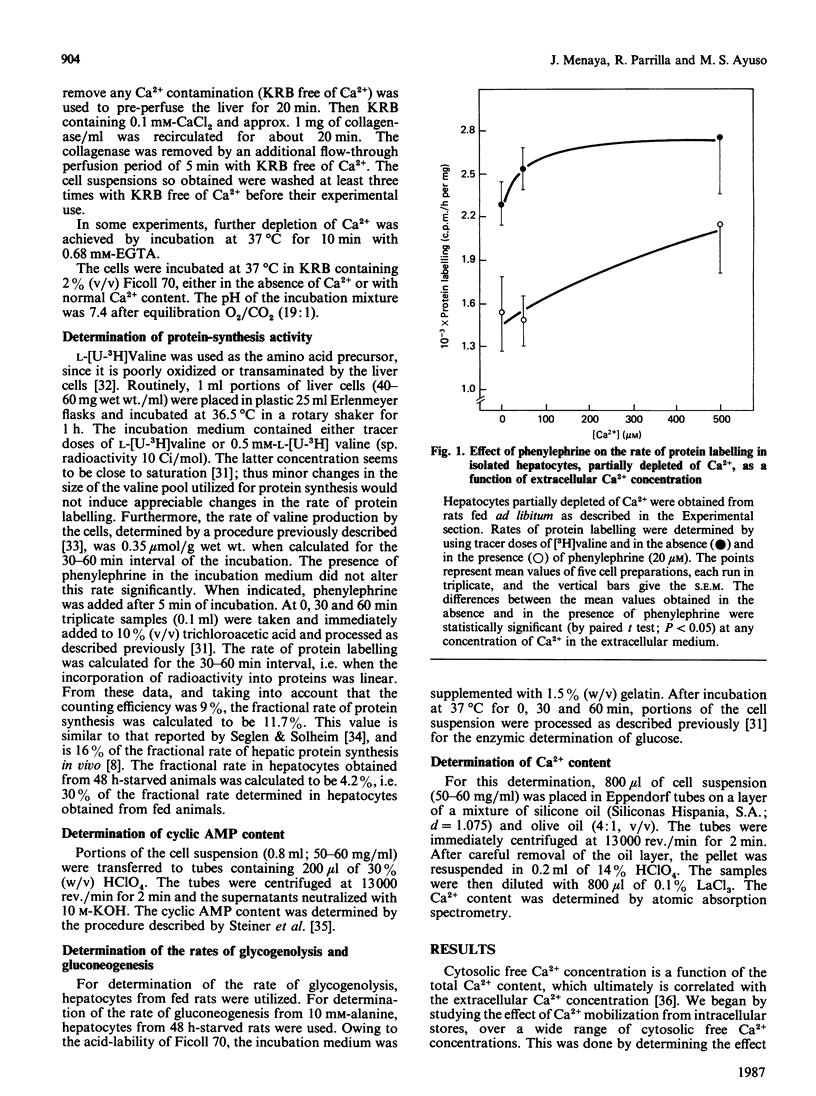
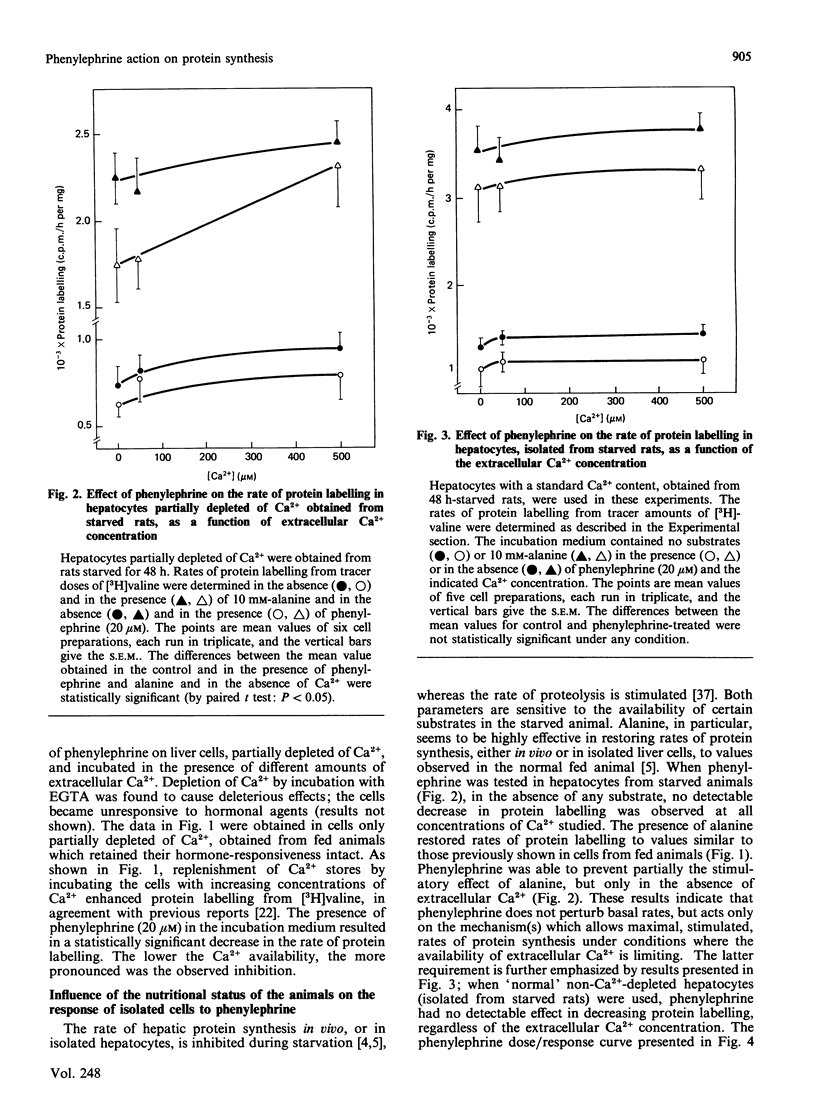
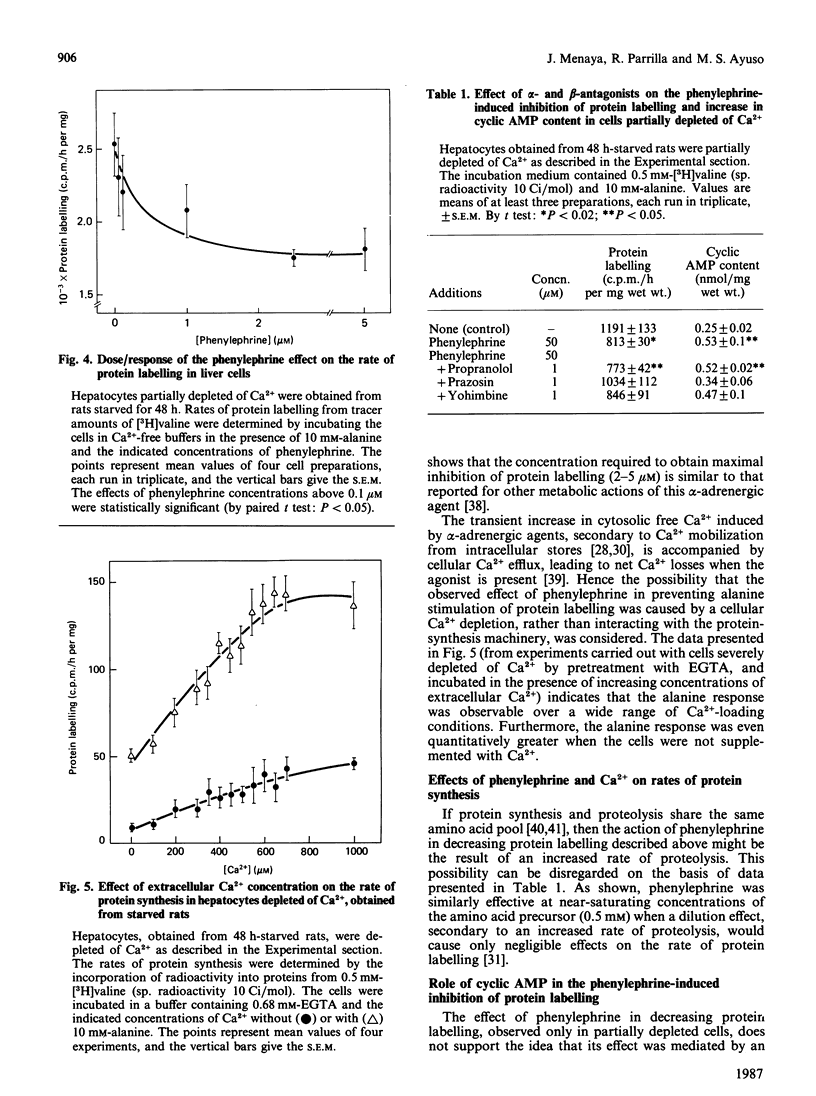
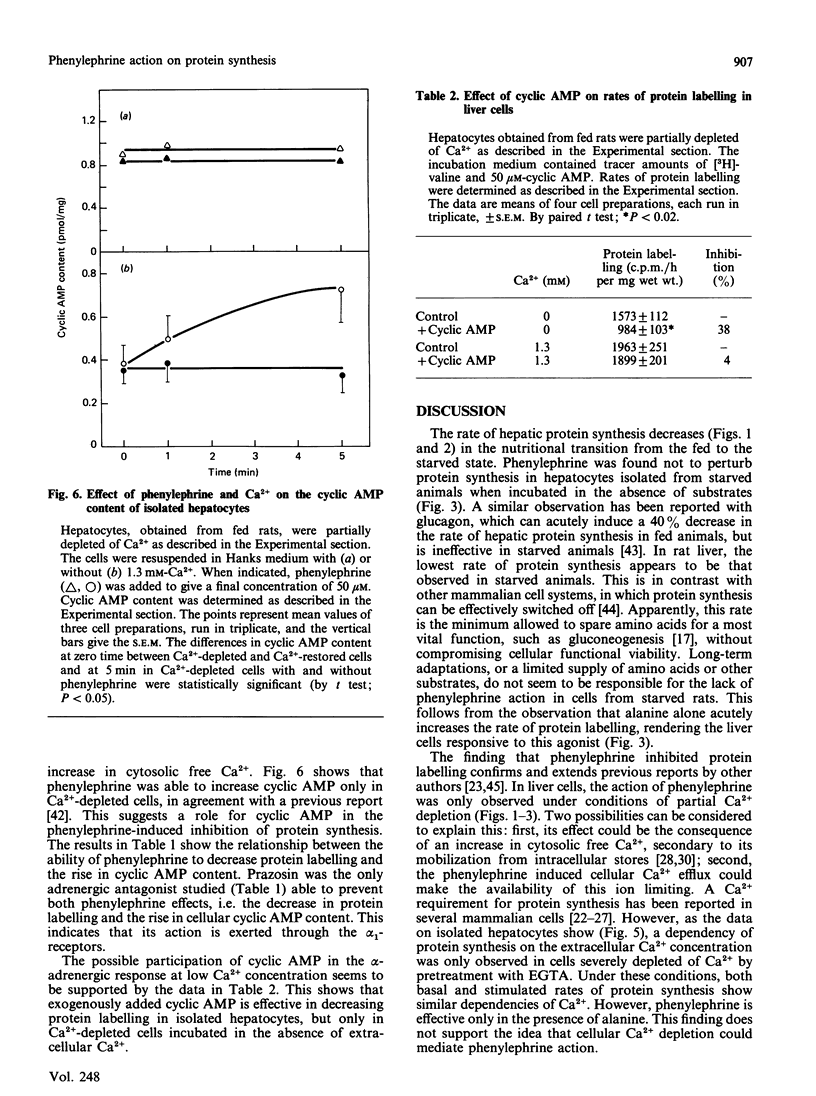
The alpha-adrenergic agonist phenylephrine was found to inhibit protein labelling from [3H]valine in isolated liver cells. This effect is only observable under conditions of partial Ca2+ depletion and in cells displaying maximal rates of protein labelling, i.e. cells isolated from fed animals or from starved animals when incubated in the presence of alanine. The ability of phenylephrine to inhibit protein labelling at near-saturating concentrations of the amino acid precursor indicates that this alpha-agonist actually decreases the rate of protein synthesis. The possibility that phenylephrine acts by making cellular Ca2+ availability further limiting can be ruled out, since alanine stimulates protein labelling under conditions of severe Ca2+ depletion obtained by pretreatment of the cells with EGTA. The following observations indicate that the phenylephrine action may be mediated by an increase in cellular cyclic AMP content: (1) a close relationship was found between the abilities of phenylephrine to inhibit protein labelling and to increase cyclic AMP content; (2) cyclic AMP mimics the phenylephrine action only in cells partially depleted of Ca2+; (3) the alpha 1-antagonist prazosin, which inhibited the phenylephrine-mediated increase in cyclic AMP, also abolished the effect on protein synthesis.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Altin J. G., Bygrave F. L. The Ca2+-mobilizing actions of vasopressin and angiotensin differ from those of the alpha-adrenergic agonist phenylephrine in the perfused rat liver. Biochem J. 1985 Dec 15;232(3):911–917. doi: 10.1042/bj2320911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayuso-Parrilla M. S., Martín-Requero A., Pérez-Días J., Parrilla R. Role of glucagon on the control of hepatic protein synthesis and degradation in the rat in vivo. J Biol Chem. 1976 Dec 25;251(24):7785–7790. [PubMed] [Google Scholar]
- Ayuso M. S., Bengoa B., Girbés T., Susin A., Parrilla R. Effect of ethanol on proteolysis in isolated liver cells. Gen Pharmacol. 1986;17(3):315–320. doi: 10.1016/0306-3623(86)90046-7. [DOI] [PubMed] [Google Scholar]
- Ayuso M. S., Vega P., Manchón C. G., Parrilla R. Interrelation between gluconeogenesis and hepatic protein synthesis. Biochim Biophys Acta. 1986 Aug 6;883(1):33–40. doi: 10.1016/0304-4165(86)90131-5. [DOI] [PubMed] [Google Scholar]
- Blackmore P. F., Dehaye J. P., Exton J. H. Studies on alpha-adrenergic activation of hepatic glucose output. The role of mitochondrial calcium release in alpha-adrenergic activation of phosphorylase in perfused rat liver. J Biol Chem. 1979 Aug 10;254(15):6945–6950. [PubMed] [Google Scholar]
- Blackmore P. F., Dehaye J. P., Strickland W. G., Exton J. H. alpha-Adrenergic mobilization of hepatic mitochondrial calcium. FEBS Lett. 1979 Apr 1;100(1):117–120. doi: 10.1016/0014-5793(79)81144-8. [DOI] [PubMed] [Google Scholar]
- Brostrom C. O., Bocckino S. B., Brostrom M. A., Galuska E. M. Regulation of protein synthesis in isolated hepatocytes by calcium-mobilizing hormones. Mol Pharmacol. 1986 Jan;29(1):104–111. [PubMed] [Google Scholar]
- Brostrom C. O., Bocckino S. B., Brostrom M. A. Identification of a Ca2+ requirement for protein synthesis in eukaryotic cells. J Biol Chem. 1983 Dec 10;258(23):14390–14399. [PubMed] [Google Scholar]
- Burgess G. M., Godfrey P. P., McKinney J. S., Berridge M. J., Irvine R. F., Putney J. W., Jr The second messenger linking receptor activation to internal Ca release in liver. Nature. 1984 May 3;309(5963):63–66. doi: 10.1038/309063a0. [DOI] [PubMed] [Google Scholar]
- Burton D. N., Collins J. M., Porter J. W. Biosynthesis of the fatty acid synthetase by isolated rat liver cells. J Biol Chem. 1969 Feb 10;244(3):1076–1077. [PubMed] [Google Scholar]
- Bygrave F. L. Properties of energy-dependent calcium transport by rat liver microsomal fraction as revealed by initial-rate measurements. Biochem J. 1978 Jan 15;170(1):87–91. doi: 10.1042/bj1700087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bygrave F. L., Tranter C. J. The subcellular location, maturation and response to increased plasma glucagon of ruthenium red-insensitive calcium-ion transport in rat liver. Biochem J. 1978 Sep 15;174(3):1021–1030. doi: 10.1042/bj1741021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan T. M., Exton J. H. alpha-Adrenergic-mediated accumulation of adenosine 3':5' monophosphate in calcium-depleted hepatocytes. J Biol Chem. 1977 Dec 10;252(23):8645–8651. [PubMed] [Google Scholar]
- Charest R., Prpić V., Exton J. H., Blackmore P. F. Stimulation of inositol trisphosphate formation in hepatocytes by vasopressin, adrenaline and angiotensin II and its relationship to changes in cytosolic free Ca2+. Biochem J. 1985 Apr 1;227(1):79–90. doi: 10.1042/bj2270079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J. L., Babcock D. F., Lardy H. A. Norepinephrine, vasopressin, glucagon, and A23187 induce efflux of calcium from an exchangeable pool in isolated rat hepatocytes. Proc Natl Acad Sci U S A. 1978 May;75(5):2234–2238. doi: 10.1073/pnas.75.5.2234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung W. Y. Cyclic 3',5'-nucleotide phosphodiesterase. Demonstration of an activator. Biochem Biophys Res Commun. 1970 Feb 6;38(3):533–538. doi: 10.1016/0006-291x(70)90747-3. [DOI] [PubMed] [Google Scholar]
- Delaunay J., Ranu R. S., Levin D. H., Ernst V., London I. M. Characterization of a rat liver factor that inhibits initiation of protein synthesis in rabbit reticulocyte lysates. Proc Natl Acad Sci U S A. 1977 Jun;74(6):2264–2268. doi: 10.1073/pnas.74.6.2264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farese R. V. Calcium as a mediator of adrenocorticotrophic hormone action on adrenal protein synthesis. Science. 1971 Jul 30;173(3995):447–450. doi: 10.1126/science.173.3995.447. [DOI] [PubMed] [Google Scholar]
- García-Sáinz J. A., Tussié-Luna M. I., Hernández-Sotomayor S. M. Phorbol esters, vasopressin and angiotensin II block alpha 1-adrenergic action in rat hepatocytes. Possible role of protein kinase C. Biochim Biophys Acta. 1986 Jun 16;887(1):69–72. doi: 10.1016/0167-4889(86)90123-0. [DOI] [PubMed] [Google Scholar]
- Girbes T., Susin A., Ayuso M. S., Parrilla R. Acute effects of ethanol in the control of protein synthesis in isolated rat liver cells. Arch Biochem Biophys. 1983 Oct 1;226(1):37–49. doi: 10.1016/0003-9861(83)90269-2. [DOI] [PubMed] [Google Scholar]
- Gross M. The control of protein synthesis by hemin in rabbit reticulocytes. Mol Cell Biochem. 1980 May 28;31(1):25–36. doi: 10.1007/BF00817888. [DOI] [PubMed] [Google Scholar]
- Henshaw E. C., Hirsch C. A., Morton B. E., Hiatt H. H. Control of protein synthesis in mammalian tissues through changes in ribosome activity. J Biol Chem. 1971 Jan 25;246(2):436–446. [PubMed] [Google Scholar]
- Hue L., Felíu J. E., Hers H. G. Control of gluconeogenesis and of enzymes of glycogen metabolism in isolated rat hepatocytes. A parallel study of the effect of phenylephrine and of glucagon. Biochem J. 1978 Dec 15;176(3):791–797. doi: 10.1042/bj1760791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes B. P., Barritt G. J. Effects of glucagon and N6O2'-dibutyryladenosine 3':5'-cyclic monophosphate on calcium transport in isolated rat liver mitochondria. Biochem J. 1978 Oct 15;176(1):295–304. doi: 10.1042/bj1760295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ireland C. B., Schmidt P. G. Proton magnetic relaxation of aspartate transcarbamylase - succinate complexes. J Biol Chem. 1977 Apr 10;252(7):2262–2270. [PubMed] [Google Scholar]
- Joseph S. K., Coll K. E., Thomas A. P., Rubin R., Williamson J. R. The role of extracellular Ca2+ in the response of the hepatocyte to Ca2+-dependent hormones. J Biol Chem. 1985 Oct 15;260(23):12508–12515. [PubMed] [Google Scholar]
- Joseph S. K., Coll K. E., Thomas A. P., Rubin R., Williamson J. R. The role of extracellular Ca2+ in the response of the hepatocyte to Ca2+-dependent hormones. J Biol Chem. 1985 Oct 15;260(23):12508–12515. [PubMed] [Google Scholar]
- Kakiuchi S., Yamazaki R. Calcium dependent phosphodiesterase activity and its activating factor (PAF) from brain studies on cyclic 3',5'-nucleotide phosphodiesterase (3). Biochem Biophys Res Commun. 1970 Dec 9;41(5):1104–1110. doi: 10.1016/0006-291x(70)90199-3. [DOI] [PubMed] [Google Scholar]
- Kaolan E., Richman H. G. Calcium enhancement of protein synthesis in rat heart ventricles. Can J Biochem. 1973 Sep;51(9):1331–1334. doi: 10.1139/o73-175. [DOI] [PubMed] [Google Scholar]
- Keppens S., Vandenheede J. R., De Wulf H. On the role of calcium as second messenger in liver for the hormonally induced activation of glycogen phosphorylase. Biochim Biophys Acta. 1977 Feb 28;496(2):448–457. doi: 10.1016/0304-4165(77)90327-0. [DOI] [PubMed] [Google Scholar]
- Mandl J., Garzo T., Antoni F. Epinephrine inhibits protein synthesis in isolated mouse hepatocytes through alpha adrenergic receptors in a calcium dependent way. Biochem Pharmacol. 1982 Apr 15;31(8):1656–1658. doi: 10.1016/0006-2952(82)90399-9. [DOI] [PubMed] [Google Scholar]
- Mauger J. P., Claret M. Mobilization of intracellular calcium by glucagon and cyclic AMP analogues in isolated rat hepatocytes. FEBS Lett. 1986 Jan 20;195(1-2):106–110. doi: 10.1016/0014-5793(86)80140-5. [DOI] [PubMed] [Google Scholar]
- Ochoa S. Regulation of protein synthesis initiation in eucaryotes. Arch Biochem Biophys. 1983 Jun;223(2):325–349. doi: 10.1016/0003-9861(83)90598-2. [DOI] [PubMed] [Google Scholar]
- Parrilla R., Goodman M. N., Toews C. J. Effect of glucagon: insulin ratios on hepatic metabolism. Diabetes. 1974 Sep;23(9):725–731. doi: 10.2337/diab.23.9.725. [DOI] [PubMed] [Google Scholar]
- Preedy V. R., Garlick P. J. The effect of glucagon administration on protein synthesis in skeletal muscles, heart and liver in vivo. Biochem J. 1985 Jun 15;228(3):575–581. doi: 10.1042/bj2280575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prpić V., Bygrave F. L. On the inter-relationship between glucagon action, the oxidation-reduction state of pyridine nucleotides, and calcium retention by rat liver mitochondria. J Biol Chem. 1980 Jul 10;255(13):6193–6199. [PubMed] [Google Scholar]
- Prpić V., Spencer T. L., Bygrave F. L. Stable enhancement of calcium retention in mitochondria isolated from rat liver after the administration of glucagon to the intact animal. Biochem J. 1978 Dec 15;176(3):705–714. doi: 10.1042/bj1760705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pérez-Sala D., Parrilla R., Ayuso M. S. Key role of L-alanine in the control of hepatic protein synthesis. Biochem J. 1987 Jan 15;241(2):491–498. doi: 10.1042/bj2410491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasmussen H. Cell communication, calcium ion, and cyclic adenosine monophosphate. Science. 1970 Oct 23;170(3956):404–412. doi: 10.1126/science.170.3956.404. [DOI] [PubMed] [Google Scholar]
- Requero A. M., Díaz J. P., Ayuso-Parrilla M. S., Parrilla R. On the mechanism of the glucagon-induced inhibition of hepatic protein synthesis. Arch Biochem Biophys. 1979 Jun;195(1):223–234. doi: 10.1016/0003-9861(79)90344-8. [DOI] [PubMed] [Google Scholar]
- Seglen P. O., Solheim A. E. Effects of aminooxyacetate, alanine and other amino acids on protein synthesis in isolated rat hepatocytes. Biochim Biophys Acta. 1978 Oct 24;520(3):630–641. doi: 10.1016/0005-2787(78)90148-x. [DOI] [PubMed] [Google Scholar]
- Seglen P. O., Solheim A. E. Valine uptake and incorporation into protein in isolated rat hepatocytes. Nature of the precursor pool for protein synthesis. Eur J Biochem. 1978 Apr;85(1):15–25. doi: 10.1111/j.1432-1033.1978.tb12208.x. [DOI] [PubMed] [Google Scholar]
- Steiner A. L., Pagliara A. S., Chase L. R., Kipnis D. M. Radioimmunoassay for cyclic nucleotides. II. Adenosine 3',5'-monophosphate and guanosine 3',5'-monophosphate in mammalian tissues and body fluids. J Biol Chem. 1972 Feb 25;247(4):1114–1120. [PubMed] [Google Scholar]
- Vidrich A., Airhart J., Bruno M. K., Khairallah E. A. Compartmentation of free amino acids for protein biosynthesis. Influence of diurnal changes in hepatic amino acid concentrations of the composition of the precursor pool charging aminoacyl-transfer ribonucleic acid. Biochem J. 1977 Feb 15;162(2):257–266. doi: 10.1042/bj1620257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilde C. J., Hasan H. R., White D. A., Mayer R. J. The effect of calcium on synthesis and degradation of mammary cytosolic proteins and casein. Biochem Biophys Res Commun. 1981 Dec 15;103(3):934–942. doi: 10.1016/0006-291x(81)90900-1. [DOI] [PubMed] [Google Scholar]
- Woodside K. H., Mortimore G. E. Suppression of protein turnover by amino acids in the perfused rat liver. J Biol Chem. 1972 Oct 25;247(20):6474–6481. [PubMed] [Google Scholar]


