Abstract
1. The precise effects of insulin, dexamethasone and lipogenic precursors on the secretion of very-low-density lipoprotein (VLDL) cholesterol and triacylglycerol were dependent on the age of the culture and the duration of treatment. 2. The rates of secretion of triacylglycerol and cholesterol gradually declined with the age of the culture, although there was no detectable decrease within a given 24 h period. 3. Between 4 h and 24 h after cell preparation, insulin inhibited VLDL secretion. Inhibition was maximal between 6 and 12 h after addition of insulin. Longer-term treatment (24-48 h) with insulin resulted in a stimulation of VLDL secretion. This effect was less apparent when dexamethasone was simultaneously present. The secretion of triacylglycerol and cholesteryl ester was more sensitive to insulin than was that of non-esterified cholesterol. 4. Dexamethasone alone stimulated the secretion of VLDL to an extent which increased with the age of the culture. In young cultures (up to 24 h old) dexamethasone protected against inhibition by insulin, but was ineffective in older cultures. 5. In young cultures the stimulatory effect of lipogenic precursors (lactate and pyruvate) on the secretion of triacylglycerol and cholesterol was more pronounced in the presence of dexamethasone. In cultures older than 24 h, the secretion of these components was less sensitive to short-term stimulation by lactate and pyruvate.
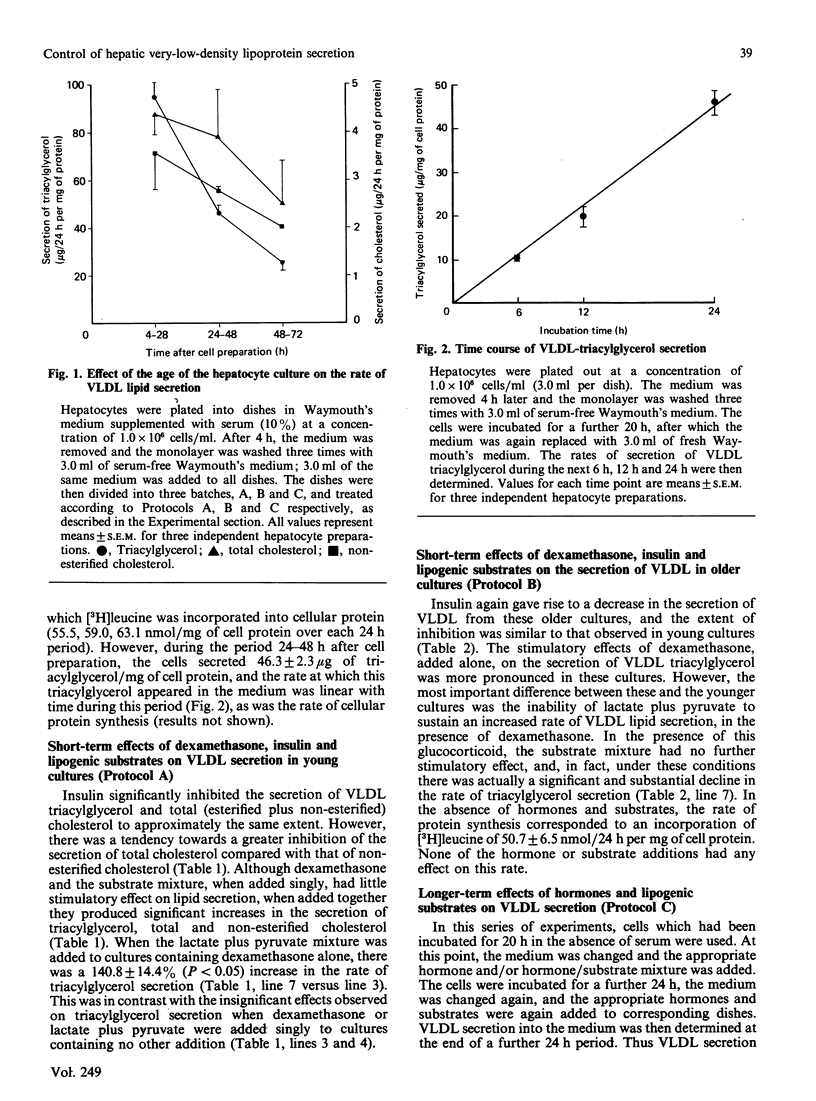
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amatruda J. M., Danahy S. A., Chang C. L. The effects of glucocorticoids on insulin-stimulated lipogenesis in primary cultures of rat hepatocytes. Biochem J. 1983 Apr 15;212(1):135–141. doi: 10.1042/bj2120135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azain M. J., Fukuda N., Chao F. F., Yamamoto M., Ontko J. A. Contributions of fatty acid and sterol synthesis to triglyceride and cholesterol secretion by the perfused rat liver in genetic hyperlipemia and obesity. J Biol Chem. 1985 Jan 10;260(1):174–181. [PubMed] [Google Scholar]
- Boogaerts J. R., Malone-McNeal M., Archambault-Schexnayder J., Davis R. A. Dietary carbohydrate induces lipogenesis and very-low-density lipoprotein synthesis. Am J Physiol. 1984 Jan;246(1 Pt 1):E77–E83. doi: 10.1152/ajpendo.1984.246.1.E77. [DOI] [PubMed] [Google Scholar]
- Boyd M. E., Albright E. B., Foster D. W., McGarry J. D. In vitro reversal of the fasting state of liver metabolism in the rat. Reevaluation of the roles of insulin and glucose. J Clin Invest. 1981 Jul;68(1):142–152. doi: 10.1172/JCI110230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caro J. F., Amatruda J. M. Glucocorticoid-induced insulin resistance: the importance of postbinding events in the regulation of insulin binding, action, and degradation in freshly isolated and primary cultures of rat hepatocytes. J Clin Invest. 1982 Apr;69(4):866–875. doi: 10.1172/JCI110526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis R. A., Boogaerts J. R. Intrahepatic assembly of very low density lipoproteins. Effect of fatty acids on triacylglycerol and apolipoprotein synthesis. J Biol Chem. 1982 Sep 25;257(18):10908–10913. [PubMed] [Google Scholar]
- Demel R. A., Jackson R. L. Lipoprotein lipase hydrolysis of trioleoylglycerol in a phospholipid interface. Effect of cholesteryl oleate on catalysis. J Biol Chem. 1985 Aug 15;260(17):9589–9592. [PubMed] [Google Scholar]
- Durrington P. N., Newton R. S., Weinstein D. B., Steinberg D. Effects of insulin and glucose on very low density lipoprotein triglyceride secretion by cultured rat hepatocytes. J Clin Invest. 1982 Jul;70(1):63–73. doi: 10.1172/JCI110604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FOLCH J., LEES M., SLOANE STANLEY G. H. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem. 1957 May;226(1):497–509. [PubMed] [Google Scholar]
- Fielding C. J., Reaven G. M., Fielding P. E. Human noninsulin-dependent diabetes: identification of a defect in plasma cholesterol transport normalized in vivo by insulin and in vitro by selective immunoadsorption of apolipoprotein E. Proc Natl Acad Sci U S A. 1982 Oct;79(20):6365–6369. doi: 10.1073/pnas.79.20.6365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fielding C. J., Reaven G. M., Liu G., Fielding P. E. Increased free cholesterol in plasma low and very low density lipoproteins in non-insulin-dependent diabetes mellitus: its role in the inhibition of cholesteryl ester transfer. Proc Natl Acad Sci U S A. 1984 Apr;81(8):2512–2516. doi: 10.1073/pnas.81.8.2512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geelen M. J., Harris R. A., Beynen A. C., McCune S. A. Short-term hormonal control of hepatic lipogenesis. Diabetes. 1980 Dec;29(12):1006–1022. doi: 10.2337/diab.29.12.1006. [DOI] [PubMed] [Google Scholar]
- Gibbons G. F. Hyperlipidaemia of diabetes. Clin Sci (Lond) 1986 Nov;71(5):477–486. doi: 10.1042/cs0710477. [DOI] [PubMed] [Google Scholar]
- Gibbons G. F., Pullinger C. R., Björnsson O. G. Changes in the sensitivity of lipogenesis in rat hepatocytes to hormones and precursors over the diurnal cycle and during longer-term starvation of donor animals. J Lipid Res. 1984 Dec 1;25(12):1358–1367. [PubMed] [Google Scholar]
- Gibbons G. F., Pullinger C. R. Regulation of hepatic very-low-density lipoprotein secretion in rats fed on a diet high in unsaturated fat. Biochem J. 1987 Apr 15;243(2):487–492. doi: 10.1042/bj2430487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goh E. H., Heimberg M. Relationship between activity of hepatic 3-hydroxy-3-methylglutaryl-coenzyme A reductase and secretion of very-low-density-lipoprotein cholesterol by the isolated perfused liver and in the intact rat. Biochem J. 1979 Oct 15;184(1):1–6. doi: 10.1042/bj1840001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein J. L., Brown M. S. Progress in understanding the LDL receptor and HMG-CoA reductase, two membrane proteins that regulate the plasma cholesterol. J Lipid Res. 1984 Dec 15;25(13):1450–1461. [PubMed] [Google Scholar]
- Juul S. M., Jones R. H. Evidence for a direct effect of bacitracin on cell-mediated insulin degradation in isolated hepatocytes. Biochem J. 1982 Aug 15;206(2):295–299. doi: 10.1042/bj2060295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz J., McGarry J. D. The glucose paradox. Is glucose a substrate for liver metabolism? J Clin Invest. 1984 Dec;74(6):1901–1909. doi: 10.1172/JCI111610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knox A. M., Sturton R. G., Cooling J., Brindley D. N. Control of hepatic triacylglycerol synthesis. Diurnal variations in hepatic phosphatidate phosphohydrolase activity and in the concentrations of circulating insulin and corticosterone in rats. Biochem J. 1979 May 15;180(2):441–443. doi: 10.1042/bj1800441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lopes-Virella M. F., Sherer G. K., Lees A. M., Wohltmann H., Mayfield R., Sagel J., LeRoy E. C., Colwell J. A. Surface binding, internalization and degradation by cultured human fibroblasts of low density lipoproteins isolated from type 1 (insulin-dependent) diabetic patients: changes with metabolic control. Diabetologia. 1982 Jun;22(6):430–436. doi: 10.1007/BF00282585. [DOI] [PubMed] [Google Scholar]
- Mangiapane E. H., Brindley D. N. Effects of dexamethasone and insulin on the synthesis of triacylglycerols and phosphatidylcholine and the secretion of very-low-density lipoproteins and lysophosphatidylcholine by monolayer cultures of rat hepatocytes. Biochem J. 1986 Jan 1;233(1):151–160. doi: 10.1042/bj2330151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsh J. B. Hepatic lipoprotein biosynthesis. Methods Enzymol. 1986;129:498–519. doi: 10.1016/0076-6879(86)29088-6. [DOI] [PubMed] [Google Scholar]
- Olefsky J. M., Farquhar J. W., Reaven G. M. Reappraisal of the role of insulin in hypertriglyceridemia. Am J Med. 1974 Oct;57(4):551–560. doi: 10.1016/0002-9343(74)90006-0. [DOI] [PubMed] [Google Scholar]
- Otway S., Robinson D. S. The use of a non-ionic detergent (Triton WR 1339) to determine rates of triglyceride entry into the circulation of the rat under different physiological conditions. J Physiol. 1967 May;190(2):321–332. doi: 10.1113/jphysiol.1967.sp008211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patsch W., Franz S., Schonfeld G. Role of insulin in lipoprotein secretion by cultured rat hepatocytes. J Clin Invest. 1983 May;71(5):1161–1174. doi: 10.1172/JCI110865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patsch W., Gotto A. M., Jr, Patsch J. R. Effects of insulin on lipoprotein secretion in rat hepatocyte cultures. The role of the insulin receptor. J Biol Chem. 1986 Jul 25;261(21):9603–9606. [PubMed] [Google Scholar]
- Pullinger C. R., Gibbons G. F. Effects of hormones and pyruvate on the rates of secretion of very-low-density lipoprotein triacylglycerol and cholesterol by rat hepatocytes. Biochim Biophys Acta. 1985 Jan 9;833(1):44–51. doi: 10.1016/0005-2760(85)90251-6. [DOI] [PubMed] [Google Scholar]
- Pullinger C. R., Gibbons G. F. The relationship between the rate of hepatic sterol synthesis and the incorporation of [3H]water. J Lipid Res. 1983 Oct;24(10):1321–1328. [PubMed] [Google Scholar]
- Salmon D. M., Bowen N. L., Hems D. A. Synthesis of fatty acids in the perused mouse liver. Biochem J. 1974 Sep;142(3):611–618. doi: 10.1042/bj1420611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlierf G., Dorow E. Diurnal patterns of triglycerides, free fatty acids, blood sugar, and insulin during carbohydrate-induction in man and their modification by nocturnal suppression of lipolysis. J Clin Invest. 1973 Mar;52(3):732–740. doi: 10.1172/JCI107235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiedgen M., Seitz H. J. Dietary control of circadian variations in serum insulin, glucagon and hepatic cyclic AMP. J Nutr. 1980 May;110(5):876–882. doi: 10.1093/jn/110.5.876. [DOI] [PubMed] [Google Scholar]
- Witztum J. L., Young S. G., Elam R. L., Carew T. E., Fisher M. Cholestyramine-induced changes in low density lipoprotein composition and metabolism. I. Studies in the guinea pig. J Lipid Res. 1985 Jan;26(1):92–103. [PubMed] [Google Scholar]


