Abstract
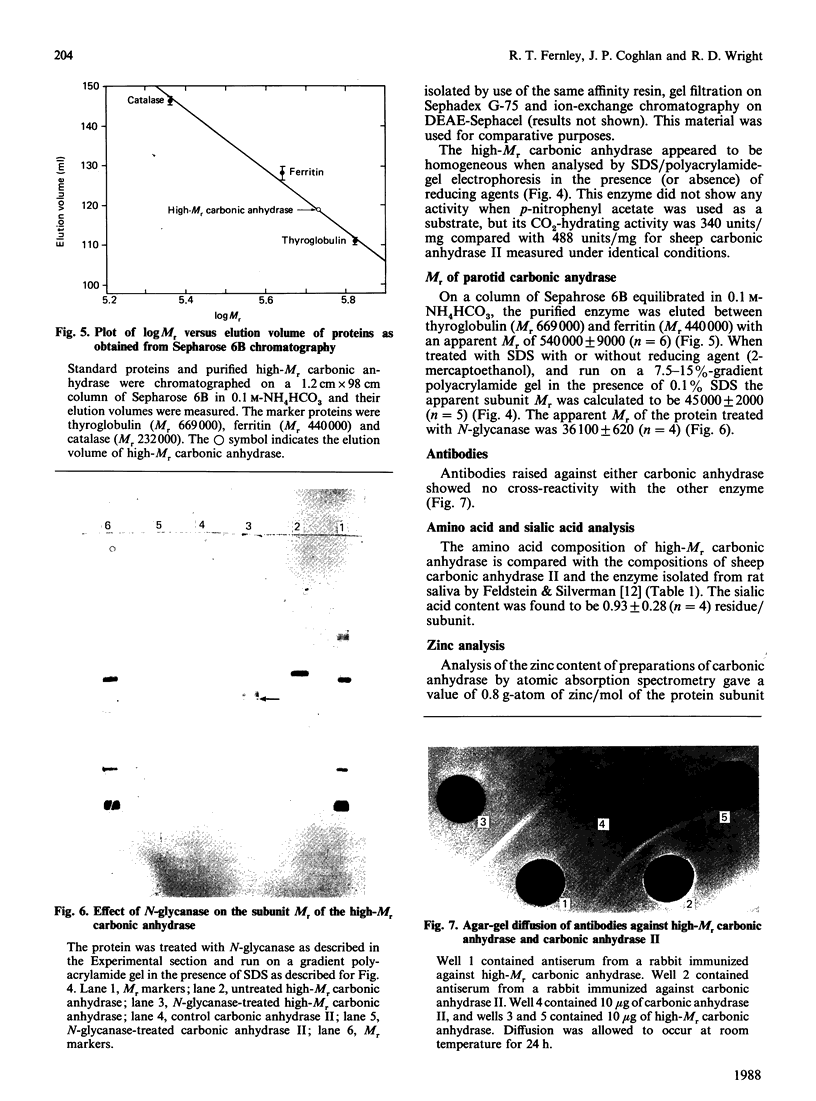
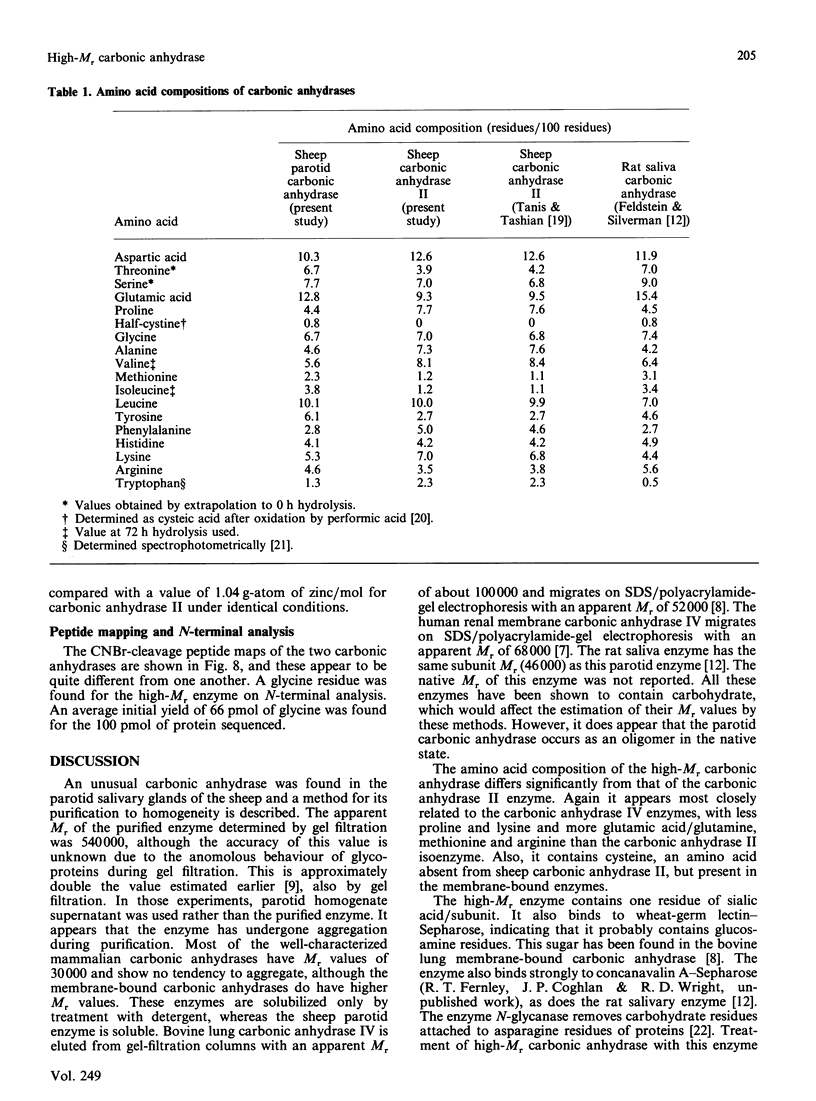
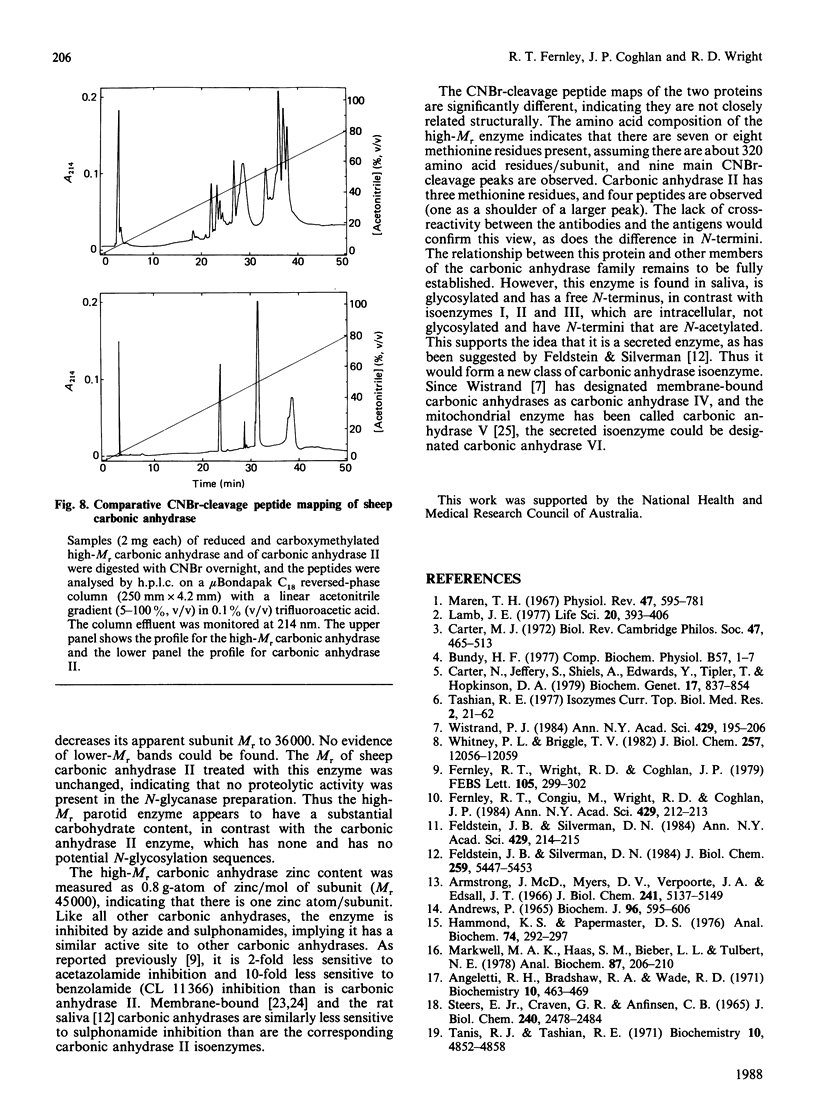
Approximately half the carbonic anhydrase activity of sheep parotid-gland homogenate is derived from a high-Mr protein [Fernley, Wright & Coghlan (1979) FEBS Lett. 105, 299-302]. This enzyme has now been purified to homogeneity, and its properties were compared with those of the well-characterized sheep carbonic anhydrase II. The protein has an apparent Mr of 540,000 as measured by gel filtration under non-denaturing conditions and an apparent subunit Mr of 45,000 as measured by SDS/polyacrylamide-gel electrophoresis. After deglycosylation with the enzyme N-glycanase the protein migrates with an apparent Mr of 36,000 on SDS/polyacrylamide-gel electrophoresis. The CO2-hydrating activity was 340 units/mg compared with 488 units/mg for sheep carbonic anhydrase II measured under identical conditions. This enzyme does not, however, hydrolyse p-nitrophenyl acetate. The enzyme contains 0.8 g-atom of zinc/mol of protein subunit. The peptide maps of the two carbonic anhydrases differ significantly from one another, indicating they are not related closely structurally. Unlike the carbonic anhydrase II isoenzyme, which has a blocked N-terminus, the high-Mr enzyme has a free glycine residue at its N-terminus.
Full text
PDF
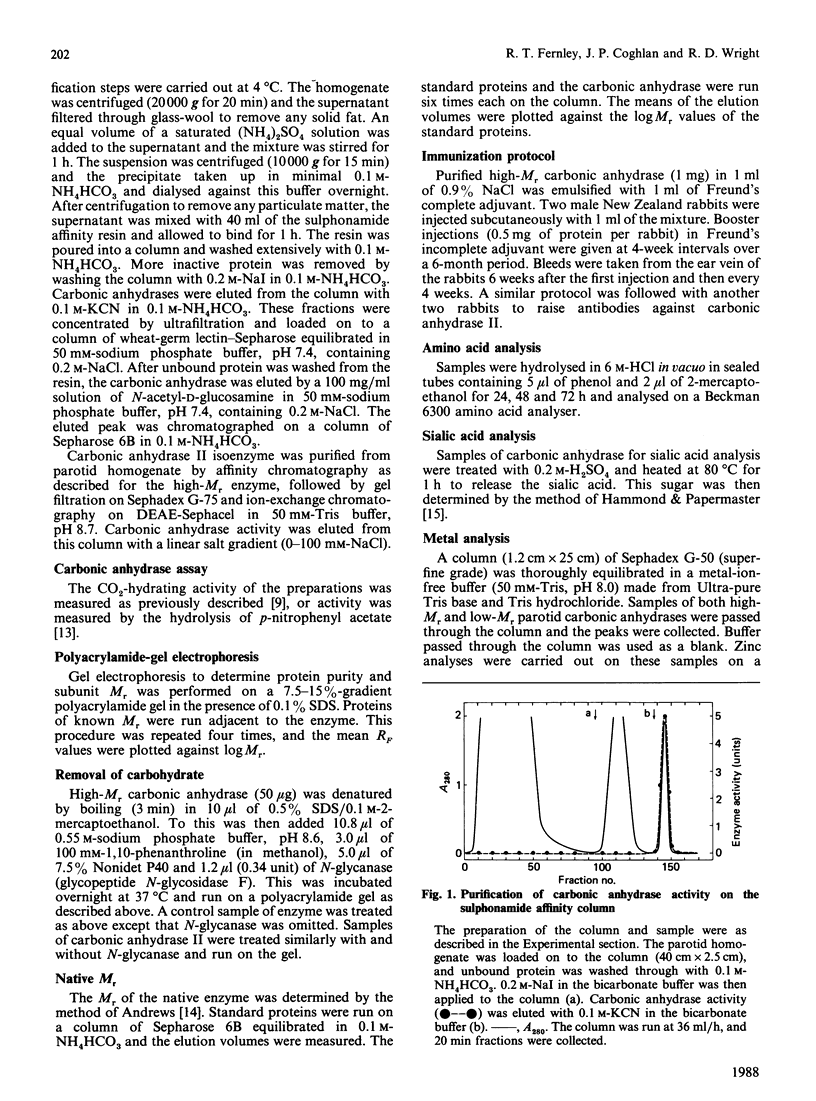
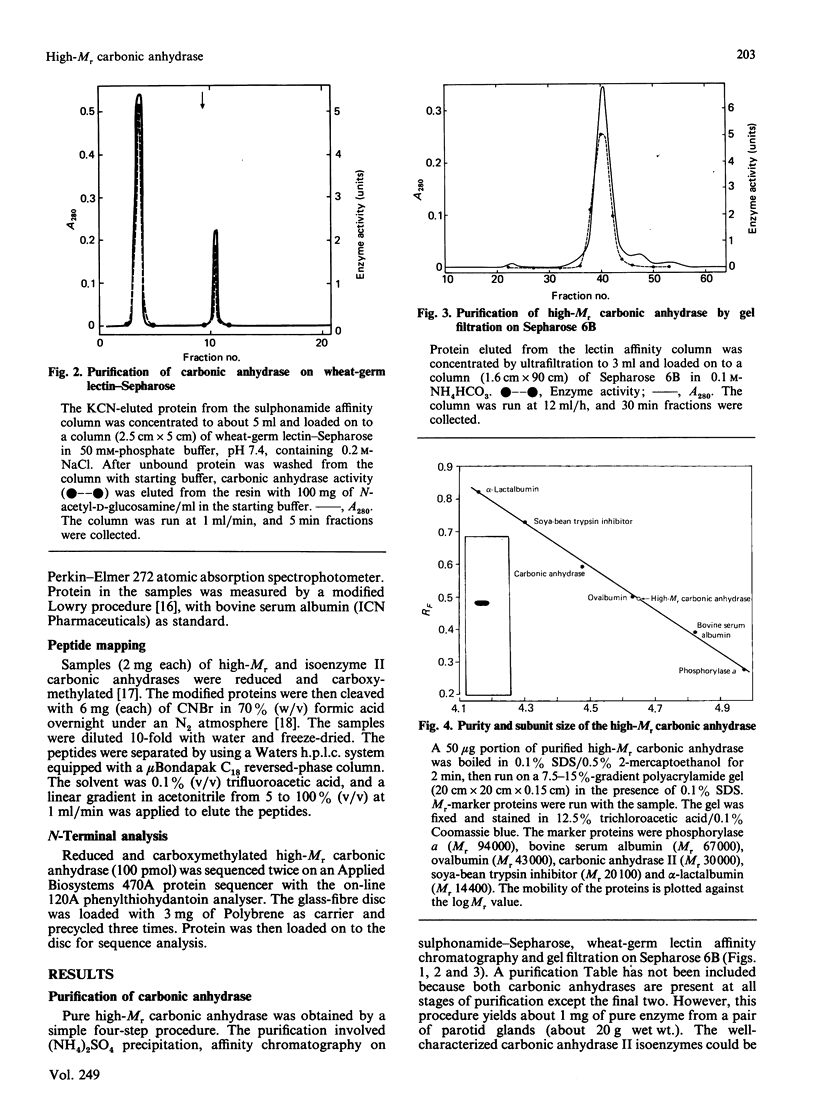

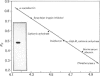
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andrews P. The gel-filtration behaviour of proteins related to their molecular weights over a wide range. Biochem J. 1965 Sep;96(3):595–606. doi: 10.1042/bj0960595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angeletti R. H., Bradshaw R. A., Wade R. D. Subunit structure and amino acid composition of mouse submaxillary gland nerve growth factor. Biochemistry. 1971 Feb 2;10(3):463–469. doi: 10.1021/bi00779a018. [DOI] [PubMed] [Google Scholar]
- Armstrong J. M., Myers D. V., Verpoorte J. A., Edsall J. T. Purification and properties of human erythrocyte carbonic anhydrases. J Biol Chem. 1966 Nov 10;241(21):5137–5149. [PubMed] [Google Scholar]
- Carter M. J. Carbonic anhydrase: isoenzymes, properties, distribution, and functional significance. Biol Rev Camb Philos Soc. 1972 Nov;47(4):465–513. doi: 10.1111/j.1469-185x.1972.tb01079.x. [DOI] [PubMed] [Google Scholar]
- Carter N., Jeffery S., Shiels A., Edwards Y., Tipler T., Hopkinson D. A. Characterization of human carbonic anhydrase III from skeletal muscle. Biochem Genet. 1979 Oct;17(9-10):837–854. doi: 10.1007/BF00504307. [DOI] [PubMed] [Google Scholar]
- Edelhoch H. Spectroscopic determination of tryptophan and tyrosine in proteins. Biochemistry. 1967 Jul;6(7):1948–1954. doi: 10.1021/bi00859a010. [DOI] [PubMed] [Google Scholar]
- Feldstein J. B., Silverman D. N. Purification and characterization of carbonic anhydrase from the saliva of the rat. J Biol Chem. 1984 May 10;259(9):5447–5453. [PubMed] [Google Scholar]
- Fernley R. T., Wright R. D., Coghlan J. P. A novel carbonic anhydrase from the ovine parotid gland. FEBS Lett. 1979 Sep 15;105(2):299–302. doi: 10.1016/0014-5793(79)80634-1. [DOI] [PubMed] [Google Scholar]
- Hammond K. S., Papermaster D. S. Fluorometric assay of sialic acid in the picomole range: a modification of the thiobarbituric acid assay. Anal Biochem. 1976 Aug;74(2):292–297. doi: 10.1016/0003-2697(76)90210-4. [DOI] [PubMed] [Google Scholar]
- Lamb J. E. Minireview plant carbonic anhydrase. Life Sci. 1977 Feb 1;20(3):393–406. doi: 10.1016/0024-3205(77)90379-4. [DOI] [PubMed] [Google Scholar]
- Maren T. H. Carbonic anhydrase: chemistry, physiology, and inhibition. Physiol Rev. 1967 Oct;47(4):595–781. doi: 10.1152/physrev.1967.47.4.595. [DOI] [PubMed] [Google Scholar]
- Maren T. H. Current status of membrane-bound carbonic anhydrase. Ann N Y Acad Sci. 1980;341:246–258. doi: 10.1111/j.1749-6632.1980.tb47176.x. [DOI] [PubMed] [Google Scholar]
- Markwell M. A., Haas S. M., Bieber L. L., Tolbert N. E. A modification of the Lowry procedure to simplify protein determination in membrane and lipoprotein samples. Anal Biochem. 1978 Jun 15;87(1):206–210. doi: 10.1016/0003-2697(78)90586-9. [DOI] [PubMed] [Google Scholar]
- Plummer T. H., Jr, Elder J. H., Alexander S., Phelan A. W., Tarentino A. L. Demonstration of peptide:N-glycosidase F activity in endo-beta-N-acetylglucosaminidase F preparations. J Biol Chem. 1984 Sep 10;259(17):10700–10704. [PubMed] [Google Scholar]
- STEERS E., Jr, CRAVEN G. R., ANFINSEN C. B., BETHUNE J. L. EVIDENCE FOR NONIDENTICAL CHAINS IN THE BETA-GALACTOSIDASE OF ESCHERICHIA COLI K12. J Biol Chem. 1965 Jun;240:2478–2484. [PubMed] [Google Scholar]
- Tanis R. J., Tashian R. E. Purification and properties of carbonic anhydrase from sheep erythrocytes. Biochemistry. 1971 Dec 21;10(26):4852–4858. doi: 10.1021/bi00802a004. [DOI] [PubMed] [Google Scholar]
- Venta P. J., Montgomery J. C., Tashian R. E. Molecular genetics of carbonic anhydrase isozymes. Isozymes Curr Top Biol Med Res. 1987;14:59–72. [PubMed] [Google Scholar]
- Whitney P. L., Briggle T. V. Membrane-associated carbonic anhydrase purified from bovine lung. J Biol Chem. 1982 Oct 25;257(20):12056–12059. [PubMed] [Google Scholar]
- Wistrand P. J., Kinne R. Carbonic anhydrase activity of isolated brush border and basal-lateral membranes of renal tubular cells. Pflugers Arch. 1977 Aug 29;370(2):121–126. doi: 10.1007/BF00581684. [DOI] [PubMed] [Google Scholar]
- Wistrand P. J. Properties of membrane-bound carbonic anhydrase. Ann N Y Acad Sci. 1984;429:195–206. doi: 10.1111/j.1749-6632.1984.tb12333.x. [DOI] [PubMed] [Google Scholar]