Abstract
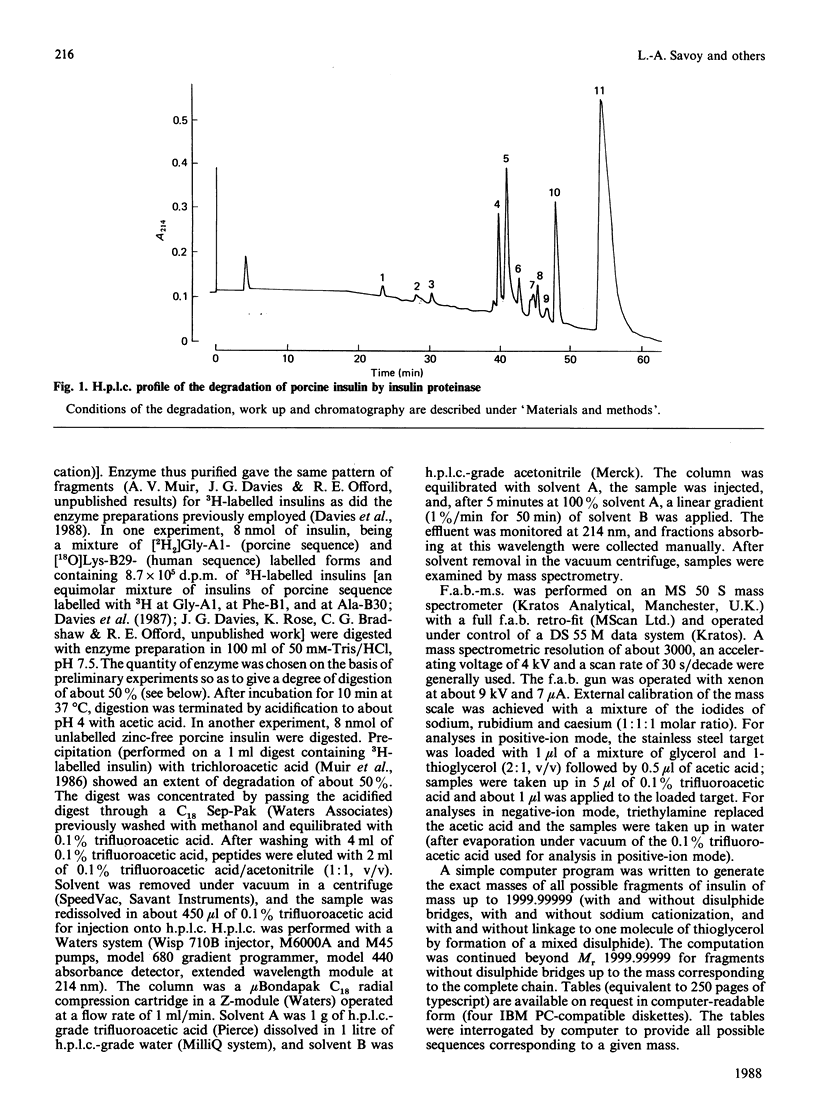
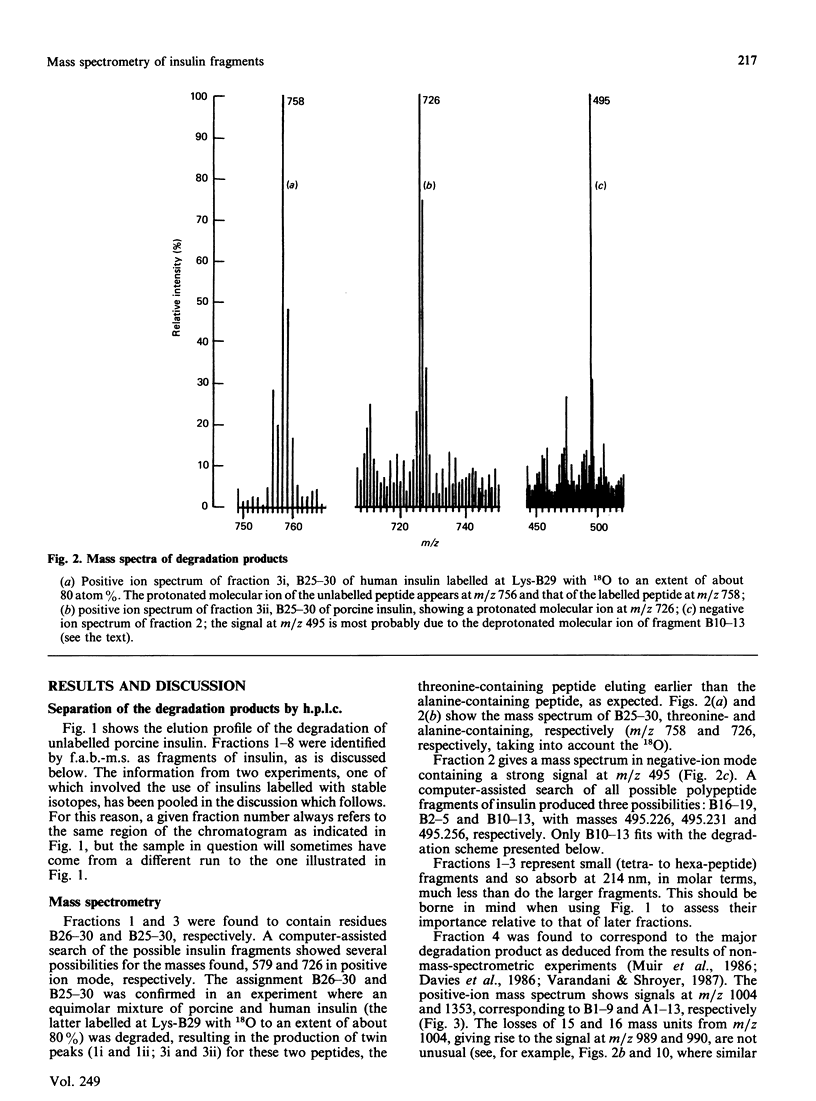
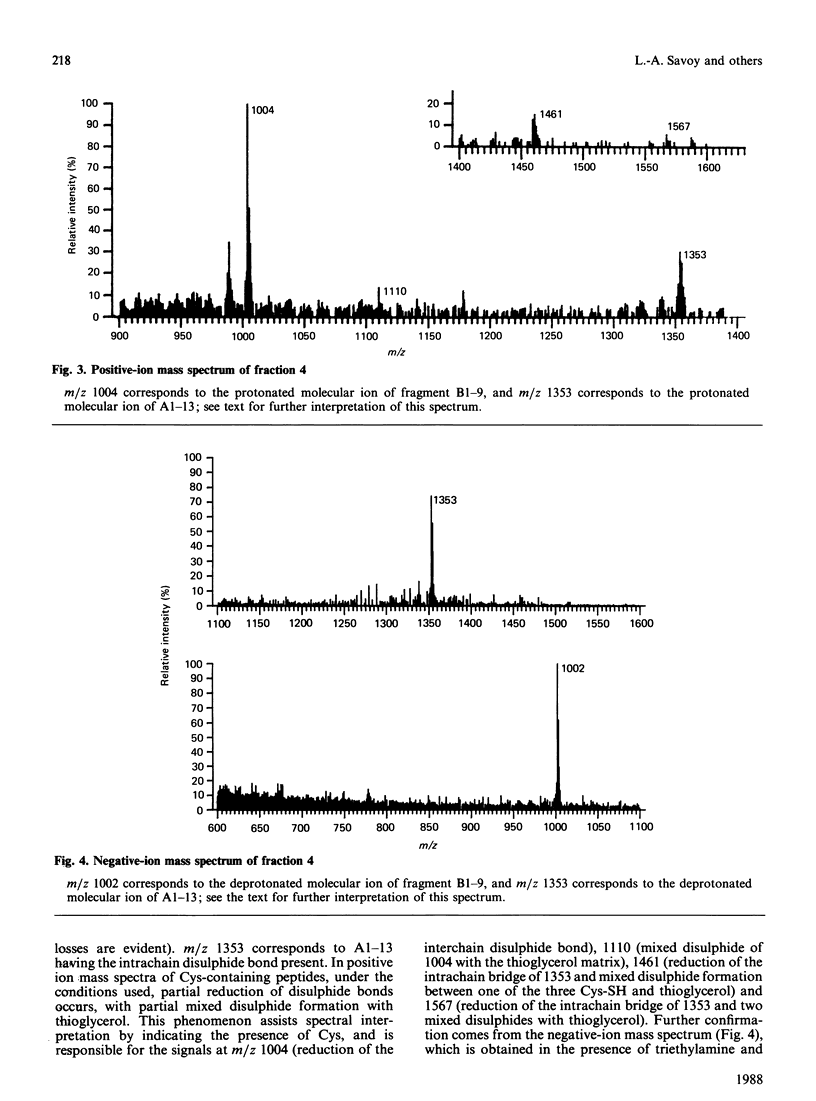
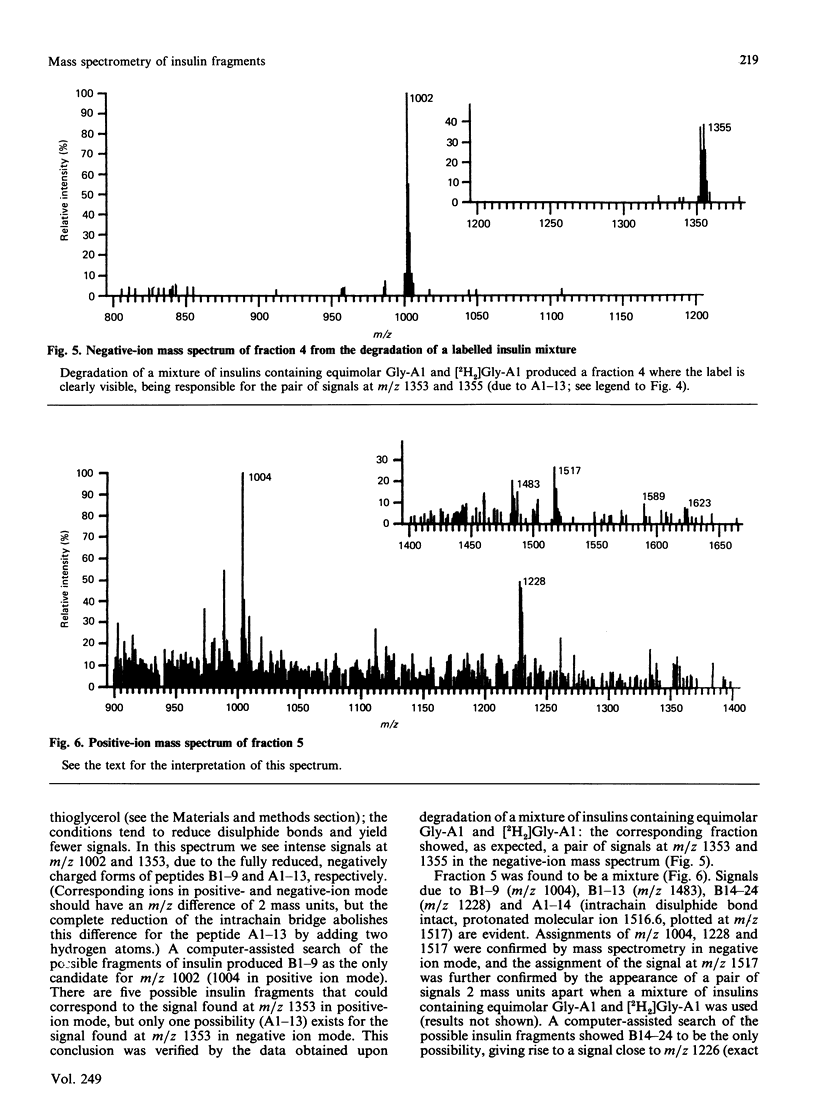
We describe the isolation by reversed-phase h.p.l.c. of a number of products of the degradation of insulin by insulin proteinase and their direct analysis by fast atom bombardment mass spectrometry (f.a.b.-m.s.). Various semisynthetically labelled insulins were used, including [[2H2]GlyA1]insulin and [18O]LysB29]insulin. The results obtained confirm and extend the results obtained by non-mass-spectrometric methods [Davies, Muir, Rose & Offord (1988) Biochem. J. 249, 209-214, and papers cited therein]. Cleavage sites were identified between positions A13-A14, A14-A15, B9-B10, B13-B14, B24-B25 and B25-B26. The advantages and disadvantages of the application of f.a.b.-m.s. to such studies are discussed.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Davies J. G., Muir A. V., Offord R. E. Identification of some cleavage sites of insulin by insulin proteinase. Biochem J. 1986 Dec 1;240(2):609–612. doi: 10.1042/bj2400609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies J. G., Muir A. V., Rose K., Offord R. E. Identification of radioactive insulin fragments liberated by insulin proteinase during the degradation of semisynthetic [3H]GlyA1]insulin and [3H]PheB1]insulin. Biochem J. 1988 Jan 1;249(1):209–214. doi: 10.1042/bj2490209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies J. G., Offord R. E. The preparation of tritiated insulin specifically labelled by semisynthesis at glycine-A1. Biochem J. 1985 Oct 15;231(2):389–392. doi: 10.1042/bj2310389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duckworth W. C., Stentz F. B., Heinemann M., Kitabchi A. E. Initial site of insulin cleavage by insulin protease. Proc Natl Acad Sci U S A. 1979 Feb;76(2):635–639. doi: 10.1073/pnas.76.2.635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamel F. G., Peavy D. E., Ryan M. P., Duckworth W. C. High performance liquid chromatographic analysis of insulin degradation by rat skeletal muscle insulin protease. Endocrinology. 1986 Jan;118(1):328–333. doi: 10.1210/endo-118-1-328. [DOI] [PubMed] [Google Scholar]
- Muir A., Offord R. E., Davies J. G. The identification of a major product of the degradation of insulin by 'insulin proteinase' (EC 3.4.22.11). Biochem J. 1986 Aug 1;237(3):631–637. doi: 10.1042/bj2370631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose K., Gladstone J., Offord R. E. A mass-spectrometric investigation of the mechanism of the semisynthetic transformation of pig insulin into an ester of insulin of human sequence. Biochem J. 1984 May 15;220(1):189–196. doi: 10.1042/bj2200189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varandani P. T., Shroyer L. A. Identification of an insulin fragment produced by an insulin degrading enzyme, neutral thiopeptidase. Mol Cell Endocrinol. 1987 Apr;50(3):171–175. doi: 10.1016/0303-7207(87)90014-1. [DOI] [PubMed] [Google Scholar]
- Williams D. H., Bradley C. V., Santikarn S., Bojesen G. Fast-atom-bombardment mass spectrometry. A new technique for the determination of molecular weights and amino acid sequences of peptides. Biochem J. 1982 Jan 1;201(1):105–117. doi: 10.1042/bj2010105. [DOI] [PMC free article] [PubMed] [Google Scholar]


