Abstract
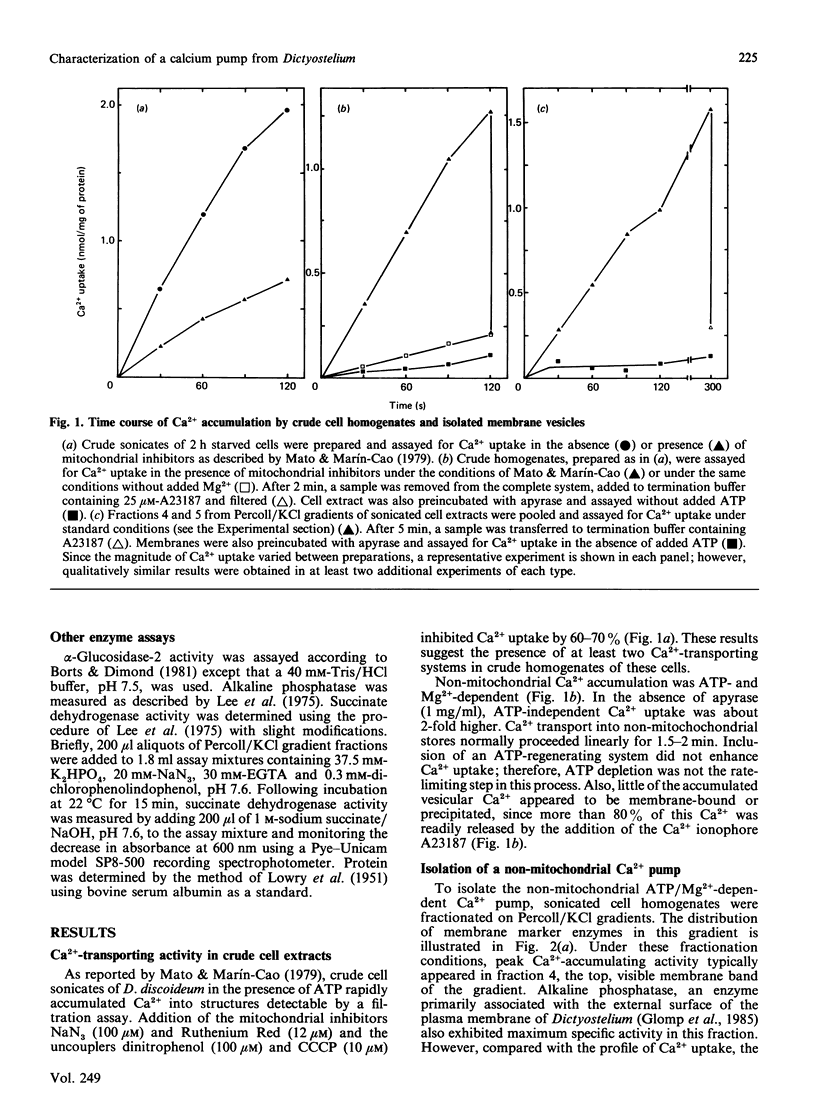
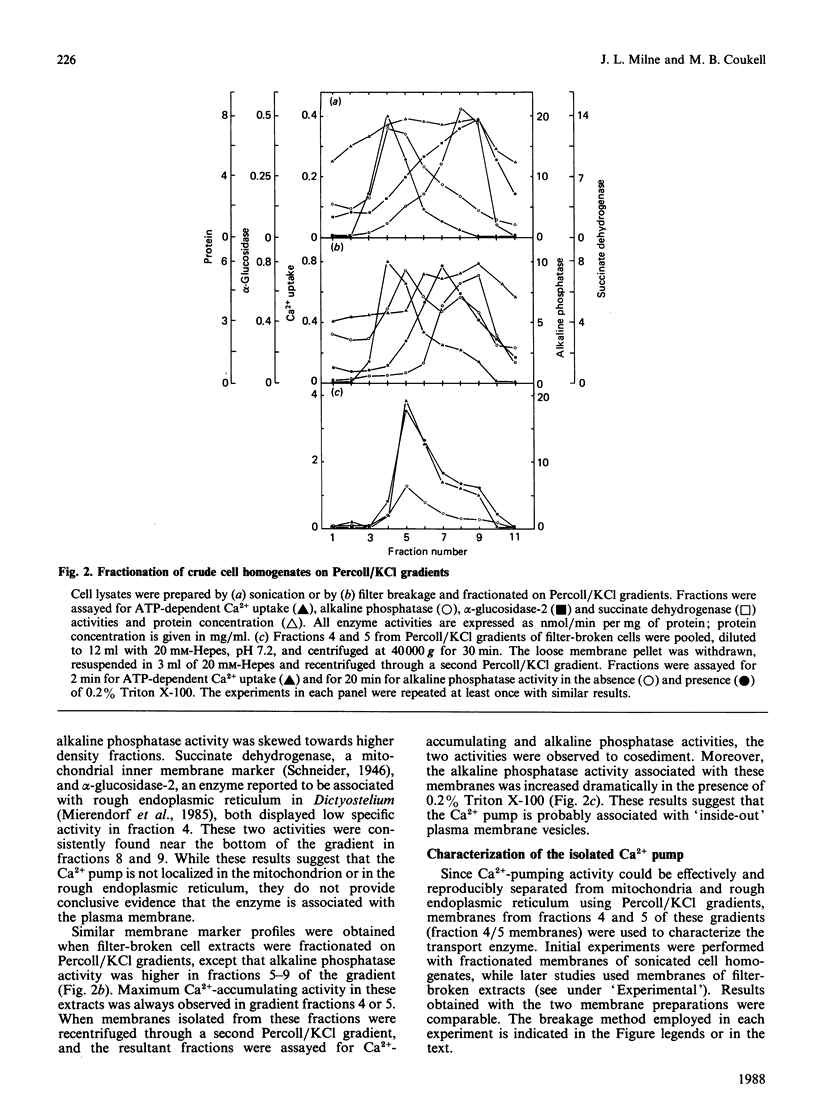
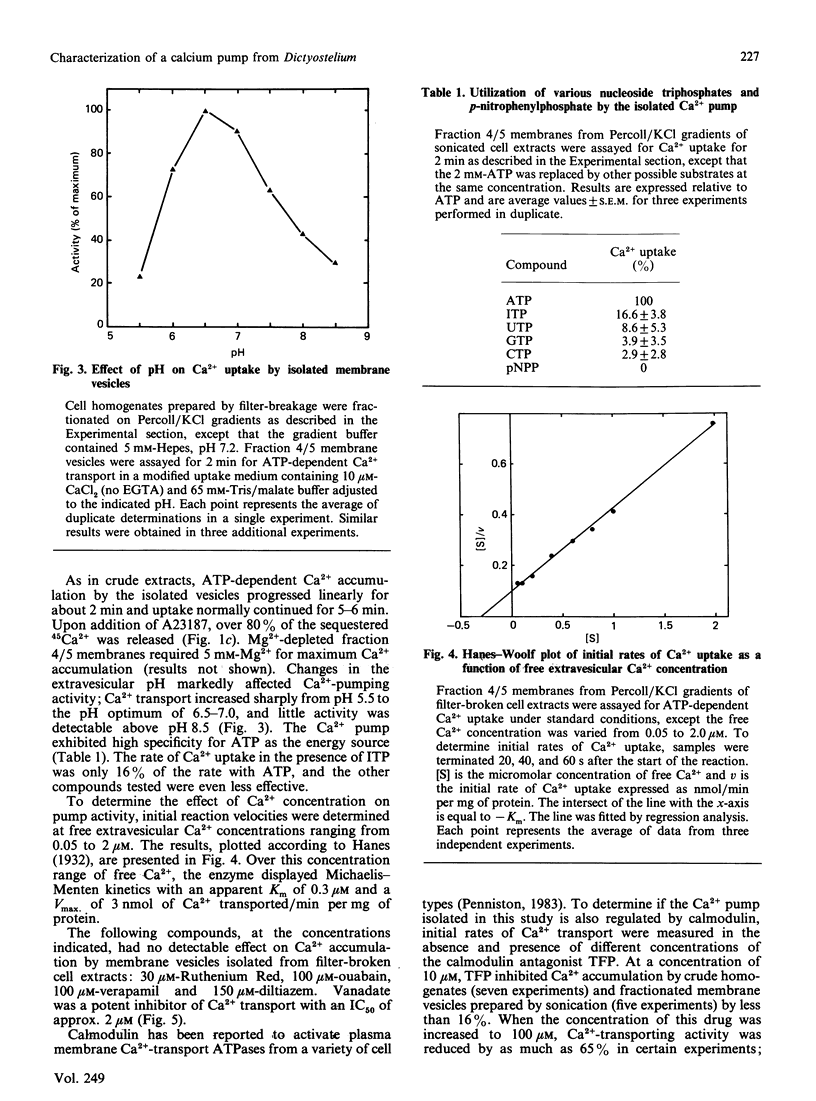
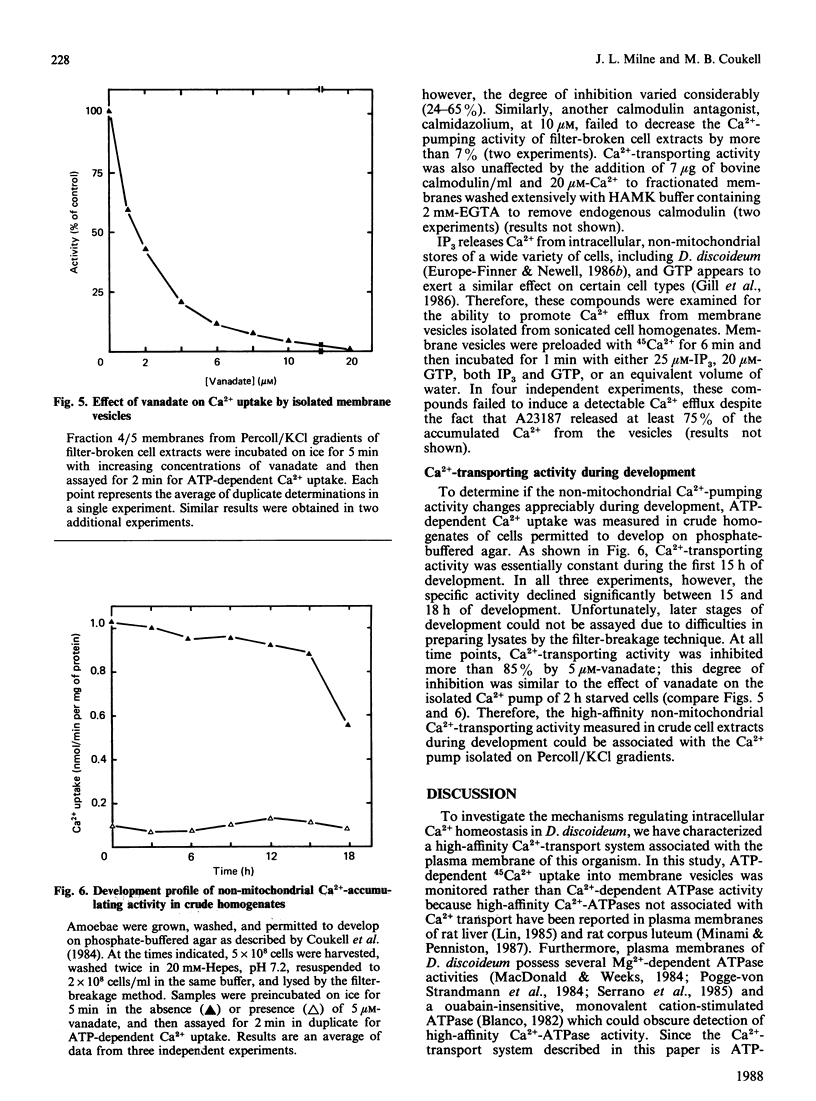
During the aggregation and differentiation of amoebae of Dictyostelium discoideum, changes in free cytosolic Ca2+ appear to regulate a number of physiological processes. To understand the mechanisms regulating free intracellular Ca2+ in this organism, we have isolated and characterized an ATP/Mg2+-dependent, high-affinity Ca2+ pump. When homogenates of 2 h starved cells were fractionated on Percoll/KCl gradients, one peak of high-affinity Ca2+-pumping activity was detected. This activity was resolved from enzyme markers of the mitochondrion and the rough endoplasmic reticulum but it cosedimented with the plasma membrane marker, alkaline phosphatase. Further studies suggested that the pump was associated with 'inside-out' plasma membrane vesicles. Like plasma membrane Ca2+-transport ATPases from other systems, this isolated Ca2+ pump: (1) was Mg2+-dependent, (2) displayed a high specificity for ATP as an energy source, (3) exhibited a high affinity for free Ca2+ with a Km of 0.3 microM, and (4) was very sensitive to inhibition by vanadate (IC50 2 microM) but was unaffected by mitochondrial inhibitors, ouabain and Ca2+-channel blockers. Unlike plasma membrane Ca2+ pumps from most other systems, this enzyme appeared not to be regulated by calmodulin. During development, non-mitochondrial, vanadate-sensitive, high-affinity Ca2+-pumping activity in crude lysates remained relatively constant for at least 15 h. These observations suggest that this plasma membrane Ca2+ pump probably functions in Dictyostelium to maintain Ca2+ homeostasis by extruding free cytosolic Ca2+ from the cells.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blanco M. Evidence for the existence of a monovalent cation-stimulated, magnesium-dependent adenosine triphosphatase activity in the isolated plasma membranes of amoebas of the slime mold dictyostelium discoideum. Biochim Biophys Acta. 1982 Apr 23;687(1):94–96. doi: 10.1016/0005-2736(82)90173-0. [DOI] [PubMed] [Google Scholar]
- Borts R. H., Dimond R. L. The alpha-glucosidases of Dictyostelium discoideum. I. Identification and characterization. Dev Biol. 1981 Oct 15;87(1):176–184. doi: 10.1016/0012-1606(81)90070-1. [DOI] [PubMed] [Google Scholar]
- Bumann J., Wurster B., Malchow D. Attractant-induced changes and oscillations of the extracellular Ca++ concentration in suspensions of differentiating Dictyostelium cells. J Cell Biol. 1984 Jan;98(1):173–178. doi: 10.1083/jcb.98.1.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caroni P., Carafoli E. The Ca2+-pumping ATPase of heart sarcolemma. Characterization, calmodulin dependence, and partial purification. J Biol Chem. 1981 Apr 10;256(7):3263–3270. [PubMed] [Google Scholar]
- Cocucci S. M., Sussman M. RNA in cytoplasmic and nuclear fractions of cellular slime mold amebas. J Cell Biol. 1970 May;45(2):399–407. doi: 10.1083/jcb.45.2.399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coukell M. B., Cameron A. M., Pitre C. M., Mee J. D. Developmental regulation and properties of the cGMP-specific phosphodiesterase in Dictyostelium discoideum. Dev Biol. 1984 May;103(1):246–257. doi: 10.1016/0012-1606(84)90026-5. [DOI] [PubMed] [Google Scholar]
- Europe-Finner G. N., McClue S. J., Newell P. C. Inhibition of aggregation in Dictyostelium by EGTA-induced depletion of calcium. FEMS Microbiol Lett. 1984 Jan;21(1):21–25. doi: 10.1111/j.1574-6968.1984.tb00179.x. [DOI] [PubMed] [Google Scholar]
- Europe-Finner G. N., Newell P. C. Calcium transport in the cellular slime mould Dictyostelium discoideum. FEBS Lett. 1985 Jul 1;186(1):70–74. doi: 10.1016/0014-5793(85)81341-7. [DOI] [PubMed] [Google Scholar]
- Europe-Finner G. N., Newell P. C. Inositol 1,4,5-triphosphate induces calcium release from a non- mitochondrial pool in amoebae of Dictyostelium. Biochim Biophys Acta. 1986 Aug 1;887(3):335–340. doi: 10.1016/0167-4889(86)90163-1. [DOI] [PubMed] [Google Scholar]
- Europe-Finner G. N., Newell P. C. Inositol 1,4,5-trisphosphate and calcium stimulate actin polymerization in Dictyostelium discoideum. J Cell Sci. 1986 Jun;82:41–51. doi: 10.1242/jcs.82.1.41. [DOI] [PubMed] [Google Scholar]
- Fabiato A., Fabiato F. Calculator programs for computing the composition of the solutions containing multiple metals and ligands used for experiments in skinned muscle cells. J Physiol (Paris) 1979;75(5):463–505. [PubMed] [Google Scholar]
- Gietzen K., Wüthrich A., Bader H. R 24571: a new powerful inhibitor of red blood cell Ca++-transport ATPase and of calmodulin-regulated functions. Biochem Biophys Res Commun. 1981 Jul 30;101(2):418–425. doi: 10.1016/0006-291x(81)91276-6. [DOI] [PubMed] [Google Scholar]
- Gilkes N. R., Weeks G. The purification and characterization of Dictyostelium discoideum plasma membranes. Biochim Biophys Acta. 1977 Jan 4;464(1):142–156. doi: 10.1016/0005-2736(77)90377-7. [DOI] [PubMed] [Google Scholar]
- Gill D. L., Ueda T., Chueh S. H., Noel M. W. Ca2+ release from endoplasmic reticulum is mediated by a guanine nucleotide regulatory mechanism. Nature. 1986 Apr 3;320(6061):461–464. doi: 10.1038/320461a0. [DOI] [PubMed] [Google Scholar]
- Glomp I., Schäfer D., Hess B. Cytochemical localization of alkaline phosphatase in the cell membrane of Dictyostelium discoideum amebae. Histochemistry. 1985;83(3):251–255. doi: 10.1007/BF00953993. [DOI] [PubMed] [Google Scholar]
- Goodloe-Holland C. M., Luna E. J. Purification and characterization of Dictyostelium discoideum plasma membranes. Methods Cell Biol. 1987;28:103–128. doi: 10.1016/s0091-679x(08)61639-8. [DOI] [PubMed] [Google Scholar]
- Hanes C. S. Studies on plant amylases: The effect of starch concentration upon the velocity of hydrolysis by the amylase of germinated barley. Biochem J. 1932;26(5):1406–1421. doi: 10.1042/bj0261406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lee A., Chance K., Weeks C., Weeks G. Studies on the alkaline phosphatase and 5'-nucleotidase of Dictyostelium discoideum. Arch Biochem Biophys. 1975 Dec;171(2):407–417. doi: 10.1016/0003-9861(75)90049-1. [DOI] [PubMed] [Google Scholar]
- Lin S. H. Novel ATP-dependent calcium transport component from rat liver plasma membranes. The transporter and the previously reported (Ca2+-Mg2+)-ATPase are different proteins. J Biol Chem. 1985 Jul 5;260(13):7850–7856. [PubMed] [Google Scholar]
- Macdonald J. I., Weeks G. A plasma membrane Mg2+-ATPase in the cellular slime mold Dictyostelium discoideum. Arch Biochem Biophys. 1984 Nov 15;235(1):1–7. doi: 10.1016/0003-9861(84)90248-0. [DOI] [PubMed] [Google Scholar]
- Maeda Y. Influence of ionic conditions on cell differentiation and morphogenesis of the cellular slime molds. Dev Growth Differ. 1970 Dec;12(3):217–227. doi: 10.1111/j.1440-169x.1970.00217.x. [DOI] [PubMed] [Google Scholar]
- Maeda Y., Maeda M. The calcium content of the cellular slime mold, Dictyostelium discoideum, during development and differentiation. Exp Cell Res. 1973 Nov;82(1):125–130. doi: 10.1016/0014-4827(73)90253-x. [DOI] [PubMed] [Google Scholar]
- Malchow D., Böhme R., Rahmsdorf H. J. Regulation of phosphorylation of myosin heavy chain during the chemotactic response of Dictyostelium cells. Eur J Biochem. 1981 Jun;117(1):213–218. doi: 10.1111/j.1432-1033.1981.tb06324.x. [DOI] [PubMed] [Google Scholar]
- Maruta H., Baltes W., Dieter P., Marmé D., Gerisch G. Myosin heavy chain kinase inactivated by Ca2+/calmodulin from aggregating cells of Dictyostelium discoideum. EMBO J. 1983;2(4):535–542. doi: 10.1002/j.1460-2075.1983.tb01459.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mato J. M., Marín-Cao D. Protein and phospholipid methylation during chemotaxis in Dictyostelium discoideum and its relationship to calcium movements. Proc Natl Acad Sci U S A. 1979 Dec;76(12):6106–6109. doi: 10.1073/pnas.76.12.6106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mato J. M., Van Haastert P. J., Krens F. A., Rhijnsburger E. H., Dobbe F. C., Konijn T. M. Cyclic AMP and folic acid mediated cyclic GMP accumulation in Dictyostelium discoideum. FEBS Lett. 1977 Jul 15;79(2):331–336. doi: 10.1016/0014-5793(77)80814-4. [DOI] [PubMed] [Google Scholar]
- McRobbie S. J., Newell P. C. Chemoattractant-mediated changes in cytoskeletal actin of cellular slime moulds. J Cell Sci. 1984 Jun;68:139–151. doi: 10.1242/jcs.68.1.139. [DOI] [PubMed] [Google Scholar]
- Mierendorf R. C., Jr, Cardelli J. A., Dimond R. L. Pathways involved in targeting and secretion of a lysosomal enzyme in Dictyostelium discoideum. J Cell Biol. 1985 May;100(5):1777–1787. doi: 10.1083/jcb.100.5.1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minami J., Penniston J. T. Ca2+ uptake by corpus-luteum plasma membranes. Evidence for the presence of both a Ca2+-pumping ATPase and a Ca2+-dependent nucleoside triphosphatase. Biochem J. 1987 Mar 15;242(3):889–894. doi: 10.1042/bj2420889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Rourke F. A., Halenda S. P., Zavoico G. B., Feinstein M. B. Inositol 1,4,5-trisphosphate releases Ca2+ from a Ca2+-transporting membrane vesicle fraction derived from human platelets. J Biol Chem. 1985 Jan 25;260(2):956–962. [PubMed] [Google Scholar]
- Prentki M., Wollheim C. B., Lew P. D. Ca2+ homeostasis in permeabilized human neutrophils. Characterization of Ca2+-sequestering pools and the action of inositol 1,4,5-triphosphate. J Biol Chem. 1984 Nov 25;259(22):13777–13782. [PubMed] [Google Scholar]
- Schaap P., Van Lookeren Campagne M. M., Van Driel R., Spek W., Van Haastert P. J., Pinas J. Postaggregative differentiation induction by cyclic AMP in Dictyostelium: intracellular transduction pathway and requirement for additional stimuli. Dev Biol. 1986 Nov;118(1):52–63. doi: 10.1016/0012-1606(86)90072-2. [DOI] [PubMed] [Google Scholar]
- Small N. V., Europe-Firmer G. N., Newell P. C. Calcium induces cyclic GMP formation in Dictyostelium. FEBS Lett. 1986 Jul 14;203(1):11–14. doi: 10.1016/0014-5793(86)81426-0. [DOI] [PubMed] [Google Scholar]
- Varnum B., Soll D. R. Chemoresponsiveness to cAMP and folic acid during growth, development, and dedifferentiation in Dictyostelium discoideum. Differentiation. 1981;18(3):151–160. doi: 10.1111/j.1432-0436.1981.tb01116.x. [DOI] [PubMed] [Google Scholar]
- Watts D. J., Ashworth J. M. Growth of myxameobae of the cellular slime mould Dictyostelium discoideum in axenic culture. Biochem J. 1970 Sep;119(2):171–174. doi: 10.1042/bj1190171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wick U., Malchow D., Gerisch G. Cyclic-AMP stimulated calcium influx into aggregating cells of Dictyostelium discoideum. Cell Biol Int Rep. 1978 Jan;2(1):71–79. doi: 10.1016/0309-1651(78)90086-3. [DOI] [PubMed] [Google Scholar]
- Yamasaki F., Hayashi H. Participation of calcium in the induction of phosphodiesterase by cyclic adenosine 3',5'-monophosphate in Dictyostelium discoideum. J Biochem. 1982 Dec;92(6):1911–1917. doi: 10.1093/oxfordjournals.jbchem.a134121. [DOI] [PubMed] [Google Scholar]


