Abstract
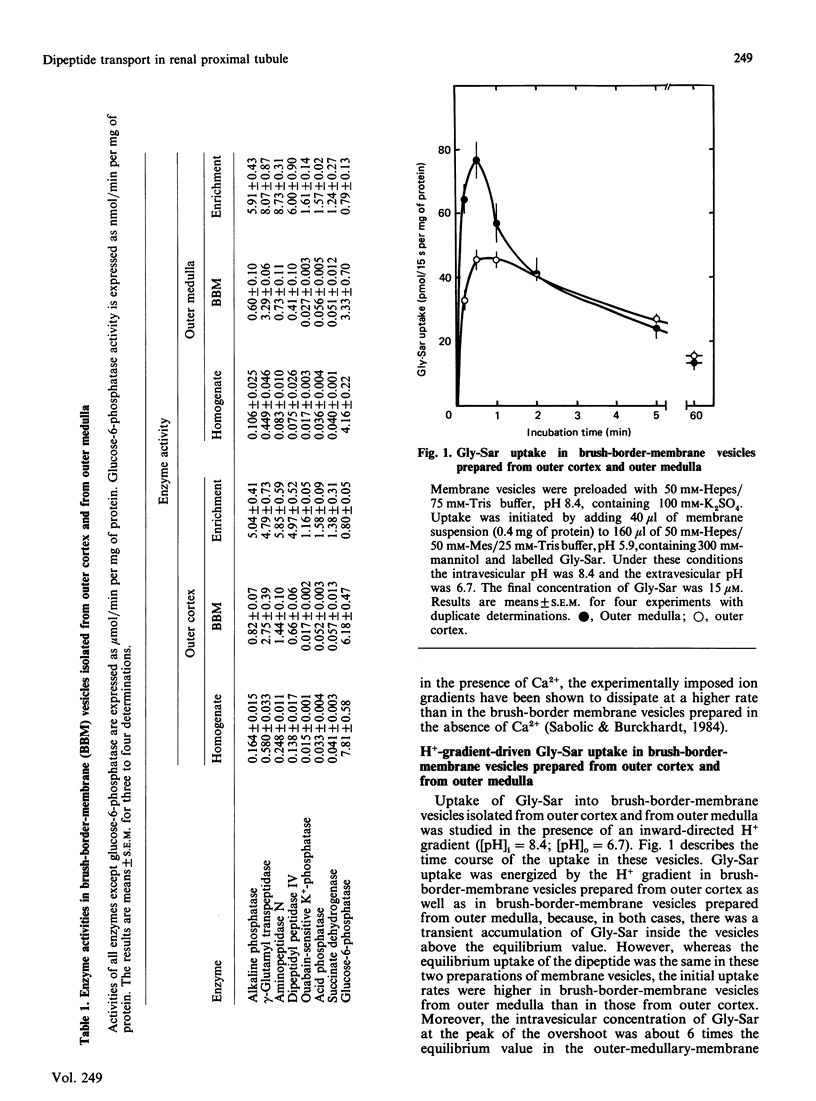
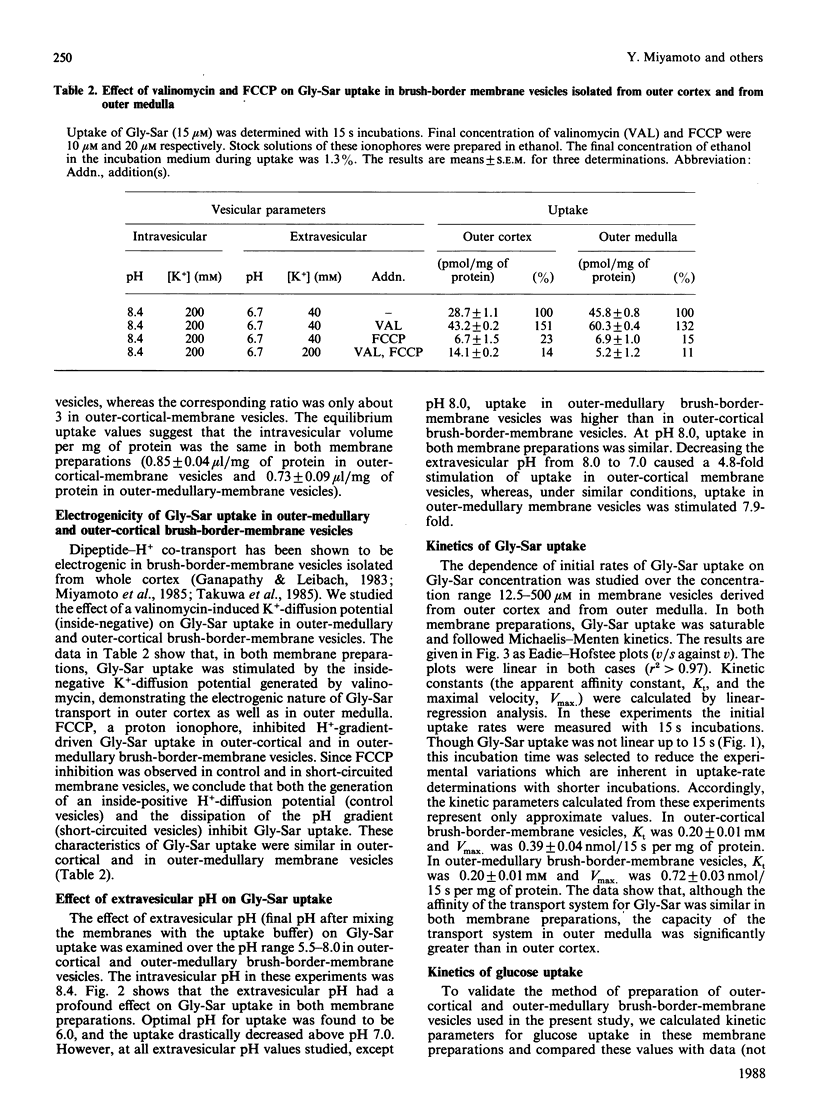
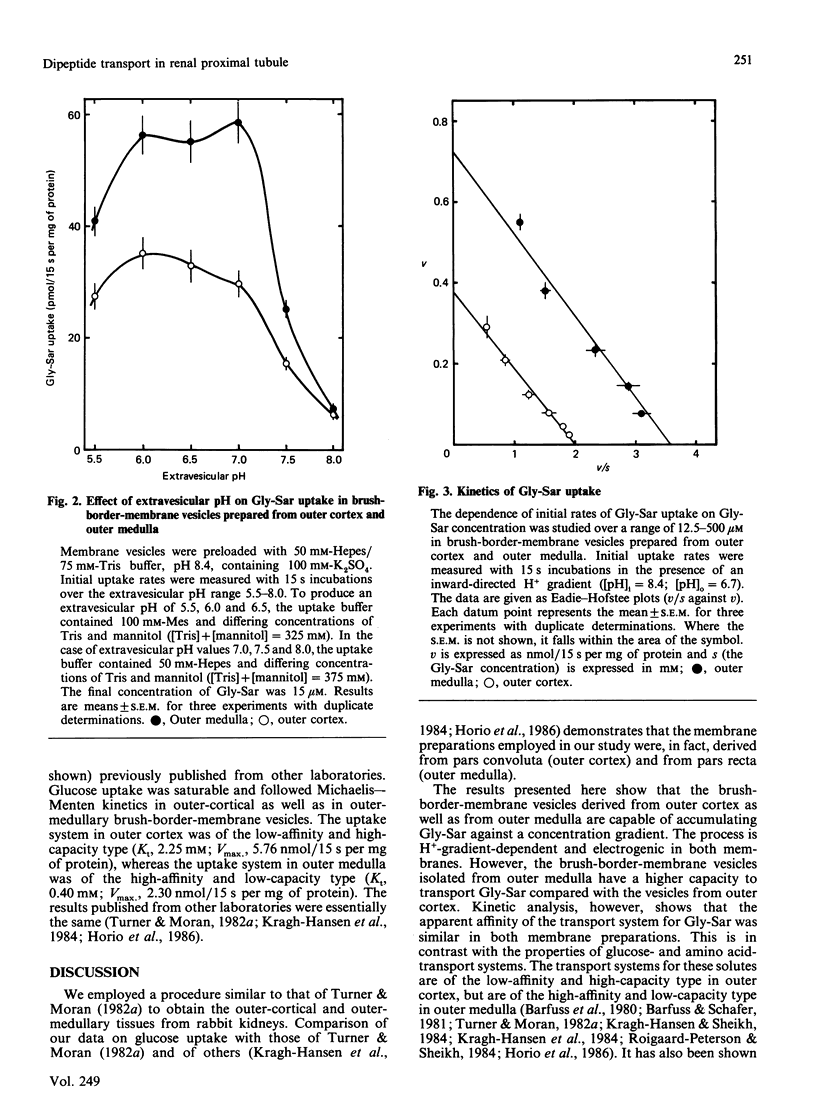
The distribution and properties of the peptide-transport system in rabbit renal proximal tubule was examined with glycylsarcosine as the substrate and using brush-border-membrane vesicles derived from pars convoluta (outer cortex) and pars recta (outer medulla). The dipeptide was transported into these vesicles against a concentration gradient in the presence of an inward-directed H+ gradient, demonstrating the presence of a H+-coupled peptide-transport system in outer-cortical as well as outer-medullary brush-border membranes. Even though the transport was electrogenic and was energized by a H+ gradient in both membranes, the system was more active in outer medullary membranes than in outer cortical membranes. Kinetic analysis showed that, although the affinity of the transport system for glycylsarcosine was similar in both membrane preparations, the capacity of the system was significantly greater in outer medulla than in outer cortex. In addition, the pH profiles of the peptide-transport systems in these membrane preparations also showed dissimilarities. The greater dipeptide uptake in one membrane vis-à-vis the other may probably be due to the difference in the affinity of the transport system for H+ and/or the difference in peptide/H+ stoichiometry.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barfuss D. W., Mays J. M., Schafer J. A. Peritubular uptake and transepithelial transport of glycine in isolated proximal tubules. Am J Physiol. 1980 Apr;238(4):F324–F333. doi: 10.1152/ajprenal.1980.238.4.F324. [DOI] [PubMed] [Google Scholar]
- Barfuss D. W., Schafer J. A. Differences in active and passive glucose transport along the proximal nephron. Am J Physiol. 1981 Sep;241(3):F322–F332. doi: 10.1152/ajprenal.1981.241.3.F322. [DOI] [PubMed] [Google Scholar]
- Ganapathy M. E., Mahesh V. B., Devoe L. D., Leibach F. H., Ganapathy V. Dipeptide transport in brush-border membrane vesicles isolated from normal term human placenta. Am J Obstet Gynecol. 1985 Sep 1;153(1):83–86. doi: 10.1016/0002-9378(85)90600-3. [DOI] [PubMed] [Google Scholar]
- Ganapathy V., Leibach F. H. Carrier-mediated reabsorption of small peptides in renal proximal tubule. Am J Physiol. 1986 Dec;251(6 Pt 2):F945–F953. doi: 10.1152/ajprenal.1986.251.6.F945. [DOI] [PubMed] [Google Scholar]
- Ganapathy V., Leibach F. H. Peptide transport in intestinal and renal brush border membrane vesicles. Life Sci. 1982 Jun 21;30(25):2137–2146. doi: 10.1016/0024-3205(82)90287-9. [DOI] [PubMed] [Google Scholar]
- Ganapathy V., Leibach F. H. Role of pH gradient and membrane potential in dipeptide transport in intestinal and renal brush-border membrane vesicles from the rabbit. Studies with L-carnosine and glycyl-L-proline. J Biol Chem. 1983 Dec 10;258(23):14189–14192. [PubMed] [Google Scholar]
- Ganapathy V., Mendicino J. F., Leibach F. H. Transport of glycyl-L-proline into intestinal and renal brush border vesicles from rabbit. J Biol Chem. 1981 Jan 10;256(1):118–124. [PubMed] [Google Scholar]
- Ganapathy V., Mendicino J., Leibach F. H. Evidence for a dipeptide transport system in renal brush border membranes from rabbit. Biochim Biophys Acta. 1981 Apr 6;642(2):381–391. doi: 10.1016/0005-2736(81)90454-5. [DOI] [PubMed] [Google Scholar]
- Ganapathy V., Pashley D. H., Fonteles M. C., Leibach F. H. Peptidases and peptide transport in mammalian kidney. Contrib Nephrol. 1984;42:10–18. doi: 10.1159/000409956. [DOI] [PubMed] [Google Scholar]
- Gustin M. C., Goodman D. B. Isolation of brush-border membrane from the rabbit descending colon epithelium. Partial characterization of a unique K+-activated ATPase. J Biol Chem. 1981 Oct 25;256(20):10651–10656. [PubMed] [Google Scholar]
- Hama T., Okada M., Kojima K., Kato T., Matsuyama M., Nagatsu T. Purification of dipeptidyl-aminopeptidase IV from human kidney by anti dipeptidyl-aminopeptidase IV affinity chromatography. Mol Cell Biochem. 1982 Mar 5;43(1):35–42. doi: 10.1007/BF00229537. [DOI] [PubMed] [Google Scholar]
- Horio M., Fukuhara Y., Orita Y., Nakanishi T., Nakahama H., Moriyama T., Kamada T. Gentamicin inhibits Na+-dependent D-glucose transport in rabbit kidney brush-border membrane vesicles. Biochim Biophys Acta. 1986 Jun 13;858(1):153–160. doi: 10.1016/0005-2736(86)90301-9. [DOI] [PubMed] [Google Scholar]
- Kenny A. J., Booth A. G. Organization of the kidney proximal-tubule plasma membrane. Biochem Soc Trans. 1976;4(6):1011–1017. doi: 10.1042/bst0041011. [DOI] [PubMed] [Google Scholar]
- Kragh-Hansen U., Røigaard-Petersen H., Jacobsen C., Sheikh M. I. Renal transport of neutral amino acids. Tubular localization of Na+-dependent phenylalanine- and glucose-transport systems. Biochem J. 1984 May 15;220(1):15–24. doi: 10.1042/bj2200015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kragh-Hansen U., Sheikh M. I. Serine uptake by luminal and basolateral membrane vesicles from rabbit kidney. J Physiol. 1984 Sep;354:55–67. doi: 10.1113/jphysiol.1984.sp015361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyamoto Y., Ganapathy V., Barlas A., Neubert K., Barth A., Leibach F. H. Role of dipeptidyl peptidase IV in uptake of peptide nitrogen from beta-casomorphin in rabbit renal BBMV. Am J Physiol. 1987 Apr;252(4 Pt 2):F670–F677. doi: 10.1152/ajprenal.1987.252.4.F670. [DOI] [PubMed] [Google Scholar]
- Miyamoto Y., Ganapathy V., Leibach F. H. Proton gradient-coupled uphill transport of glycylsarcosine in rabbit renal brush-border membrane vesicles. Biochem Biophys Res Commun. 1985 Nov 15;132(3):946–953. doi: 10.1016/0006-291x(85)91899-6. [DOI] [PubMed] [Google Scholar]
- PENNINGTON R. J. Biochemistry of dystrophic muscle. Mitochondrial succinate-tetrazolium reductase and adenosine triphosphatase. Biochem J. 1961 Sep;80:649–654. doi: 10.1042/bj0800649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pillion D. J., Ganapathy V., Leibach F. H. Identification of insulin receptors on the mucosal surface of colon epithelial cells. J Biol Chem. 1985 May 10;260(9):5244–5247. [PubMed] [Google Scholar]
- Røigaard-Petersen H., Sheikh M. I. Renal transport of neutral amino acids. Demonstration of Na+-independent and Na+-dependent electrogenic uptake of L-proline, hydroxy-L-proline and 5-oxo-L-proline by luminal-membrane vesicles. Biochem J. 1984 May 15;220(1):25–33. doi: 10.1042/bj2200025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabolić I., Burckhardt G. Effect of the preparation method on Na+-H+ exchange and ion permeabilities in rat renal brush-border membranes. Biochim Biophys Acta. 1984 May 16;772(2):140–148. doi: 10.1016/0005-2736(84)90037-3. [DOI] [PubMed] [Google Scholar]
- Takuwa N., Shimada T., Matsumoto H., Hoshi T. Proton-coupled transport of glycylglycine in rabbit renal brush-border membrane vesicles. Biochim Biophys Acta. 1985 Mar 28;814(1):186–190. doi: 10.1016/0005-2736(85)90435-3. [DOI] [PubMed] [Google Scholar]
- Turner R. J., Moran A. Further studies of proximal tubular brush border membrane D-glucose transport heterogeneity. J Membr Biol. 1982;70(1):37–45. doi: 10.1007/BF01871587. [DOI] [PubMed] [Google Scholar]
- Turner R. J., Moran A. Heterogeneity of sodium-dependent D-glucose transport sites along the proximal tubule: evidence from vesicle studies. Am J Physiol. 1982 Apr;242(4):F406–F414. doi: 10.1152/ajprenal.1982.242.4.F406. [DOI] [PubMed] [Google Scholar]
- Turner R. J., Moran A. Stoichiometric studies of the renal outer cortical brush border membrane D-glucose transporter. J Membr Biol. 1982;67(1):73–80. doi: 10.1007/BF01868649. [DOI] [PubMed] [Google Scholar]


