Abstract
Background:
The high recurrent rate after liver transplantation (LT) remains a clinical challenge, especially for those exceeding the Milan criteria (MC) and with high RETREAT scores. Therefore, the authors aim to investigate whether neoadjuvant systemic therapy allows safely administered and effectively reduces post-LT recurrence for those patients.
Methods:
In this prospective, randomized, open-label, pilot study, patients with HCC exceeding the MC were randomly assigned to PLENTY or control group before LT. The primary endpoint of the study was the recurrence-free survival after LT.
Results:
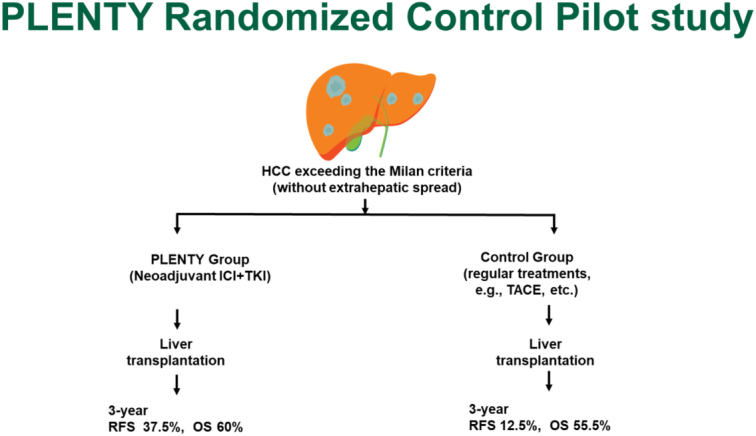
Twenty-two patients were enrolled and randomly assigned: 11 to the PLENTY group and 11 to the control group. The 30-month tumor-specific RFS was 37.5% in the PLENTY group and 12.5% in the control group. The 12-month tumor-specific RFS after LT was significantly improved in the PLENTY group (87.5%) compared to the control group (37.5%) (P=0·0022). The objective response rate in the PLENTY group was 30 and 60% when determined by RECIST 1.1 and mRECIST, respectively. Six patients (60%) had significant tumor necrosis, including three (30%) who had complete tumor necrosis at histopathology. No acute allograft rejection after LT occurred in the PLENTY and Control group.
Conclusion:
Neoadjuvant pembrolizumab plus lenvatinib before LT appears to be safe and feasible, associated with significantly better RFS for patients exceeding the MC. Despite the limitations of small sample size, this is the first RCT to evaluate neoadjuvant PD-1 blockade combined with tyrosine kinase inhibitors in LT recipients, the results of this study will inform future research.
Keywords: hepatocellular carcinoma, immunotherapy, liver transplantation, neoadjuvant therapy, recurrence-free survival
Introduction
Highlights
Up to now, only 25 cases, including seven patients in our center, underwent neoadjuvant immunotherapy before liver transplantation (LT) was published, and neither high-quality trial nor evidence of long-term prognosis has been published.
Neoadjuvant therapy with pembrolizumab and lenvatinib yielded favorable objective response rates and significantly improved recurrence-free survival without increasing graft rejection after LT.
This is the first randomized controlled trial to evaluate neoadjuvant PD-1 blockade combined with tyrosine kinase inhibitors in LT recipients with hepatocellular carcinoma presenting beyond the Milan criteria, provides more evidence of the efficacy and safety of the neoadjuvant systematic treatment, especially the superior recurrence-free survival.
Hepatocellular carcinoma (HCC) is a leading cause of cancer-related death worldwide1. Liver transplantation (LT) is indeed become the most effective treatment for HCC as it not only eliminates the tumors but also the diseased liver with the underlying hepatocarcinogenic factors. Even so, recurrence is still inevitable for some patients; the 24-month cumulative incidence of recurrence is as high as 24.2%2. The Milan criteria (MC) are the most common criteria for LT in HCC patients3, but in recent years, significant efforts have been made to modify selection criteria with the goal of maximizing transplant benefits for patients with HCC. However, it also results in an increase in the risk of tumor recurrence4. Thus, many centers began to employ tumor down-staging (DS) or bridging strategies with locoregional therapy (LRT) for LT to reduce the viable tumor burden to meet acceptable LT criteria. LRT-DS patients had superior RFS (60 vs. 54%) compared with not being down-staged5.
Recently, the treatment landscape of advanced HCC has been evolving rapidly, notably, there are no guidelines or consensus on the application of immune checkpoint inhibitors (ICIs) with tyrosine kinase inhibitors (TKIs) before LT due to safety concerns of acute graft rejection6–8. Our previous research9 preliminarily proved that the combination of PD-1 blockades with lenvatinib and subsequent LT were feasible and effective under close monitoring. We assume that ICIs combined with lenvatinib as neoadjuvant therapy prior to LT could reduce the risk of post-LT recurrence and improve the RFS as well as OS rate in patients with HCC. Therefore, we assigned the prospective, pilot study to investigate whether pembrolizumab plus lenvatinib as a neoadjuvant therapy before LT can be safely administered and effectively reduce post-LT recurrence for patients with HCC exceeding the MC. This trial is registered with ClinicalTrial.gov.
Methods
Study design and participants
PLENTY study was a prospective, pilot study designed to investigate the efficacy and safety of pembrolizumab plus lenvatinib as pre-LT neoadjuvant therapy for patients with HCC beyond MC and without extrahepatic spread, conducted at the department of Liver Surgery. Patients aged 18–80 years with HCC exceeding MC and planned for LT were eligible for inclusion. Major inclusion criteria included Eastern Cooperative Oncology Group (ECOG) performance status 0-1, Child-Pugh A-B7 liver score (5 to 7 points), and adequate organ function. A full list of eligibility criteria was referred to in the Supplement protocol (Supplemental Digital Content 1, http://links.lww.com/JS9/C976). The study was reviewed and approved by the Ethical Committee of Hospital. Written informed consent on study aims, participation requirements, and the right to refuse was obtained from all participants. The trial was conducted in accordance with the principles of the Declaration of Helsinki and Good Clinical Practice. All procedures were in accordance with the STROBE guidelines. The work has been reported in line with Consolidated Standards of Reporting Trials (CONSORT) Guidelines10,11.
Study procedures
Following written informed consent and completion of baseline assessments, patients deemed suitable candidates for the study were enrolled and subsequently randomized into the two groups with a ratio of 1:1. Randomization was performed at the Clinical Research Unit of Hospital using the computer-generated random number code. The randomization codes were concealed in sequentially numbered opaque envelopes by the clinical research nurse, who was not involved in data analysis. In addition, the clinical research coordinators were in charge of randomly assigning the patients to different groups based on allocation sequence. No masking was applied to clinical outcome assessors.
Interventions
Patients who assigned to PLENTY group received 200 mg of pembrolizumab intravenously every 3 week until ~6 weeks before LT according to pharmacokinetic concentration-time profiles of pembrolizumab12, or unacceptable toxicity developed. According to the estimated LT waiting time of 2 months in our center [mostly, when the patient was ranked fourth (blood type O) to seventh (blood type AB) on the waiting list], the pembrolizumab was discontinued. Simultaneously, lenvatinib was given orally at 8 mg once a day until 1–2 weeks before LT. Patients assigned to the control group received LRT such as transarterial chemoembolization (TACE), etc. Both groups received the best supportive care at the discretion of the investigator. The tumor stage was evaluated with enhanced CT or MRI scan, and restaging scans were done at least every 6 weeks until LT for patients. Tumor necrosis on pathological examination was assessed by consensus of two dedicated hepatopathologists (F.H. and L.Z.B.) who visually estimated and agreed upon the percentage of necrosis seen within the resected tumor bed.
All the patients underwent donor after cardiac death whole graft orthotopic LT13, including a tapered dose of methylprednisolone, a drug regimen of tacrolimus, and mycophenolate mofetil. Two more days longer for those patients using pre-LT pembrolizumab in the methylprednisolone tapering schedule. Gradual mycophenolate mofetil withdrawal was assessed on a case-by-case basis; the tapering-off time was generally controlled within 2 months after LT. Sirolimus was administrated 2 mg once a day from ~6–8 weeks after LT14. For patients who relapsed more than 6 months after LT, lenvatinib was reused until progression.
Follow-up protocol
Median follow-up was 37.1 months (range, 26.2–45.6). Enhanced CT or MRI scan was done every 6–8 weeks in the first year after LT and at least every 12 weeks after 1 year. Diagnosis of recurrence was assessed by two trained and experienced radiologists (Z.Z.G. and Q.L.J.) or by pathology consistent with HCC if suspected of recurrence but could not be confirmed by imaging. Patients with tumor recurrence received optimal treatment assigned by the multidisciplinary team in our center.
Study measures
The primary endpoint of the study was the recurrence-free survival (RFS) after LT between the two groups. RFS was defined as the time from LT until the first documentation of HCC recurrence (local, regional, or distant), which was assessed by two trained and experienced radiologists (Z.Z.G. and Q.L.J.) or by pathology consistent with HCC if suspected of recurrence but could not be confirmed by imaging, or death due to any cause (both cancerous and noncancerous causes of death). Secondary endpoints included (1) overall survival (OS, from randomization to death, and after LT); (2) objective response rate (ORR, according to Response Evaluation Criteria in Solid Tumors [RECIST] version 1.1 and modified RECIST [mRECIST]15), defined as the proportion of patients with a complete response or partial response (PR); (3) adverse event, defined as any unfavorable and unintended sign, symptom, or disease (new or worsening) associated with the therapy. Adverse events were graded according to the Common Terminology Criteria for Adverse Events (CTCAE, version 5.0). The exploratory endpoints were immunological/biomarker changes in the peripheral blood and significant tumor necrosis, defined as more than 70% necrosis of resected tumor under pathological examination16.
Sample size calculation and statistical analysis
Survival analyses with the Kaplan–Meier method and log-rank test were done to assess various time-to-event endpoints. Proportions of patients with an overall response (according to RECIST 1.1 and mRECIST) were estimated along with 95% CIs. R version 4.2.0 was used for the statistical analysis. This study is registered with ClinicalTrials.gov.
Concerning the sample size of the pilot study, since no previous reference on RFS or OS after LT in patients who received ICIs before LT is available, the sample size for this pilot study was determined by ORR. In the previous retrospective study, the ORR who received ICIs before LT was 70%9. At 90% power and a 5% significance level, 20 patients (10 patients in each group) were recruited for this pilot study. Data analysis was performed when 20 patients completed the liver transplant procedure.
Results
Baseline and waitlist characteristics
A total of 22 patients with HCC beyond MC were enrolled between 3 February 2020 and 5 September 2021. All patients received protocol-assigned treatment except one patient in the PLENTY group failed to be followed up after four cycles of combined therapy when ready to undergo LT. He was found to recontact 15 months later and showed a PR, thus, he was excluded from the efficacy and safety analysis (Fig. 1). Randomization provided a reasonable balance of demographic and clinical factors in both groups (Table 1). Individual details are provided in the Supplement Tables (Supplemental Digital Content 2, http://links.lww.com/JS9/C977). One patient in the control group died from tumor progression while on the waiting list (Fig. 1). No LRT was received during the study period for the treatment arm patients. Each arm included four patients with a history of LRT prior to enrollment (more than 3 months before enrollment). Finally, 20 patients received LT, and the median time on the waiting list was longer in the PLENTY group compared to the control group (P<0.001; Table 1) due to four cycles (range, 2–5) of combined therapy, and a washout period of 60.5 days (range, 25–193) for pembrolizumab.
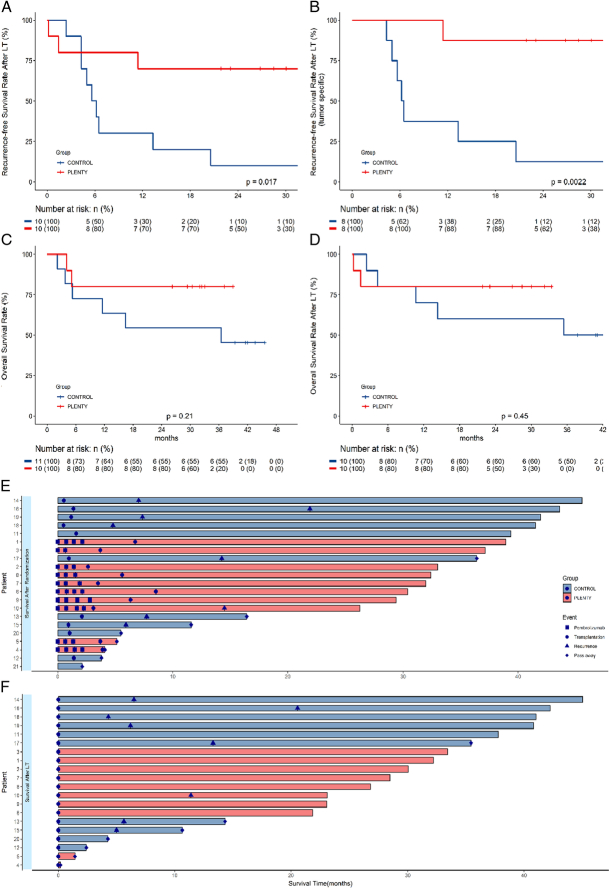
Figure 1.
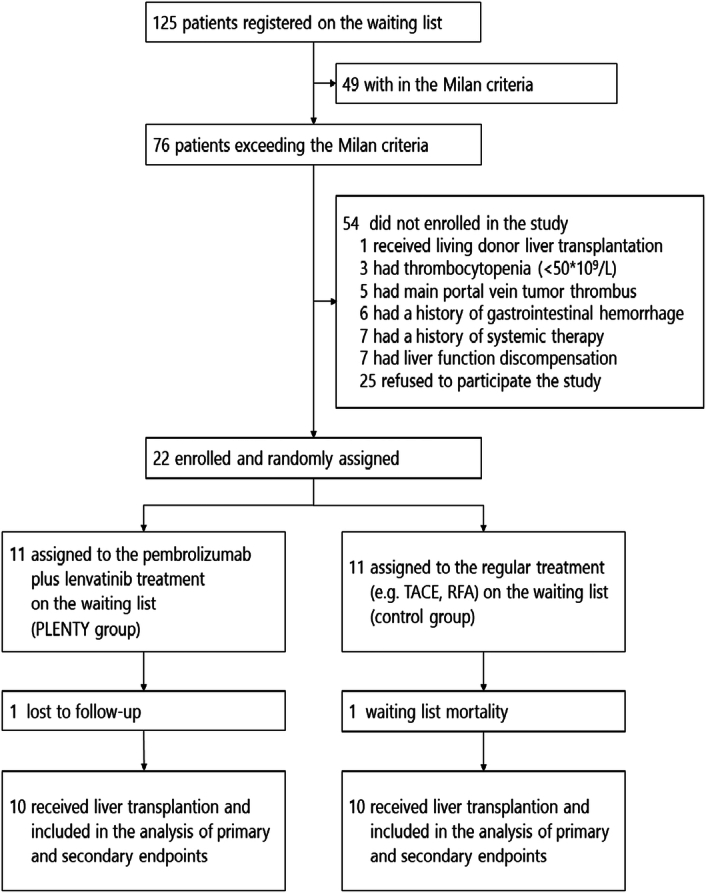
Trial profile.
Table 1.
Demographics and clinical characteristics.
| Characteristics | PLENTY (n=10) | CONTROL (n=11) | P |
|---|---|---|---|
| Age at randomization, years | 57.5 (38–68) | 51 (38–66) | 0.440 |
| Sex | 0.944 | ||
| Male | 9 (90.0%) | 10 (90.1%) | |
| Female | 1 (10.0%) | 1 (9.1%) | |
| ECOG performance status | 0.329 | ||
| 0 | 10 (100%) | 10 (90.1%) | |
| 1 | 0 (0) | 1 (9.1%) | |
| HBV background | 10 (100%) | 11 (100%) | / |
| Maximum tumor size (cm) | 7.01 (1.655–16.94) | 7.5 (2.3–14.5) | 0.446 |
| Number of lesions | 3.5 (1–23) | 3 (1–12) | 0.753 |
| PVTT | 0.835 | ||
| Vp0 | 5 (50.0%) | 5 (45.5%) | |
| Vp1-3 | 5 (50.0%) | 6 (54.5%) | |
| BCLC stage | 0.528 | ||
| B | 5 (50%) | 4 (36.4%) | |
| C | 5 (50%) | 7 (63.6%) | |
| Alpha-fetoprotein | 0.466 | ||
| ≤400 ng/ml | 3 (30.0%) | 5 (45.5%) | |
| >400 ng/ml | 7 (70.0%) | 6 (54.5%) | |
| Des-γ-carboxyprothrombin | 0.890 | ||
| ≤400 mAU/ml | 7 (70.0%) | 8 (72.7%) | |
| >400 mAU/ml | 3 (30.0%) | 3 (27.3%) | |
| ALT (U/l) | 47 (23–88) | 53 (21–111) | 0.841 |
| AST (U/l) | 72 (21–99) | 55 (22–162) | 0.479 |
| Child-Pugh class | 0.217 | ||
| A | 8 (80.0%) | 6 (54.5%) | |
| B | 2 (20.0%) | 5 (45.5%) | |
| Waiting time (Day) | 114 (89–256) | 33 (15–63) | 0.001 |
| Waiting list mortality | 0.329 | ||
| No | 10 (100%) | 10 (90.1%) | |
| Yes | 0 (0) | 1 (9.1%) | |
| Treatment cycles | 4 (2–5) | / | / |
| Washout period (Day) | 60.5 (25–193) | / | / |
Data are n (%) or median (range).
ALT, alanine transaminase; AST, aspartate transaminase; BCLC, Barcelona Clinic Liver Cancer; ECOG, Eastern Cooperative Oncology Group; HBV, Hepatitis B Virus; LRT, loco-regional therapy; PVTT, portal vein tumor thrombus; TB, total bilirubin.
Recurrence and survival
Until November 2023, tumor recurrence after LT was observed in eight patients (seven in the control group and one in the PLENTY group) during a median follow-up of 33.4 months (range, 23.1–45.0), and three patients in the control group died from tumor progression. The median RFS after LT was 12.4 months (95% CI: 5.63-NA).
RFS was significantly improved in patients who received neoadjuvant pembrolizumab plus lenvatinib treatment compared with those in the control group (log-rank P=0·017; Fig. 2A). The 12-month RFS rate was 70% (95% CI: 34.8–93.3) in PLENTY group and 30% (95% CI: 6.67–65.2) in the control group, respectively. Concerning the 12-month tumor-specific RFS, it was 87.5% in the PLENTY group and 37.5% in the control group (P=0·0022; Fig. 2B). The 30-month tumor-specific RFS was 37.5% in the PLENTY group and 12.5% in the control group.
Figure 2.
Survival and recurrence outcomes (A) Recurrence-free survival after liver transplantation (LT), per patient (n=20). (B) Tumor-specific recurrence-free survival after LT, per patient (n=16). (C) Overall survival after randomization, per patient (n=21). (D) Overall survival after LT, per patient (n=20). (E) Swimmer plot showing the time to treatment, recurrence and pass away after randomization. (F) Swimmer plot showing the time to recurrence and pass away after LT.
During a median follow-up of 37.1 months after randomization, two patients in the PLENTY group and five patients in the control group died. One patient in the PLENTY group died from GVHD 43 days after LT. He had a 51-day washout period before LT, and GVHD was diagnosed 18 days after LT. The patient developed a mild decrease in white blood cells and hyponatremia on day 18 after LT. On postoperative day 23, the patient developed skin rash, accompanied by fever and dysphagia. Methylprednisolone, rucotinib, balliximab, and immunoglobulin are used to treat the disease. The liver function indexes such as transaminase and bilirubin did not appear abnormal during the course of treatment, and immune related hepatitis was excluded. The diagnosis of GvHD was confirmed by skin biopsy. The fatal adverse event was evaluated as unlikely to be associated with pembrolizumab according to the WHO-UMC system by the multidisciplinary team. Another patient in the PLENTY group died from intra-abdominal coagulopathic hemorrhage 6 days after LT. Neither of the deaths was considered to be related to the neoadjuvant treatment.
No statistical difference was observed in OS between the two groups (Fig. 2C, D). To gain further insight into the durability of response, swimming plots for each patient were generated, including the time points of combined therapy, LT, recurrence, and death (Fig. 2E, F).
Tumor responses
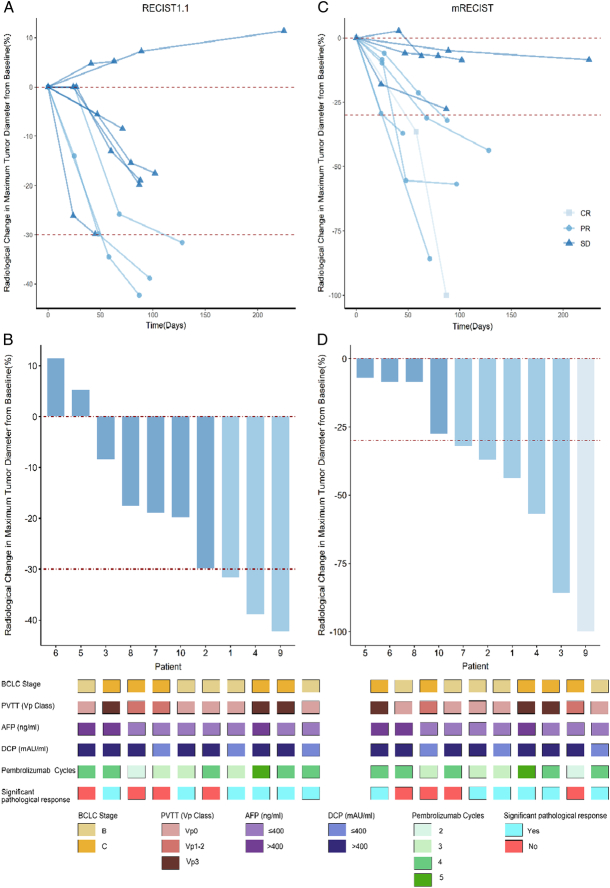
The average Risk Estimation of Tumor Recurrence After Transplant (RETREAT) score was 5 in the PLENTY group and 4.9 in the control group from recruitment, respectively. And the RETREAT score at LT was 4.8 in the PLENTY group and 4.9 in the control group, respectively. Radiological response before LT was evaluated in 10 patients in the PLENTY group. Thirty percent of patients had a PR and 70% had stable disease (SD) according to RECIST 1.1. While as for mRECIST, 10% of patients had a complete response, 50% had a PR, and 40% had SD. Thus, the ORR was 30% (95% CI: 6.67–65.2) and 60% (95% CI: 26.2–87.8) when determined by RECIST 1.1 and mRECIST, respectively. The median time to response was 63 days (range, 45–88) per mRECIST. Spider plots and waterfall plots of patients’ target lesion changes from baseline were presented in Figure 3. Pathological response to the neoadjuvant therapy was also evaluated after LT in 10 patients in the PLENTY group; six patients had significant tumor necrosis (60%, 95% CI: 26.2–87.8), including three who had complete tumor necrosis (30%, 95% CI: 6.67–65.2) at histopathology. Decreasing serum alpha-fetoprotein (AFP) concentrations, AFP-L3 percentages, and des-gamma-carboxyprothrombin (DCP) concentrations were observed in most patients [8 (80%), 7 (70%), and 6 (60%) of 10, respectively] in the PLENTY group. However, there was no correlation between any biomarker changes over time and pathological responses (Supplement Figure, Supplemental Digital Content 3, http://links.lww.com/JS9/C978).
Figure 3.
Tumor responses (A) Spider plot showing the percentage change from baseline in maximum diameter of target lesions over time (months) in each of the 10 patients in the PLENTY group, according to treatment response per RECIST 1.1. (B) Waterfall plot showing the percentage change from baseline in maximum diameter of target lesions in each of the 10 patients in the PLENTY group, according to treatment response per RECIST 1.1. (C) Spider plot showing the percentage change from baseline in maximum diameter of target lesions over time (months) in each of the 10 patients in the PLENTY group, according to treatment response per mRECIST. (D) Waterfall plot showing the percentage change from baseline in maximum diameter of target lesions in each of the 10 patients in the PLENTY group, according to treatment response per mRECIST.
Adverse events
All 10 patients who received pembrolizumab plus lenvatinib treatment were included in the safety analysis. The most common adverse events were hypertension (80%), elevated transaminases (60%), diarrhea (50%), and thrombocytopenia (50%). Grade 3 adverse effects were observed in 3 (30%) patients: one had fatigue, one had diarrhea, and one had pruritus. No grade 4 or 5 adverse events were observed (Table 2). There were no treatment discontinuation or surgical cancellations due to treatment-related adverse events (TRAEs). No acute allograft rejection after LT occurred in either group.
Table 2.
Treatment-related adverse events and serious adverse events.
| Adverse events | Any grade | Grade 1 | Grade 2 | Grade 3 | Grade 4–5 |
|---|---|---|---|---|---|
| Any | 10 (100%) | 8 (80%) | 5 (50%) | 3 (30%) | 0 |
| Hypertension | 8 (80%) | 8 (80%) | 0 | 0 | 0 |
| Elevated transaminases | 6 (60%) | 5 (50%) | 1 (10%) | 0 | 0 |
| Diarrhea | 5 (50%) | 2 (20%) | 2 (20%) | 1 (10%) | 0 |
| Thrombocytopenia | 5 (50%) | 3 (30%) | 2 (20%) | 0 | 0 |
| Elevated bilirubin | 4 (40%) | 3 (30%) | 1 (10%) | 0 | 0 |
| Fatigue | 3 (30%) | 0 (0) | 2 (20%) | 1 (10%) | 0 |
| Pruritus | 2 (20%) | 0 | 1 (10%) | 1 (10%) | 0 |
| Proteinuria | 2 (20%) | 0 | 2 (20%) | 0 | 0 |
| Neutropenia | 2 (20%) | 1 (10%) | 1 (10%) | 0 | 0 |
| Pneumonitis | 2 (20%) | 1 (10%) | 1 (10%) | 0 | 0 |
| Abdominal pain | 2 (20%) | 1 (10%) | 1 (10%) | 0 | 0 |
| Elevated pancreatic enzymes | 2 (10%) | 2 (10%) | 0 | 0 | 0 |
| Elevated creatinine | 2 (20%) | 2 (20%) | 0 | 0 | 0 |
| Anemia | 1 (10%) | 1 (10%) | 0 | 0 | 0 |
Data are n (%) and represent the highest grades assigned.
Extension neoadjuvant ICI cohort
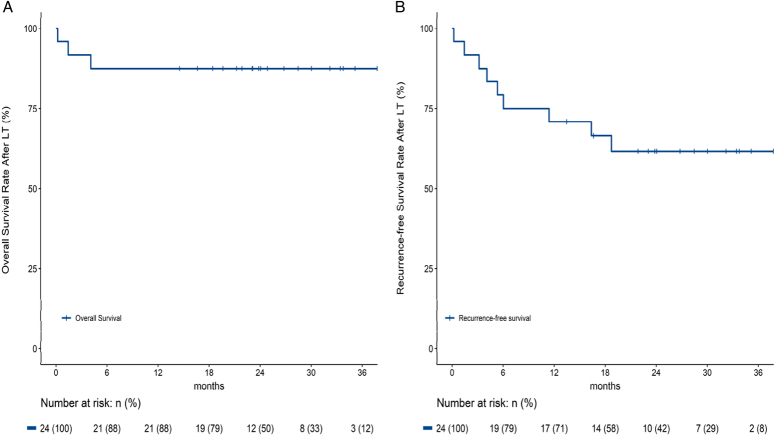
Up to November 2022, a total of 24 patients with HCC beyond MC had received LT after neoadjuvant ICIs with or without TKIs in our center (Fig. 4 and Table 3), including 14 extra patients during the same period in our center. Acute rejection was found in three patients, one patient was confirmed by biopsy, the other two were considered clinical suspected acute rejection due to elevated transaminases, and all of them was responded well from methylprednisolone. No allograft loss occurred (Supplementary Table 3, Supplemental Digital Content 2, http://links.lww.com/JS9/C977).
Figure 4.
Overall survival and recurrence-free survival of 24 patients with hepatocellular carcinoma beyond Milan criteria who received liver transplantation after neoadjuvant immune checkpoint inhibitors in our center.
Table 3.
All the patients in our center received LT after bridging/downstaging with immunotherapy.
| Characteristics | LT after immunotherapy (n=24) |
|---|---|
| ICI molecules | |
| Pembrolizumab | 12 (50.0%) |
| Camrelizumab | 7 (29.2%) |
| Tislelizumab | 4 (16.7%) |
| Nivolumab | 1 (4.1%) |
| Combination with lenvatinib | 20 (83.3%) |
| Treatment cycles | 3 (1–5) |
| Washout period (Day) | 69.5 (18–206) |
| Rejection | 3 (12.5%) |
| Recurrence | 6 (25.0%) |
| Death | 3 (12.5%) |
Data are n (%) or median (Range).
ICI, immune checkpoint inhibitors; LRT, loco-regional therapy.
The OS rate was 33.3% and the RFS was 29.2% for these 24 patients. This is one of the largest single-center sample all over the world for neoadjuvant ICIs prior to LT, proving the application of ICIs could be a feasible and effective DS protocol for advanced HCC before LT.
Discussion
The high recurrent rate of HCC after LT remains a considerable clinical challenge. The application of neoadjuvant ICIs before LT in patients with advanced HCC has been explored since 2019 in our center. We reported here, to our knowledge, for the first RCT providing evidence of the benefit of neoadjuvant systemic therapy in patients with HCC who are planned for LT. Although several clinical trials are ongoing, the results of these studies are not yet available (Supplementary Table 2, Supplemental Digital Content 2, http://links.lww.com/JS9/C977). This prospective trial was designed based on our retrospective study9 and a series of literature review17–22.
Downstaging treatments before LT have mostly been limited to LRT, including TACE, RFA, transarterial radioembolization (TARE), and combined different treatments5,23,24. The probability of successful DS to meet MC for patients within UNOS-DS criteria23, was 89 and 86% with TACE and TARE, respectively24. However, the neoadjuvant treatment using a combination of sorafenib and TACE before LT had not significantly improved time-to-progression (71 vs. 85 days), ORR (20.8 vs. 26.9%) or DCR (66.7 vs. 73.1%) compared to TACE alone25. Several neoadjuvant treatment strategies with lenvatinib, instead of sorafenib26,27, with higher DS intent that permit subsequent salvage hepatectomy due to its greater antiangiogenic effect, have been reported28,29. In addition, the P-161 Trial (NCT05171335), in order to explore the efficacy and safety of neoadjuvant therapy of lenvatinib plus TACE for transplant-eligible patients with large HCC is enrolled. However, high-quality evidence of pre-LT neoadjuvant systematic therapy still needs to be provided (Supplementary Table 4, Supplemental Digital Content 2, http://links.lww.com/JS9/C977).
Although the tumor was completely removed with the diseased liver, the invisible circulating tumor cells (CTCs) are believed to become the seeds of tumor recurrence in the immunosuppressive microenvironment after LT. The preoperative CTCs level has become a valuable predictor of recurrent HCC after LT30,31. LRT was unsatisfactory in eradicating CTCs32,33. More recently, a multicenter study showed that CTC levels decreased after immunotherapy in patients with metastatic renal cell carcinoma34,35. However, in consideration of the potential risk of post-LT rejection induced by ICIs and delays in surgery due to TRAEs, the clinical data regarding peri-operative administration of ICIs in the context of solid organ transplantation are scarce. A meta-analysis including 52 patients treated with ICIs after LT36 discovered that acute rejection occurred in 28.8% of patients, and nearly half of them died because of graft loss. Compared to postoperative, the preoperative application of ICIs might be safer because of a controllable washout period in LT recipients. Up to now, only 25 cases of patients with HCC receiving ICIs prior to LT have been published9,17–22. Twelve percent had suffered severe rejection and allograft loss; two patients died17,18, and one survived with retransplantation19. Besides, 16% of patients had mild to moderate rejection.9,19,20 Schnickel and Sogbe suggested that ICIs should be terminated at least 3 months before LT19,22. In this pilot study, the median washout period was 2 months (range, 25–193 days); five patients (50%) had an even shorter washout period without any rejection after LT, indicating that the termination time point of ICIs needed further exploration. Referring to the prescribing information for pembrolizumab for intravenous injection, the terminal half-life (t1/2) of the drug is documented to be 22 days. Recent studies have demonstrated that the washout period for the immunotherapy agent pembrolizumab must exceed a minimum duration of 1 month to mitigate the risk of graft rejection. A literature review regarding the washout period for pembrolizumab was shown in Supplementary Table 5 (Supplemental Digital Content 2, http://links.lww.com/JS9/C977).
In the extension cohort, the median washout period was 73 days. Surprisingly, biopsy proved acute rejection (BPAR, RAI=5) was found in the patient underwent camrelizumab treatment and the washout period was 106 days. The patient underwent tislelizumab with the washout period of 18 days and the patient underwent nivolumab with the washout period of 70 days experienced clinical suspected acute rejection (Supplementary Table 3, Supplemental Digital Content 2, http://links.lww.com/JS9/C977).
TACE and stereotactic body radiotherapy followed by TKI-ICI is also expected to be potential neoadjuvant therapy options for HCC LT. Combination therapies with locoregional therapies have also been actively explored to enhance ICI efficacy by promoting the release of tumor-associated antigens and cytokines. The ORR were reported higher in the TACE-TKI-ICI group compared to TKI-ICI group for unresectable HCC37,38. In the CHANCE001 real-world study, 556 advanced HCC patients receiving either TACE-TKI-ICI or TACE monotherapy. ORR were significantly higher in the combination group (60.1 vs. 32.0%), though Grade 3/4 adverse events rate were also elevated (15.8 vs 7.5%)39.
Our study results tentatively suggest that although immunotherapy in LT recipients is associated with an ineligible risk of rejection and even mortality, it should not be discarded in this particular fragile population. In the present study, grade 3 TRAEs were observed in 30% of patients, similar to previous reports8,40, and did not increase the mortality, delay, or cancellation of LT waiting list by TRAEs.
The RFS benefit from neoadjuvant systemic therapy in patients with HCC has been preliminarily proved in hepatectomy. The 12-month RFS after hepatectomy was about 61.5–75% in patients with initially unresectable HCC who had received ICIs plus TKIs as conversion therapy; furthermore, achieving a pathological complete response to systemic therapy was associated with a favorable RFS after resection35,41. Our results showed that neoadjuvant pembrolizumab and lenvatinib could improve RFS with a low incidence of TRAEs. Despite the small sample size, it is notable that RFS differed significantly between the two groups, regardless of whether excluded ‘nontumor-related’ deaths (P=0.03, P=0.0057). The predefined primary endpoint of RFS was met, which could support further studies to investigate the efficacy of these regimens42,43.
Cucchetti et al.44 revealed that ‘tumor-related death’ after LT was not only correlated with tumor number and tumor size but also correlated with the effect of neoadjuvant therapy evaluated by mRECIST. Several studies have demonstrated that the level of CTCs observably decreased in patients with well-radiological response45,46. In our study, no patient had progressive disease after neoadjuvant therapy, although they were still exceeding the MC. Nonetheless, the goal of reducing the risk of post-LT recurrence was achieved, maybe because of the necrosis of the intrahepatic lesions and the decrease in CTCs.
Instead of radiological responses, pathological responses have been adopted as surrogate endpoints for neoadjuvant immunotherapy trials in patients with resectable HCC44,45. Kaseb et al.47 used an exploratory cutoff of 70% tumor necrosis as an endpoint significant tumor necrosis. Then, Marron et al.48 agreed with this cutoff value and found that 20% of patients had significant tumor necrosis with two cycles of neoadjuvant cemiplimab. The results in our study suggest remarkable clinical activity of pembrolizumab plus lenvatinib for HCC with a significant pathological response rate at 60%, higher than previous studies of neoadjuvant ICIs therapy in HCC47,48.
Limitations
Limitations of our study include the fact that it was a single-center study with a relatively small sample size. Initially, the study was designed with a sample size of 192, unluckily, the COVID-19 pandemic had hampered the enrollment of patients and the donor liver allocation. Particularly, due to the increasing time periods of neoadjuvant therapy and washout period, the follow-up time after LT was inevitably shorter in the PLENTY group than in the control group. Although a statistically significant difference was observed only in RFS but not in OS, which may be associated with the heterogeneity of participants caused by the small sample size and nonsystemic therapy-related death, the OS of the plenty group was still better than that of the control group. Additionally, the patients in this study had a larger tumor burden, and some patients had higher RETREAT scores or portal vein tumor thrombus, which may also lead to a certain bias. The extension cohort was also provided so that the transplant community could make a more comprehensive analysis of this issue. Despite the limitations, the results of this study will inform future research. On the one hand, the large-sample RCT is still recruiting and ongoing, and longer survival outcomes will be verified.
Conclusion
Our findings provide updated evidence that neoadjuvant ICIs combined with TKIs before LT is associated with better RFS for patients with HCC beyond MC, without increasing post-LT graft rejection. Systemic therapy in the preoperative setting for patients with HCC on the waiting list warrants further studies, which will promote the DS and bridging strategies into a new era.
Ethical approval
The study was reviewed and approved by the Ethical Committee of Renji Hospital(reference number KY2020-083). The trial was conducted in accordance with the principles of the Declaration of Helsinki and Good Clinical Practice. All procedures were in accordance with the STROBE guidelines.
Consent
Written informed consent on study aims, participation requirements, and the right to refuse was obtained from all participants.
Source of funding
This study was funded by Shanghai Science and Technology Development Foundation (Outstanding academic leader) (HF, 23XD1423100, National Natural Science Foundation, China (XQ, 82241221, 92059205), and Innovative research team of high-level local universities in Shanghai (XQ, HF, SHSMU-ZLCX20211602).
Author contribution
F.H., X.Q., and L.Z.C.: participated in the conception and design of this study; F.H.: was the project manager and coordinated patient recruitment; L.Z.C., Z.Z.J., Y.J., X.L., T.Y., T.H., L.L., Z.J., W.Y., Z.Z.G., L.Z.B., and W.T.: were involved in the acquisition, analysis, or interpretation of data; L.Z., Y.J., J.Y., and Z.Z.J.: drafted the manuscript. All the authors contributed to the critical review and final approval of the manuscript. F.H., X.Q., and L.Z.C.: accessed and verified the underlying study data. All authors were responsible for the decision to submit the manuscript.
Conflicts of interest disclosure
The authors declare no conflicts of interests.
Research registration unique identifying number (UIN)
ClinicalTrial.gov Identifier: NCT04425226.
Guarantor
Hao Feng and Qiang Xia.
Data availability statement
The data of this study are available under a transfer agreement from the corresponding author based on a reasonable request. There will be limitations on how data can be used or how long data will be available for.
Provenance and peer review
Not commissioned, externally peer-reviewed.
Supplementary Material
Acknowledgements
This study was funded by the Shanghai Science and Technology Development Foundation (Outstanding academic leader) (HF, 23XD1423100, National Natural Science Foundation, China (XQ, 82241221, 92059205), and an Innovative research team of high-level local universities in Shanghai (XQ, HF, SHSMU-ZLCX20211602). The Renji Clinical Research Unit supported the study set-up, site identification, and delivery of this study. We acknowledge the study coordinators of the Department of Liver Surgery, Renji Hospital, Jian-Jun ZHANG, Kang HE, Wei GENG, Jie CAO, and Han-Yong SUN, for their ongoing administrative support of the study.
Footnotes
Zicheng Lv, June-kong Yong, and Xuelin Xiang contributed equally to this work.
Sponsorships or competing interests that may be relevant to content are disclosed at the end of this article.
Supplemental Digital Content is available for this article. Direct URL citations are provided in the HTML and PDF versions of this article on the journal’s website, www.lww.com/international-journal-of-surgery.
Published online 12 July 2024
Contributor Information
Zicheng Lv, Email: lyuzicheng@163.com.
Xuelin Xiang, Email: mmmikasa.xxl@situ.edu.cn.
June-kong Yong, Email: yongjunekong@aliyun.com.
Yi Zhou, Email: zhouyi_0823@qq.com.
Yichi Wu, Email: wuyichi_0804@163.com.
Linman Li, Email: 742048047@qq.com.
Yuanhao Wang, Email: wangyh2000@sjtu.edu.cn.
Zijie Zhang, Email: sjtuzzj@163.com.
Qiang Xia, Email: xiaqiang@medmail.com.cn.
Hao Feng, Email: surgeonfeng@live.com.
References
- 1. Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2021;71:209–249. [DOI] [PubMed] [Google Scholar]
- 2. Guo DZ, Cheng JW, Yan JY, et al. Efficacy and safety of lenvatinib for preventing tumor recurrence after liver transplantation in hepatocellular carcinoma beyond the Milan criteria. Ann Transl Med 2022;10:1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Mazzaferro V, Regalia E, Doci R, et al. Liver transplantation for the treatment of small hepatocellular carcinomas in patients with cirrhosis. N Engl J Med 1996;334:693–699. [DOI] [PubMed] [Google Scholar]
- 4. Zheng SS, Xu X, Wu J, et al. Liver transplantation for hepatocellular carcinoma: Hangzhou experiences. Transplantation 2008;85:1726–1732. [DOI] [PubMed] [Google Scholar]
- 5. Kardashian A, Florman SS, Haydel B, et al. Multicenter cohort of 789 patients with hepatocellular carcinoma presenting beyond Milan criteria. Hepatology 2020;72:2014–2028. [DOI] [PubMed] [Google Scholar]
- 6. Kudo M, Finn RS, Qin S, et al. Lenvatinib versus sorafenib in first-line treatment of patients with unresectable hepatocellular carcinoma: a randomised phase 3 non-inferiority trial. Lancet 2018;391:1163–1173. [DOI] [PubMed] [Google Scholar]
- 7. Brahmer JR, Lacchetti C, Schneider BJ, et al. National Comprehensive Cancer Network. Management of immune-related adverse events in patients treated with immune checkpoint inhibitor therapy: American Society of Clinical Oncology Clinical Practice Guideline. J Clin Oncol 2018;36:1714–1768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Finn RS, Ikeda M, Zhu AX, et al. Phase Ib study of lenvatinib plus pembrolizumab in patients with unresectable hepatocellular carcinoma. J Clin Oncol 2020;38:2960–2970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Qiao ZY, Zhang ZJ, Lv ZC, et al. Neoadjuvant programmed cell death 1 (PD-1) inhibitor treatment in patients with hepatocellular carcinoma before liver transplant: a cohort study and literature review. Front Immunol 2021;12:653437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Moher D, Hopewell S, Schulz KF, et al. CONSORT . CONSORT 2010 explanation and elaboration: updated guidelines for reporting parallel group randomised trials. Int J Surg 2012;10:28–55. [DOI] [PubMed] [Google Scholar]
- 11. Schulz KF, Altman DG, Moher D, CONSORT Group . CONSORT 2010 statement: updated guidelines for reporting parallel group randomised trials. BMJ 2010;340:c332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ribas A, Lawrence D, Atkinson V, et al. Combined BRAF and MEK inhibition with PD-1 blockade immunotherapy in BRAF-mutant melanoma. Nat Med 2019;25:936–940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Xia L, Qiao ZY, Zhang ZJ, et al. Transplantation for EASL-CLIF and APASL acute-on-chronic liver failure (ACLF) patients: The TEA cohort to evaluate long-term post-Transplant outcomes. EClinicalMedicine 2022;49:101476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Lencioni R, Llovet JM. Modified RECIST (mRECIST) assessment for hepatocellular carcinoma. Semin Liver Dis 2010;30:52–60. [DOI] [PubMed] [Google Scholar]
- 15. Allard MA, Sebagh M, Ruiz A, et al. Does pathological response after transarterial chemoembolization for hepatocellular carcinoma in cirrhotic patients with cirrhosis predict outcome after liver resection or transplantation? J Hepatol 2015;63:83–92. [DOI] [PubMed] [Google Scholar]
- 16. Han B, Ding H, Zhao S, et al. Potential role of adjuvant lenvatinib in improving disease-free survival for patients with high-risk hepatitis B virus-related hepatocellular carcinoma following liver transplantation: a retrospective, case control study. Front Oncol 2020;10:562103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Nordness MF, Hamel S, Godfrey CM, et al. Fatal hepatic necrosis after nivolumab as a bridge to liver transplant for hepatocellular carcinoma: are checkpoint inhibitors safe for the pretransplant patient? Am J Transplant 2020;20:879–883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Chen GH, Wang GB, Huang F, et al. Pretransplant use of toripalimab for hepatocellular carcinoma resulting in fatal acute hepatic necrosis in the immediate postoperative period. Transpl Immunol 2021;66:101386. [DOI] [PubMed] [Google Scholar]
- 19. Schnickel GT, Fabbri K, Hosseini M, et al. Liver transplantation for hepatocellular carcinoma following checkpoint inhibitor therapy with nivolumab. Am J Transplant 2022;22:1699–1704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Tabrizian P, Florman SS, Schwartz ME. PD-1 inhibitor as bridge therapy to liver transplantation? Am J Transplant 2021;21:1979–1980. [DOI] [PubMed] [Google Scholar]
- 21. Schwacha-Eipper B, Minciuna I, Banz V, et al. Immunotherapy as a downstaging therapy for liver transplantation. Hepatology 2020;72:1488–1490. [DOI] [PubMed] [Google Scholar]
- 22. Sogbe M, López-Guerra D, Blanco-Fernández G, et al. Durvalumab as a successful downstaging therapy for liver transplantation in hepatocellular carcinoma: the importance of a washout period. Transplantation 2021;105:e398–e400. [DOI] [PubMed] [Google Scholar]
- 23. Yao FY, Mehta N, Flemming J, et al. Downstaging of hepatocellular cancer before liver transplant: long-term outcome compared to tumors within Milan criteria. Hepatology 2015;61:1968–1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Mehta N, Frenette C, Tabrizian P, et al. Downstaging outcomes for hepatocellular carcinoma: results from the multi-center evaluation of reduction in tumor size before liver transplantation (MERITS-LT) consortium. Gastroenterology 2021;161:1502–1512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Hoffmann K, Ganten T, Gotthardtp D, et al. Impact of neo-adjuvant Sorafenib treatment on liver transplantation in hepatocellular carcinoma patients - a prospective, randomized, double-blind, phase III trial. BMC Cancer 2015;15:392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Golse N, Radenne S, Rode A, et al. Liver transplantation after neoadjuvant sorafenib therapy: preliminary experience and literature review. Exp Clin Transplant 2018;16:227–236. [DOI] [PubMed] [Google Scholar]
- 27. Minoux K, Lassailly G, Ningarhari M, et al. Neo-adjuvant use of sorafenib for hepatocellular carcinoma awaiting liver transplantation. Transpl Int 2022;35:10569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Hidaka M, Hara T, Soyama A, et al. The outcome of conversion liver resection surgery by lenvatinib treatment: a single center experience. Anticancer Res 2022;42:3049–3054. [DOI] [PubMed] [Google Scholar]
- 29. Colón Rodríguez A, Velasco Sánchez E, Rodríguez-Bachiller L, et al. Neoadjuvant combined strategy to surgery based on chemoembolization and lenvatinib in hepatocellular carcinoma. Gastroenterol Hepatol 2022;45:490–491. [DOI] [PubMed] [Google Scholar]
- 30. Xue F, Shi S, Zhang Z, et al. Application of a novel liquid biopsy in patients with hepatocellular carcinoma undergoing liver transplantation. Oncol Lett 2018;15:5481–5488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Chen Z, Lin X, Chen C, et al. Analysis of preoperative circulating tumor cells for recurrence in patients with hepatocellular carcinoma after liver transplantation. Ann Transl Med 2020;8:1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Li Y, Huang N, Wang C, et al. Impact of liver tumor percutaneous radiofrequency ablation on circulating tumor cells. Oncol Lett 2018;16:2839–2850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Fang ZT, Zhang W, Wang GZ, et al. Circulating tumor cells in the central and peripheral venous compartment - assessing hematogenous dissemination after transarterial chemoembolization of hepatocellular carcinoma. Onco Targets Ther 2014;7:1311–1318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Bootsma M, McKay RR, Emamekhoo H, et al. Longitudinal molecular profiling of circulating tumor cells in metastatic renal cell carcinoma. J Clin Oncol 2022;40:3633–3641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Zhu XD, Huang C, Shen YH, et al. Downstaging and resection of initially unresectable hepatocellular carcinoma with tyrosine kinase inhibitor and anti-PD-1 antibody combinations. Liver Cancer 2021;10:320–329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Kayali S, Pasta A, Plaz Torres MC, et al. Immune checkpoint inhibitors in malignancies after liver transplantation: a systematic review and pooled analysis. Liver Int 2023;43:8–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Zhang JX, Hua HJ, Cheng Y, et al. Role of transarterial chemoembolization in the era of tyrosine kinase inhibitor and immune checkpoint inhibitor combination therapy for unresectable hepatocellular carcinoma: a retrospective propensity score matched analysis. Acad Radiol 2023;S1076-6332:00469–5. [DOI] [PubMed] [Google Scholar]
- 38. Xin Y, Zhang X, Liu N, et al. Efficacy and safety of lenvatinib plus PD-1 inhibitor with or without transarterial chemoembolization in unresectable hepatocellular carcinoma. Hepatol Int 2023;17:753–764. [DOI] [PubMed] [Google Scholar]
- 39. Zhu HD, Li HL, Huang MS, et al. Transarterial chemoembolization with PD-(L)1 inhibitors plus molecular targeted therapies for hepatocellular carcinoma (CHANCE001). Signal Transduct Target Ther 2023;8:58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Finn RS, Ryoo BY, Merle P, et al. Pembrolizumab as second-line therapy in patients with advanced hepatocellular carcinoma in KEYNOTE-240: a randomized, double-blind, phase III trial. J Clin Oncol 2020;38:193–202. [DOI] [PubMed] [Google Scholar]
- 41. Yi Y, Sun BY, Weng JL, et al. Lenvatinib plus anti-PD-1 therapy represents a feasible conversion resection strategy for patients with initially unresectable hepatocellular carcinoma: A retrospective study. Front Oncol 2022;12:1046584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Tan DJH, Lim WH, Yong JN, et al. UNOS down-staging criteria for liver transplantation of hepatocellular carcinoma: systematic review and meta-analysis of 25 studies. Clin Gastroenterol Hepatol 2022:S1542–S3565 00144–6. [DOI] [PubMed] [Google Scholar]
- 43. Lee DD, Samoylova M, Mehta N, et al. The mRECIST classification provides insight into tumor biology for patients with hepatocellular carcinoma awaiting liver transplantation. Liver Transpl 2019;25:228–241. [DOI] [PubMed] [Google Scholar]
- 44. Cucchetti A, Serenari M, Sposito C, et al. Including mRECIST in the Metroticket 2.0 criteria improves prediction of hepatocellular carcinoma-related death after liver transplant. J Hepatol 2020;73:342–348. [DOI] [PubMed] [Google Scholar]
- 45. Wu X, Yang C, Yu H. The predictive values of serum dickkopf-1 and circulating tumor cells in evaluating the efficacy of transcatheter arterial chemoembolization treatment on hepatocellular carcinoma. Medicine (Baltimore) 2019;98:e16579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Zhou J, Zhu Y, Li Y, et al. Combined detection of circulating tumor cells, α-fetoprotein heterogene-3 and α-fetoprotein in the early diagnosis of HCC for the prediction of efficacy, prognosis, recurrence after microwave ablation. Infect Agent Cancer 2021;16:28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Kaseb AO, Hasanov E, Cao HST, et al. Perioperative nivolumab monotherapy versus nivolumab plus ipilimumab in resectable hepatocellular carcinoma: a randomised, open-label, phase 2 trial. Lancet Gastroenterol Hepatol 2022;7:208–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Marron TU, Fiel MI, Hamon P, et al. Neoadjuvant cemiplimab for resectable hepatocellular carcinoma: a single-arm, open-label, phase 2 trial. Lancet Gastroenterol Hepatol 2022;7:219–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data of this study are available under a transfer agreement from the corresponding author based on a reasonable request. There will be limitations on how data can be used or how long data will be available for.