In parallel with rising mortality from drug overdoses, hospitalizations for infectious complications from substance use are increasing [1]. One in 9 hospitalized adults in the United States has a substance use disorder (SUD) [2]. Traditionally, patients with injection drug use (IDU)–associated infections such as infective endocarditis or osteomyelitis may be subjected to prolonged hospitalizations for intravenous (IV) antimicrobial treatment. Treating SUDs in hospitalized patients is now considered standard of care, though SUD care is still suboptimal in many hospitals [3, 4]. While hospitalizations can and should give patients the opportunity to engage in treatment for SUDs, some patients may prefer not to pursue SUD treatment options at that time or may lack SUD treatment options. A growing body of evidence supports inclusion of patient preferences in antimicrobial treatment decisions, considering multiple antimicrobial treatment options for effective treatment of hospitalized people with SUD [5–7], incorporating multidisciplinary management, and flattening barriers, such as stigma and distrust, that lead to delayed presentation and less favorable outcomes. We represent infectious diseases (ID) and addiction medicine clinicians, researchers, and a person who formerly used drugs/harm reduction outreach specialist who are sounding the alarm that increased attention is needed to support equitable interventions and improve patient outcomes and well-being.
Barriers to accessing harm reduction services and SUD treatment range from restrictive policies to logistic challenges, such as lack of transportation, to fear of law enforcement [8–12]. Patients, including parents and pregnant individuals who use drugs, may also delay or avoid medical care because of stigma and distrust of clinicians [13, 14]. Stigmatizing encounters can also contribute to poor outcomes, such as self-directed discharges [15, 16]. Understanding how we can reduce stigma and bias, as well as provide trauma-informed care, is therefore crucial to optimize engagement in care.
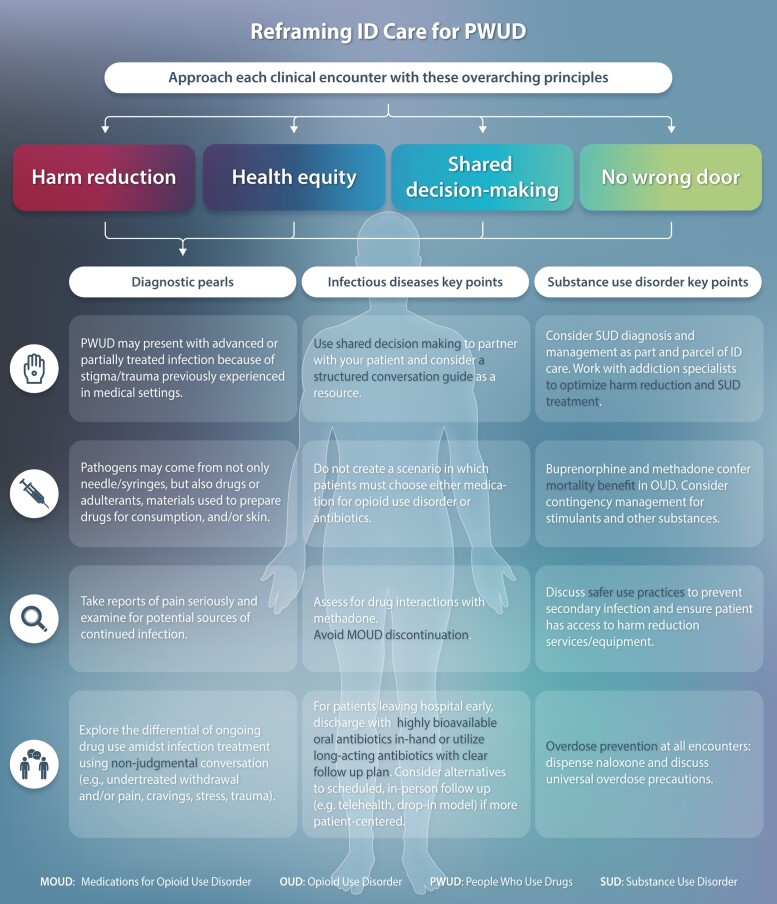
An important barrier to care for people with SUD is implicit racial and ethnic bias by healthcare professionals. Systemic racism and sexism influence healthcare professionals’ decisions and access to healthcare and insurance and fuels mistrust of the healthcare system [17]. Racial inequities are present in the diagnosis and prevention of infections, as well as in the management of infections and SUD [18]. Systemic racism [19], particularly against Black individuals, as well as Latina/Latino, Native American, and people of multiple races, has oppressed these populations and places them at higher risk for infections, such as human immunodeficiency virus (HIV) and coronavirus disease 2019 (COVID-19) [20]. Further, mass incarceration, particularly among people of color, has been associated with distrust in healthcare and can result in late diagnoses of infectious diseases [18]. There is also evidence of racial inequities in treatment, such as choice of antibiotic therapy for skin and soft tissue infections [21], antiretroviral prescribing [21], buprenorphine and methadone prescribing [22], and uptake of pre-exposure prophylaxis (PrEP) for HIV. Notably, while race/ethnicity is typically a focal point in inequity discussions, there are also health inequities at the intersections of gender and sexual orientation. To this extent, people who use drugs (PWUD) and identify as a sexual minority also experience stigma and oppression [23]. As ID clinicians, we must recognize and act to address these inequities (Figure 1).
Figure 1.
Key tenets of improving infectious diseases care of PWUD include reframing our clinical decisions around principles of shared decision making, health equity, and harm reduction and ensuring that all doors remain open to engagement in infection and/or substance use disorder treatment. Abbreviation: PWUD, people who use drugs.
Integration of harm reduction–based addiction care in the inpatient and general medicine settings may be a unique opportunity to reach patients and offer more equitable treatment access and mitigate disparities. Adopting a harm reduction–based approach to caring for PWUD involves prioritizing patient preferences and being sensitive to how inequities and stigmatizing encounters can worsen harm and health outcomes. Harm reduction is both a philosophy of care and a pragmatic set of strategies that helps PWUD take protective and proactive measures for themselves, their families, and communities [24]. A harm reduction–based approach to treating infectious complications of drug use should include patient preferences in antimicrobial treatment plans. For example, clinicians may consider prescribing a less bioavailable oral antimicrobial instead of IV antimicrobials (or no antimicrobials) if that aligns with patient preferences [5].
In clinical situations where more than 1 treatment option is clinically appropriate, shared decision making (SDM) has been evolving as an approach to care that incorporates patient preferences. SDM is a collaborative process where the patient and provider work together to choose the best path forward, with the patient and their values at the center of the decision [25]. Increasingly, treatment guidelines recommend an approach that involves SDM to allow patient participation where evidence is still emerging or is ambiguous and where options include trade-offs [26]. Rabi and colleagues eloquently state, “By recommending SDM, experts recognize the critical role that factors other than research evidence have in forming plans of care, including the experience and expertise of patients, their priorities, and the particulars of their situation, such as comorbidities, existing burdens of illness and treatment, social support, and personal capacity to safely enact the care plan” [26]. In the case of IDU-associated infections, this scenario is the norm. The clinical situation is complex and life-threatening, and most or all patients in this situation have complex psychosocial issues in their social systems and limited capacity to care for themselves.
In this review, we use a case-based structure to illustrate a spectrum of scenarios and provide viewpoints, particularly where robust data are lacking. We offer strategies for patient-centered healthcare that incorporate the tenets of SDM, harm reduction, and health equity.
CASE 1: A PATIENT WHO PREFERS TO BE DISCHARGED HOME WITH INTRAVENOUS ANTIMICROBIALS
Clinical Summary
A 35-year-old woman is hospitalized for fevers. Prior to presentation, she had fevers and rigors for 1 week and took several days of doxycycline that she acquired from a friend. Blood cultures drawn in the emergency department grow Streptococcus mitis. Further workup shows a dental infection. She has had 3 days of bacteremia. Co-occurring conditions include anxiety and opioid use disorder (OUD), with a history of IDU. Her OUD treatment includes methadone and recovery group attendance, and she denies any recent drug use or cravings. Transthoracic and transesophageal echocardiograms are negative for vegetation, and there are no signs or symptoms of infective endocarditis. She undergoes dental extraction. Her home dose of methadone is continued, and she attends virtual recovery group meetings during her hospitalization. She is unable to tolerate oral antimicrobial options. After 4 days, she expresses her desire to be discharged home to be with her children and complete IV antimicrobial treatment. She feels she can safely care for her peripherally inserted central catheter (PICC) and is eligible for home health services. She understands that the risks of incomplete treatment include recurrence of infection and, potentially, death. She reports good family support and is also able to follow up with her methadone clinic and recovery group after discharge.
DISCUSSION POINTS
People With SUD May Delay Seeking Care Due to Several Barriers, Thus Impacting Clinical Presentation
Patients may also delay or avoid medical care because of stigma and distrust of clinicians [13], in addition to other barriers such as restrictive policies and lack of transportation [8–12]. It is also not unusual for some individuals to acquire antimicrobials from nontraditional sources, such as peers, community partners, or even veterinary stores [27]. Asking patients whether they took antimicrobials prior to seeking care is important, as blood cultures could be “falsely” negative. If blood cultures are negative, performing a detailed history and physical is crucial so as not to delay diagnosis of severe IDU-associated infections, such as infective endocarditis [28]. Additionally, it is important to offer screening for sexually transmitted infections, such as HIV; prior studies have shown that PWUD are less likely to seek HIV counseling and testing and thus more likely to receive late HIV diagnoses and present with CD4 < 200cells/μL [29].
Substance Use Is Not a Contraindication to Discharging People Home With Outpatient Parenteral Antimicrobial Therapy
A recent systematic review showed that most people (72%–100%) with a history of IDU completed outpatient parenteral antimicrobial therapy (OPAT), which is similar to outcomes in patients without a history of IDU [30]. While large, randomized, controlled trials are lacking, there is still evidence that patients with IDU-associated infections complete OPAT, particularly when it is combined with SUD treatment [31, 32]. Thus, OPAT should be considered for people with SUD. Even when OPAT is deemed an ideal option with multidisciplinary support that includes addiction care, nursing facilities or home health agencies may reject patients due to their underlying SUD [33]. In these situations, it is important to consult with legal teams and consider patient protections under the American Disabilities Act and advocate to change policies [34] and reduce stigma and discrimination [35].
Integrate an SDM Approach Into Clinical Encounters
Prolonged hospitalizations for IV antimicrobial treatment are isolating for many patients and create challenges in retaining employment and fulfilling family and financial responsibilities [36]. While some clinicians may perceive the hospital as a protective environment [5], these prolonged hospitalizations can be harmful, whether due to stigmatizing encounters or nosocomial infections [16]. Because each patient may have different values, preferences, and goals for treatment, engaging patients in SDM can help patients meet their treatment goals [37]). Patients with a history of IDU may have low health literacy, which does not exclude SDM, and there is evidence that people with lower literacy may have greater benefit from SDM [38].
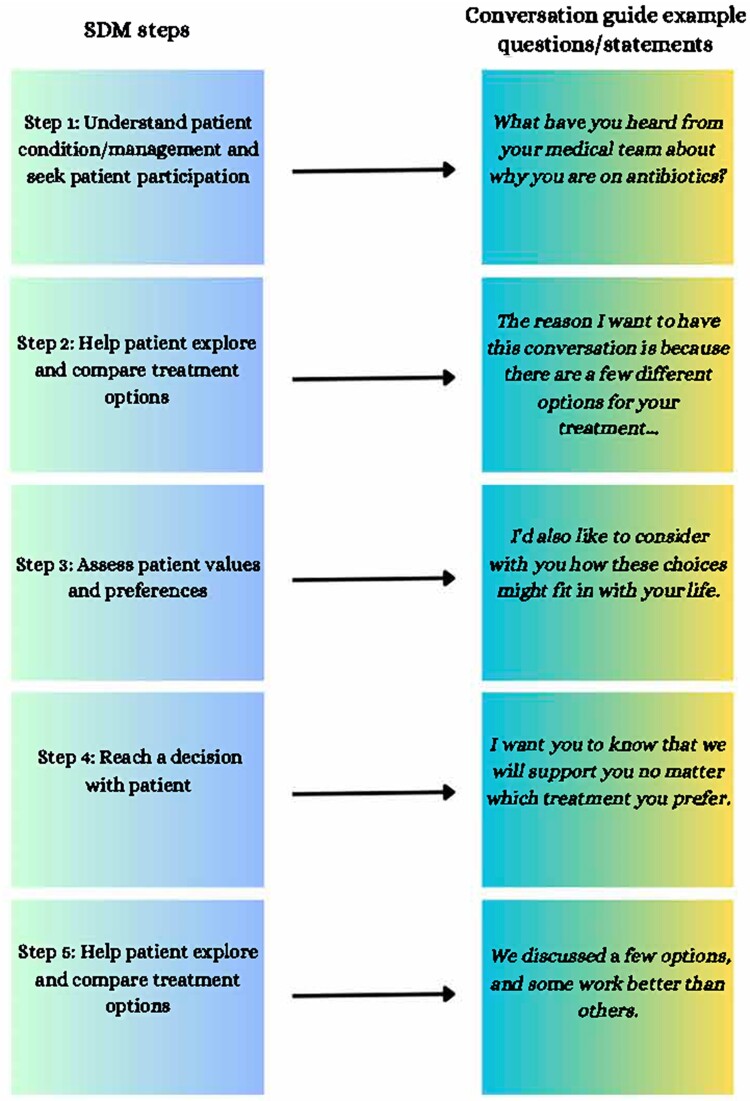
One commonly accepted SDM model includes 5 steps: seeking patient participation, helping the patient to explore and compare treatment options, assessing the patient's values and preferences, reaching a decision with the patient, and evaluating the patient's decision. Building on the SDM model and the Serious Illness Conversation Guide in the palliative care field [39, 40], a pilot study of a conversation guide developed specifically for patients hospitalized with IDU-associated infections was shown to be feasible and acceptable to both patients and healthcare professionals, while also improving patient autonomy and self-efficacy [6]. The conversation guide includes the following steps: setting up the conversation, assessing understanding, sharing the prognosis, exploring key topics, discussing antibiotic option trade-offs, closing the conversation, documenting the conversation, and communicating with key clinicians (Figure 2). If clinicians understand patient values and goals (eg, successful infection treatment, but prefers early discharge for childcare reasons) and if patients have a good understanding of these benefits and risks (eg, potential risk of infection, overdose, or even death associated with using a PICC to use drugs), we recommend documenting conversations around antimicrobial treatment options in the electronic health record and proceeding with the shared decision. Communication about serious illness is time-consuming, and recommendations for best practices include training healthcare professionals and using conversation guides [41].
Figure 2.
SDM steps [25] and conversation guide [6, 40] example questions/statements. Abbreviation: SDM, shared decision making.
RECOMMENDATIONS
We would have a structured conversation with the patient to understand her preferences and discuss antimicrobial trade-offs, including the risks and benefits of having a PICC. Given the patient's preferences, goals, and good understanding of her infection and treatment options, as well as stable housing and family support, we would recommend that the care team pursue discharge with OPAT. Given the current methadone regulatory environment, our patient may need to attend a methadone clinic daily, so we would recommend ceftriaxone 2 g IV daily to mitigate dosing schedule complexity (Table 1).
Table 1.
Case Examples of People Who Use Drugs, With Diagnostic and Management Recommendations
| Case No. | Case Summary | Key Diagnostic Lessons | Key Infection Management Lessons | Key Substance Use Disorder Management Lessons |
|---|---|---|---|---|
| 1 | 35-year-old woman with OUD on methadone hospitalized for Staphylococcus mitis bacteremia related to dental infection; presentation delayed by 1 wk (and complicated by prehospital antibiotics she got from a friend) due to fear of being labeled as “drug-seeking” | Recognize that people with SUD may present with advanced and/or partially treated infection because of stigma, trauma previously experienced in medical settings | Use shared decision making to partner with your patient and choose the best antimicrobial option, including setting and route | For patients with OUD on methadone, consider the potential need to attend a methadone clinic daily upon discharge |
| Do not create a scenario in which patients must choose either MOUD or antibiotics (eg, once-daily antibiotic push doses or continuous infusion pumps for patients needing to attend methadone clinic may be optimal) | ||||
| 2 | 44-year-old man with OUD and methamphetamine use and chronic back pain experiencing homelessness in rural Maine after release from jail, hospitalized with MSSA mitral valve endocarditis and continued back pain on day 8 of positive blood cultures | Take reports of pain seriously and examine for potential sources of continued infection such as epidural abscess | Assess for drug interactions with methadone; avoid MOUD discontinuation; consider alternatives to scheduled, in-person follow-up visits (eg, telehealth, drop-in model) if more patient-centered | Buprenorphine and methadone confer mortality benefit in OUD; contingency management could be considered if resources available |
| 3 | 35-year-old transgender woman experiencing homelessness with opioid and stimulant use disorders, hospitalized with Serratia bacteremia; source of infection from injecting with nonsterile water and reused needles given no access to SSPs in her primarily Black neighborhood; wants to leave hospital before antibiotic plan finalized | Pathogens may come from not only needles/syringes but also drugs or adulterants, materials used to prepare drugs for consumption, and/or skin; consider providing sexually transmitted infection screening and relevant vaccinations early in hospital stay |
For patients leaving hospital early, discharge with highly bioavailable oral antibiotics in hand and the option to make follow-up outpatient appointments prior to leaving | Discuss safer use practices to prevent secondary infection and ensure patient has access to harm reduction services/equipment; prescribe naloxone and discuss universal overdose precautions (eg, use small “test” doses, avoid using alone when possible, stagger drug use with others, use drug-checking services, if available) |
| Recognize and address health inequities (eg, ensure providing equitable access to harm reduction services as well as infectious disease/SUD treatment options) and partner with the community to provide low-barrier care | ||||
| 4 | 25-year-old man experiencing homelessness with opioid and stimulant use disorders, and MSSA prosthetic valve endocarditis, receiving intravenous cefazolin through PICC at respite care center; treated with methadone and mirtazapine for stimulant use disorder; experienced triggering event and used PICC line to inject methamphetamine once | Nonjudgmental conversation about injection drug use to explore the differential of ongoing injection drug use (eg, undertreated withdrawal and/or pain, cravings, stress, trauma) | Use a structured conversation guide to review risks and benefits of PICC vs other antimicrobial routes (oral, long-acting injection) | Work with addiction medicine/psychiatry providers and modify substance use treatment plan if needed; implement and advocate for harm reduction services (eg, safe consumption sites) |
Abbreviations: MOUD, medication for opioid use disorder; MSSA, methicillin-susceptible Staphylococcus aureus; OUD, opioid use disorder; PICC, peripherally inserted central catheter; SSPs, syringe services programs; SUD, substance use disorder.
CASE 2: A PATIENT IN A RURAL STATE WHO IS EXPERIENCING HOMELESSNESS AND FEELS READY TO LEAVE THE HOSPITAL
Clinical Summary
A 44-year-old man who injects fentanyl and is experiencing homelessness in rural Maine after recently being released from jail is hospitalized with methicillin-resistant Staphylococcus aureus mitral valve infective endocarditis. A 0.5-cm mitral valve vegetation is noted on transesophageal echocardiogram, without any other cardiac abnormalities. The multidisciplinary endocarditis team, which includes the primary team, cardiology, cardiothoracic surgery, infectious diseases, addiction medicine, and case management, recommends medical management. The inpatient addiction consult service diagnosed him with OUD, and the patient identifies his goal as abstaining from further fentanyl use. After discussing the risks and benefits of medications for OUD, they initiate methadone with plans to up-titrate the dose during hospitalization. The patient also meets with a licensed clinical social worker to facilitate housing applications. After 4 weeks in the hospital, the patient feels he is at a stable methadone dose and is not experiencing significant cravings. Though he has not yet secured housing, he expresses his desire to leave the hospital. He does not want to be discharged with a PICC but wants his infection to be treated. He notably has a sulfa allergy (anaphylaxis). His medication list also includes sertraline 100 mg daily.
DISCUSSION POINTS
Multidisciplinary Teams Should Be Standard of Care When Managing Infectious Complications of Drug Use
Multidisciplinary endocarditis teams have been associated with favorable outcomes, including a reduction in mortality and hospital readmissions, in addition to high antimicrobial completion rates and retention in SUD treatment [42–44]. Step-by-step guides to developing a multidisciplinary endocarditis team have been described elsewhere [45]. While few studies have been published on multidisciplinary teams for other infections, there are nevertheless some data from single-site studies. For example, in Conte et al's study on multidisciplinary teams, of 153 patients, approximately 29% and 21% had vertebral osteomyelitis and epidural abscesses, respectively. Patients who received a multidisciplinary discharge planning care team consult had a higher proportion of antimicrobial completion and fewer unplanned discharges [46].
Provide Treatment for SUDs and Create Organizational Policies to Enhance Access to Services
Hospitalization is an important indicator of increased risk for overdose and death, and it is an opportunity to offer patients lifesaving medications and treatment [47]. Models for hospital-based addiction care are diverse, ranging from consult models that include integrated ID and addiction medicine teams, stand-alone addiction medicine or addiction psychiatric consult services [48], individual consultants, and community provider-in reach (ex: facilitating referrals to community providers after discharge) to practice-based models where opioid and alcohol treatment are integrated into general hospitalist care [49]. Notably, addiction medicine consultation is associated with a mortality benefit following hospitalization [50]. Thus, regardless of the model, there is a pressing need to provide evidence-based addiction care as part of high-quality ID care [51].
Ideally, ID clinicians are comfortable diagnosing SUD (eg, OUD, stimulant use disorder) and understand available treatment options. The US Food and Drug Administration (FDA)–approved medications for OUD are buprenorphine (partial opioid agonist), methadone (full opioid agonist), and intramuscular naltrexone (opioid antagonist). All are associated with a mortality benefit, and use of buprenorphine and methadone is associated with more than 80% lower hazard of overdose death compared with nonmedication treatment [52]. In an effort to increase treatment access, in January 2023, the Drug Enforcement Administration (DEA) eliminated the X-waiver federal requirement, allowing any provider with a DEA license to prescribe buprenorphine. Buprenorphine can be accessed in both inpatient and outpatient settings, including lower barrier harm reduction centers, primary care, and via addiction and ID specialists. The most common formulations used for OUD are sublingual films or tabs, though buprenorphine is also available as a subcutaneous injection administered monthly. Clinical trials have demonstrated noninferiority to sublingual formulations with high patient satisfaction [53], suggesting potential opportunities for augmented use before hospital discharge and/or coupling with infection treatment. Traditionally, starting buprenorphine required patients to abstain from opioids and develop withdrawal before initiating buprenorphine. However, increasing evidence supports use of either “low-dose” or “high-dose” methods to start buprenorphine without requiring a patient to suffer through withdrawal [54, 55]. That said, particularly among those who use fentanyl heavily, precipitated withdrawal is a potential challenge that ID clinicians may encounter and are likely to seek support from addiction consultants to prevent and/or manage. If addiction services are not available on site, potential resources include seeking outside support through peers/programs such as the Providers Clinical Support System or the National Clinician Consultation Substance Use Warmline [56, 57].
Methadone, a long-acting full agonist opioid, is another highly effective option for OUD that has been supported by decades of evidence of effectiveness in reducing mortality, reducing time in carceral settings, and reducing HIV transmission [52]. Prescribing methadone outside of the hospital or emergency department setting is unfortunately limited to highly regulated, federally licensed opioid treatment programs. There is an urgent need for regulatory reform to increase methadone access. While highly variable based on the relationship between ID clinicians and local opioid treatment programs, there have been many successful examples of leveraging the structured nature of methadone clinics to provide directly observed therapy for HIV and/or hepatitis C medications that may have utility to support oral antibiotic treatment completion [58–60].
Finally, it is worth noting that naltrexone, an injectable opioid antagonist, often has less utility for hospitalized patients, many of whom have acute pain management needs. Moreover, retention in treatment is relatively low. If monthly injections are inconsistent and a patient returns to opioid use after loss of opioid tolerance, they are at high risk of overdose death [61].
In the face of sharply rising morbidity and mortality due to methamphetamine use [62], treatment for stimulant use disorder remains a challenge. There are no FDA-approved medications for stimulant use disorder; both mirtazapine and oral (PO) bupropion/intramuscular naltrexone have been studied with modest effect [63] but without results robust enough to recommend universally. As such, the current gold-standard treatment for those interested in reducing or stopping stimulant use is a behavioral intervention called contingency management (CM). CM is an incentive-based intervention anchored in operant conditioning; tangible reinforcers are provided for objective behavior change (commonly, stimulant-negative urine tests) 2–3 times weekly, typically over 12–16 weeks. At least 20 studies have documented efficacy in methamphetamine use disorder specifically, and more than 100 studies have demonstrated CM efficacy in reducing substance use more broadly [64–70]. Contingent incentives have also been used with success in supporting adherence to HIV, hepatitis, and tuberculosis treatment [71]. While efficacious, implementation has unfortunately lagged for multiple reasons including heavy staffing needs and cost concerns; however, leaders at the federal and state levels have called for more widespread use of CM as a priority [72].
Hospitals must ensure that the institutional policies and clinicians’ education are aligned with national recommendations for SUD treatment [73]. Hospital leadership should review and update SUD care policies to ensure they align with national treatment recommendations, are not discriminatory or punitive, and are trauma-informed, ideally with input from individuals with lived experience [74]. Care management teams should maintain relationships and open lines of communication with outpatient treatment teams to facilitate timely transition to services in the community. For example, case management can use contacts with methadone clinics to facilitate direct-admit intakes for high-risk patients on hospital discharge. In addition to promoting principles of harm reduction and discussing secondary prevention of infections, ID clinicians can play an important role in facilitating SUD treatment along with infection treatment.
Offer “Nontraditional” Antimicrobial Treatment Options, Such as Long-Acting Antimicrobial Injections and Oral Antimicrobials, and Explain the Trade-offs
Treatment options, both first-line and “nontraditional” antimicrobial options, should be discussed with PWUD as part of SDM and as a harm reduction strategy. A consensus approach to choosing a nontraditional antimicrobial treatment option for PWUD with infective endocarditis is described elsewhere [75]. Self-efficacy and autonomy are improved when all relevant treatment options are presented to hospitalized PWUD using a structured conversation guide [6].
Staphylococcus aureus (methicillin-susceptible [MSSA] and methicillin-resistant [MRSA]) and Streptococcus spp. are commonly isolated organisms in IDU-related infection. IV antistaphylococcal penicillins (ie, nafcillin or oxacillin) or first-generation cephalosporins (ie, cefazolin) are first-line antibiotics for MSSA and streptococcal infections, whereas IV vancomycin is standard treatment for MRSA infections. However, the frequent dosing interval of these agents may limit feasibility of outpatient infusions, either in an ambulatory clinic or infusion center. Furthermore, lack of transportation, as well as lack of health insurance to subsidize the cost of home infusion services, can inhibit use of these agents among PWUD. Daptomycin is therefore a reasonable alternative for outpatient treatment of streptococcal, MRSA, or MSSA infections due to its long half-life that allows for once-daily dosing [76]. Of interest, dalbavancin, a newer lipoglycopeptide drug, is currently approved by the FDA for use in skin and soft tissue infections. With a longer half-life, 1 dose of dalbavancin can last 14 days, with 2 doses of 1500 mg 1 week apart associated with therapeutic serum levels for up to 8 weeks [77]. Dalbavancin has been used in PWUD effectively, though sometimes with challenges related to cost and follow-up. Thus, care teams should troubleshoot potential treatment barriers with patients prior to discharge [78]. Though not currently approved, in vitro and clinical data suggest that dalbavancin can be an effective treatment in bacteremia and infective endocarditis [79], and a randomized trial is in progress to address its efficacy in this context [80].
Partial use of oral antibiotics (following initial treatment with IV antibiotics) in PWUD resulted in noninferior treatment when compared with patients who were treated with all IV therapy [81]. If being discharged with oral antimicrobials, having a nonjudgmental discussion about the feasibility (ie, twice daily cefadroxil vs 4 times daily cephalexin), potential side effects (ie, signs/symptoms of serotonin syndrome with medications such as selective serotonin reuptake inhibitor and linezolid), and efficacy (ie, acknowledging lack of randomized control data) is imperative. Historically, many oral antimicrobials have been avoided in the treatment of bone and joint infections due to their inability to achieve the required minimum inhibitory concentrations (MICs) at the site of infection. Fluoroquinolones, linezolid, and sulfamethoxazole/trimethoprim can exceed MICs of targeted organisms in bone and joint infections and should be considered as alternatives to IV antibiotics [7].
Regardless of which treatment regimen is chosen, it is imperative to discuss the risks and benefits of each option. Preferably, these conversations should occur early during the hospitalization and when patients are medically stable and ready to engage in treatment conversations [6]. If all the necessary information is not available during these conversations, openly discussing potential options (eg, dalbavancin if/once source control is achieved) and potential challenges (eg, ensuring dalbavancin is covered by insurance, arranging transportation) is important to improve patient involvement in decisions and possibly avoid adverse outcomes, such as self-discharge [82]. It is also important to consider structural drivers of health and the feasibility of treatment options; for example, access to transportation and/or patient copay assistance programs if a long-acting antimicrobial infusion such as dalbavancin is pursued. Nevertheless, the patient's healthcare insurance status and appropriate antimicrobial agent should be prioritized [83].
If in Line With Patient Preferences, Prioritize Medications for SUD When Considering Antimicrobial Drug–Drug Interactions
Risk of overdose is high at certain touchpoints, such as hospital discharge or release from a county jail or state prison [47]. If patients plan to pursue SUD treatment after hospital discharge, every effort should be made to ensure smooth transition to SUD treatment in the community. For example, while the Partial Oral versus Intravenous Antibiotic Treatment (POET) trial [84] studied linezolid 600 mg PO twice daily plus rifampin 600 mg PO twice daily, rifampin can markedly reduce methadone levels [75] and may also reduce buprenorphine levels, leading to opioid withdrawal symptoms [85]. Alternative considerations include treating with rifabutin instead of rifampin (which is associated with preserved methadone and buprenorphine levels in most but not all cases) or offering linezolid monotherapy, which has not been prospectively studied in infective endocarditis [86, 87]. We recommend that clinicians discuss the interactions between antimicrobials and SUD treatment, ensure that patients understand the interactions and trade-offs, and make a shared decision with patients regarding antimicrobial options. We recommend against stopping SUD treatment, such as methadone, to accommodate antimicrobial regimens.
Accommodate and Advocate for Antimicrobial and SUD Treatment Access, Particularly in Rural Settings
Rural areas are disproportionately burdened by SUD and drug overdose deaths [88]. Traumatic experiences, such as personal loss, sexual assault, or loss of child custody, as well as tapering of opioid prescriptions and normalization of drug use within families and communities are several environmental factors that PWUD in rural settings have identified as reasons for opioid misuse or transition to IDU [89]. Prevention and substance use treatment services for PWUD are unfortunately often limited in rural areas. For example, while many PWUD may prefer methadone treatment in the era of fentanyl, restrictive regulatory policies, distance, and limited opioid treatment programs are among the many barriers to methadone treatment in rural areas [90, 91]. Similarly, antimicrobial treatment options, including OPAT, may be limited in rural areas due to Medicaid nonexpansion and lack of resources such as transportation and staffing [92, 93].
As supported by the Infectious Diseases Society of America (IDSA), telehealth options should be offered to patients [94]. Given the limited number of ID clinicians in rural areas and the long distance individuals must travel to the closest health center or hospital, telehealth is an important option clinicians should offer to reduce patient travel costs. During the COVID-19 pandemic, relaxed telehealth policies for addiction care, such as allowing telehealth visits for the first 30 days of treatment for ongoing buprenorphine prescribing, improved access to treatment [95]. However, there are still limitations to telehealth in rural counties, such as lack of broadband infrastructure for video calls in some areas and potential need for phone cards. To address all of these challenges, there is a need for more advocacy to incentivize telehealth services [96], support Medicaid expansion, and reduce barriers to prescribing medications for SUD, such as methadone and buprenorphine, in rural settings [55].
Criminal–Legal Involved Individuals Have Unique Care Coordination Needs and Treatment Considerations
PWUD have high rates of detention and incarceration in carceral facilities including jails and prisons. Accessing medical records related to previous treatments for infectious diseases at jails and prisons can be challenging. Details regarding facility name, type of facility, and incarceration dates should be gathered from the patient to start the process of retrieving relevant health information. Carceral settings should respond to faxed release-of-information requests about healthcare; however, these tasks are often deprioritized with other competing medical tasks. If there is no response to faxed requests, a call to the infirmary or clinical area (“medical unit”) of the jail or prison should be made to connect to a staff person who will respond to the inquiry.
If a patient thinks they may be incarcerated, clinicians should ensure that the patient knows the details of their treatment, including a point of contact that the jail or prison should call for medical records. Notably, most carceral settings will not accept patients back to the jail’s “general population,” the least confining of statuses, with a PICC. Placing a PICC may force the person to be in isolation in the infirmary with restrictions on interactions with other people, jail programming, and visitors. Such restrictions, which people have also experienced during the COVID-19 pandemic, can be harmful to mental health [97]. Thus, the decision to place a PICC should be weighed with these potential restrictions.
RECOMMENDATIONS
In this situation, we would use a structured conversation guide to discuss the risks and benefits of various treatment options. We would present dalbavancin as a first alternative treatment option that would minimize drug interactions, minimize the need for daily antimicrobial treatment, and thus potentially optimize effectiveness. If other factors such as transportation to an infusion center or home health staffing shortages preclude use of dalbavancin, we would present select oral antimicrobials as potential treatment options. Based on the POET trial [84], we would prescribe linezolid 600 mg PO twice daily with rifabutin 300 mg daily. Because the patient is on a selective serotonin reuptake inhibitor, we would discuss signs/symptoms of serotonin syndrome. We would advise that the benefits of linezolid outweigh the risks from our perspective, particularly given that serotonin syndrome is exceedingly rare [98]. We would not recommend additional bloodwork if the patient gets 2 weeks of PO linezolid. However, for patients who require linezolid for 4 weeks or longer, we would recommend monthly bloodwork (eg, complete blood cell count, comprehensive metabolic panel to assess for thrombocytopenia and transaminitis, respectively) and counsel the patient on potential side effects (eg, peripheral neuropathy). Given his rural setting, we would offer telehealth services for follow-up, through video or audio calls. If the patient were treated with buprenorphine, we would offer telehealth for SUD treatment. Since the patient is on methadone, telehealth is not currently an option based on current opioid treatment program regulations. However, we would advocate for less restrictive regulations around methadone prescribing (eg, methadone prescriptions for OUD through primary care, pharmacy dispensing). We would also work with care team members to support methadone treatment care coordination (eg, confirming that methadone clinic intake is scheduled and that it aligns with discharge timing, last methadone dose information is provided in discharge paperwork, patient has photo identification).
CASE 3: A PATIENT IN AN URBAN AREA WHO IS EXPERIENCING HOMELESSNESS AND DECIDES TO LEAVE THE HOSPITAL
Clinical Summary
A 35-year-old transgender woman who is experiencing homelessness is admitted with pan-susceptible Serratia marcescens bacteremia in the setting of IV fentanyl and methamphetamine use. She has been injecting drugs with nonsterile water and shares needles, given there is no access to a syringe services program in her primarily Black neighborhood in Seattle. The patient occasionally engages in primary care through a local mobile health unit. She is not currently interested in outpatient SUD treatment. Her bacteremia is treated initially with cefepime, and opioid withdrawal is treated with short-acting opioids. The medical team builds a good rapport with the patient; however, 2 days into her hospitalization, she decides to leave the hospital at midnight.
DISCUSSION POINTS
Offer Oral Antimicrobial Treatment and Other Prevention and Supportive Services in the Event of Unplanned Discharges
The goal of having structured conversations about antimicrobial treatment options and their trade-offs is to minimize unplanned discharges [6]. However, if a patient has an unplanned discharge, a single-site study has shown that offering oral antimicrobial options, when possible, results in better outcomes than when no antimicrobial options are given [99]. Structured conversations about different treatment options, including oral antimicrobial options, should be documented in the chart to help guide cross-covering teams if an unplanned discharge occurs when ID clinicians are unavailable. Supportive services should also be offered, and naloxone should be prescribed. In addition to stigmatizing encounters, patients may leave the hospital because of other competing priorities, such as loss of employment, childcare responsibilities, or other financial barriers. Regardless, patients should receive expedited telehealth or in-person ID follow-up; assessment for post-exposure prophylaxis (PEP) for HIV prevention; health and harm reduction coaching (safer use and overdose prevention strategies, appointment reminders, antimicrobial verification); assistance with housing, insurance, and/or disability applications; linkage to primary care; and support for other care coordination needs [99].
Implement Preventive Strategies to Optimize Patient Health and Safety
Although the primary reason for this patient's hospitalization is bacteremia, hospitalization is an opportunity to offer ID screening and prevention. PWUD should be screened for HIV, viral hepatitis (hepatitis A, B, and C), and other sexually transmitted infections at least annually per Centers for Disease Control and Prevention guidelines [100]. Extragenital testing for gonorrhea/chlamydia, including anal and throat swabs, should also be offered. Particularly because of the high risk of HIV acquisition in certain individuals, such as transgender women [101], some PWUD may warrant more frequent screening. We recommend testing more frequently, for example, every 3 months, depending on individual practices (eg, sharing injection or smoking equipment, involvement in sex work). Additionally, vaccinations such as hepatitis A and B, Tdap, Prevnar 20, COVID-19, and other age-appropriate vaccines should be offered to all patients, even those with self-directed discharges. Viral hepatitis vaccines offer some level of protection even after 1 dose. For hospitalized patients, we recommended offering vaccinations as early as possible (once patients are medically stable) in the event they may have a self-directed discharge. Other preventive strategies include PrEP for HIV prevention, as well as screening for latent tuberculosis. Developing a checklist [102] and/or tool kits or bundled interventions can help to systematically integrate several evidence-based practices into routine inpatient care [103].
Before transitioning to the outpatient setting, clinicians should discuss safer drug use, prescribe naloxone to patients, offer vaccinations, and discuss PrEP with patients who are HIV-negative. They should link patients to community-based harm reduction services, in addition to treatment for primary care, substance use, ID, and other specialties as needed.
Use Community Partnerships to Equitably Optimize Engagement in Care
Many PWUD experience housing insecurity and have limited access to medical care [12]. This experience is amplified at the intersection of race/ethnicity and social gender minority status [104, 105]. Nevertheless, PWUD often have established trusting relationships with community-based programs, ranging from syringe services programs to shelters or other community centers. Particularly for patients who have self-directed discharges or face barriers to accessing outpatient care, we recommend partnering with the community to equitably provide low-barrier harm reduction, prevention, and treatment services. To maximize equitable outreach, in Seattle, a co-located weekly mobile clinic at a local community center staffed by an ID physician, nurse, and social worker successfully provided comprehensive services (eg, social services, medical care, reproductive health, PrEP, substance use treatment) to a population of women who have historically not engaged with healthcare for several reasons, including stigmatization and mistrust [106]. In single-site studies, working closely with community social workers, case managers, harm reductionists, and peer/outreach specialists has been helpful for equitably engaging patients. These care team members can help coordinate substance use and ID treatment plans, particularly if an antimicrobial regimen was not determined before discharge [99]. Some individuals may prefer oral antimicrobials to be prescribed weekly, rather than for longer courses, due to the potential risk of medications being lost or stolen. Partnering with community organizations to educate the community on the appropriate use of antimicrobial agents and facilitate antimicrobial medication storage and management can be helpful. In addition, community-based organizations can also provide harm reduction equipment and training to patients and clinicians [107]. Furthermore, low-barrier, community-led clinics have also been shown to be effective in promoting the equitable uptake of healthcare resources in vulnerable communities where access to preventive services is a major limitation [108]. It is therefore crucial to create alliances with community organizations that provide services to PWUD.
RECOMMENDATIONS
In this patient's situation, we would prescribe oral ciprofloxacin 750 mg by mouth twice daily prior to patient discharge. We would document this plan in the chart so that if the patient leaves early overnight, the covering night team can prescribe this regimen. If we arrived the next morning after she left, we would try to contact her and arrange pickup of a prescription at a convenient pharmacy. In addition to screening for HIV, viral hepatitis, and other sexually transmitted infections, we would also discuss and offer PEP since she is within the 72-hour exposure period and discuss PrEP for HIV prevention. If the patient accepted PEP, we would then encourage transition to PrEP. We would offer vaccinations (hepatitis A and B, influenza, Prevnar 20, and COVID-19) early during the hospitalization; if viral hepatitis serology results were not available, we would still offer hepatitis A and B vaccines. If the patient declines vaccines or screening prior to discharge, we would work with outpatient care team members (case management, social work, peer/outreach workers) to facilitate coordination of care. During hospitalization, we would discuss safe injection practices, as well as safer smoking practices, to minimize risk of future IDU-associated infections. It is important to recognize that the pathogen responsible for the clinical syndrome may come from not only needles/syringes but also drugs or adulterants, materials used to prepare drugs for consumption, and/or skin. Thus, upon discharge, we would ensure the patient has access to syringe services programs or has other means of acquiring safer equipment, prescribe naloxone, and ensure the patient knows where they can access free low-barrier naloxone. We would also discuss harm reduction practices for overdose precautions (ie, use small “test” doses, avoid using alone when possible, take turns when using with others, avoid mixing substances, use drug-checking tools and services [109] if available to detect contaminants, use fentanyl test strips to detect high potency fentanyl, carry naloxone, carry a phone to contact 911). Ideally, expedited ID and substance use follow-up would be made available to the patient through a bridge or co-located, low-barrier program.
CASE 4: A PATIENT WHO IS USING DRUGS WHILE RECEIVING IV ANTIBIOTICS
Clinical Summary
A 25-year-old man experiencing homelessness with chronic hepatitis C virus (HCV), opioid and stimulant disorders, and recent MSSA prosthetic valve endocarditis is receiving IV cefazolin through a PICC at a local medical respite care center. Through the respite care center, which serves people experiencing housing insecurity, he can receive continuous care, including IV antimicrobial treatment, following hospital discharge. His SUD treatment includes methadone for OUD and mirtazapine for stimulant use disorder, as well as counseling. Four weeks into treatment, he is still unable to secure housing and discovers he has lost his job permanently. Respite care staff are alerted that a nurse found a syringe in his bed. There is concern that he used his PICC to inject methamphetamine over the weekend. He is hemodynamically stable. Just prior to his scheduled ID follow-up appointment that week, respite care staff ask how they should proceed with treatment.
DISCUSSION POINTS
Ask Permission to Have Open, Nonjudgmental Conversations About Ongoing Substance Use
Some clinicians may perceive the hospital as a protective environment [5]. However, prior work has shown that up to 40% of patients may use drugs during their hospitalization, particularly in the setting of undertreated pain and withdrawal symptoms [110]. Per the SDM framework [39], understanding patient values, particularly whether they wish to continue to use substances, is important. Using elements of the structured conversation guide, we recommend asking permission from the patient to discuss ongoing substance use. As Martin et al discuss, using open-ended questions regarding substance use is important, as well as emphasizing concern for the patient's safety and desire to minimize any harm related to substance use (eg, overdose, infection) [74]. It is imperative to ask patients how clinical staff can best support them to tolerate their hospitalizations so that the care team can tailor interventions appropriately. For example, some potential interventions include offering adequate pain control, medications to manage withdrawal symptoms, and other supportive services (eg, phone chargers, snacks, daily visits) [74].
Continued Use of Drugs May Occur, But Harm Reduction Tools Can Support Safer Use of Drugs
Hospitals should ensure that patients are offered standard of care for SUD and antimicrobial treatment [111]. However, harm reduction strategies should always be discussed with patients, regardless of SUD treatment plans. Importantly, research has shown that PWUD prefer harm reduction approaches for safe use of drugs [112]. Though use of a PICC to inject drugs is uncommon, clinicians should have open, nonjudgmental conversations with patients about safe injection and smoking practices [24]. We also recommend that hospitals offer harm reduction kits upon discharge (eg, safer injection and smoking equipment, wound care items) [107]. Last, we support safe consumption sites to optimize the health and safety of PWUD. Safe consumption sites are settings where people can go to use drugs and are monitored for overdoses or other adverse outcomes. They reduce overdose deaths, have the potential to reduce infections, and have been widely used in Europe, Australia, and Canada. In the United States, these services were not available until 2021, when New York City opened its first safe consumption site. In the hospital setting, PWUD have reported that having a supportive environment, such as a safe consumption site, would promote retention in care and risk reduction. Only a few acute care hospitals in Europe and Canada currently provide inpatient safe consumption sites, and preliminary evidence has shown that patients who use these sites are less likely to leave before medically advised [113, 114]. We recommend consultation with institutional teams and further exploration and expansion of safe consumption sites in acute care and community settings.
Substance Use Is Not a Contraindication to Hepatitis C Treatment
Per the American Association for the Study of Liver Diseases and IDSA guidelines, neither recent nor active substance use is a contraindication to hepatitis C treatment [115]. Several studies have shown that PWUD achieved high hepatitis C cure rates [116, 117], some regardless of medication for OUD treatment. Reinfection can occur, but rates appear to be lower than incident HCV infection rates among PWUD [118]. While optimal models of treatment are still being investigated, the guidelines support promoting harm reduction services (eg, syringe services programs, access to medication for OUD, and overdose education and naloxone distribution) as key components of HCV treatment among PWUD [115]. Treating people for active HCV who are injecting drugs is necessary to get closer to ending the HCV epidemic. Some hospitals have instituted processes for discharging PWUD with prescriptions for HCV medications waiting at the outpatient pharmacy; starting the HCV treatment process either during or immediately after hospitalization should be considered if resources are available [119].
RECOMMENDATIONS
In this situation, we would invite the patient for an ID visit. We would ask for his permission to discuss potential ongoing substance use so that we could make his respite care stay more tolerable and safer. We would invite the patient to describe triggers for his recent use and work with his SUD treatment providers to modify his substance use treatment plan if needed. We would check 2 sets of blood cultures, in addition to a complete blood count with differential, comprehensive metabolic panel, and C-reactive protein. Through a structured conversation guide, we would ensure that the patient understands the risks (including new or worsening infection, death) and benefits of having a PICC, in addition to other antimicrobial treatment options (eg, long-acting antimicrobial infusions, oral antimicrobials, inpatient IV antimicrobials). We would also ensure that he knows how to access safer equipment. In this SDM process, if the patient understands the risks and benefits and decides to continue IV cefazolin through the PICC (assuming blood cultures are negative), we would document a summary of the structured conversation in the electronic health record. We would also confirm that the respite care center has naloxone on site and is trained in overdose reversal. We would not recommend readmission to the hospital if the patient were otherwise stable. We would treat the patient's chronic hepatitis C, offer appropriate vaccinations, and, per guidelines [115], recommend annual HCV RNA screening or more frequently (ie, every 3 months) if there is recurrence or ongoing substance use.
Next Steps for Shared Decision Support Tools for PWUD
As described in the 4 cases presented here, the clinical scenarios are as unique as the underlying psychosocial situations. There are no decision aids for patients with IDU-associated infections, and it is unlikely that any will be developed because the complexity of the clinical scenarios and treatment options would require endless iterations. Thus, based on current available data, we recommend using a conversation guide to help care team members engage patients in the care plan [6]. Given the worsening ID and SUD syndemic and lack of large clinical trials that incorporate patient preferences into treatment plans, there is a need for more research and training. The National Institutes of Health (National Institute on Drug Abuse, National Institute of Allergy and Infectious Diseases) should offer grant mechanisms specifically for projects that examine SDM among PWUD in order to further inform best practices. Professional organizations should include conference sessions dedicated to the care of PWUD and discussing the importance of SDM with hospitalized individuals.
CONCLUSIONS
The ID and SUD syndemic continues to worsen, as evidenced by the rising number of patients experiencing infectious complications from substance use. As ID clinicians, we must recognize and address the stigma and inequities that our patients face. In this review, we presented several clinical cases to illustrate a spectrum of scenarios and provided viewpoints, particularly where robust data are lacking. When caring for PWUD, we recommend drawing on principles of SDM and harm reduction to optimize patient autonomy, health, and safety.
Contributor Information
Kinna Thakarar, Tufts University School of Medicine, Boston, Massachusetts, USA; Center for Interdisciplinary Population & Health Research, MaineHealth Institute for Research, Portland, Maine, USA; Department of Medicine, Maine Medical Center, Portland, Maine, USA.
Ayesha Appa, Division of HIV, Infectious Diseases, and Global Medicine at San Francisco General Hospital, University of California–San Francisco, San Francisco, California, USA.
Jacinda C Abdul Mutakabbir, Division of Clinical Pharmacy, Skaggs School of Pharmacy and Pharmaceutical Sciences, University of California–San Diego, La Jolla, California, USA; Division of the Black Diaspora and African American Studies, University of California–San Diego, La Jolla, California, USA.
Amelia Goff, Section of Addiction Medicine, Department of Medicine, Oregon Health and Science University, Portland, Oregon, USA.
Jessica Brown, III, Department of Care Management, Oregon Health and Science University, Portland, Oregon, USA.
Chasity Tuell, Maine Access Points, Machias, ME, USA.
Kathleen Fairfield, Tufts University School of Medicine, Boston, Massachusetts, USA; Center for Interdisciplinary Population & Health Research, MaineHealth Institute for Research, Portland, Maine, USA; Department of Medicine, Maine Medical Center, Portland, Maine, USA.
Alysse Wurcel, Tufts University School of Medicine, Boston, Massachusetts, USA; Department of Medicine, Division of Geographic Medicine and Infectious Diseases, Tufts Medicine, Boston, Massachusetts, USA.
Notes
Author contributions. K. T., A. A., K. F., and A. W.: conceptualization; writing—original draft; writing—reviewing and editing. J. A. M. and A. G.: writing—original draft; writing—reviewing and editing. J. B. and C. T: writing—reviewing and editing.
References
- 1. Kadri AN, Wilner B, Hernandez AV, et al. Geographic trends, patient characteristics, and outcomes of infective endocarditis associated with drug abuse in the United States from 2002 to 2016. J Am Heart Assoc 2019; 8:e012969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Suen LW, Makam AN, Snyder HR, et al. National prevalence of alcohol and other substance use disorders among emergency department visits and hospitalizations: NHAMCS 2014–2018. J Gen Intern Med 2022; 37:2420–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Rosenthal ES, Karchmer AW, Theisen-Toupal J, Castillo RA, Rowley CF. Suboptimal addiction interventions for patients hospitalized with injection drug use-associated infective endocarditis. Am J Med 2016; 129:481–5. [DOI] [PubMed] [Google Scholar]
- 4. Englander H, Davis CS. Hospital standards of care for people with substance use disorder. N Engl J Med 2022; 387:672–5. [DOI] [PubMed] [Google Scholar]
- 5. Moore N, Kohut M, Stoddard H, et al. Health care professional perspectives on discharging hospitalized patients with injection drug use-associated infections. Ther Adv Infect Dis 2022; 9:20499361221126868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Thakarar K, Kohut M, Stoddard H, et al. “I feel like they’re actually listening to me”: a pilot study of hospital discharge-decision making for patients with injection drug use-associated infections. Ther Adv Infect Dis 2023; 10:20499361231165108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Wald-Dickler N, Holtom PD, Phillips MC, et al. Oral is the new IV. Challenging decades of blood and bone infection dogma: a systematic review. Am J Med 2022; 135:369–379.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Canary L, Hariri S, Campbell C, et al. Geographic disparities in access to syringe services programs among young persons with hepatitis C virus infection in the United States. Clin Infect Dis 2017; 65:514–7. [DOI] [PubMed] [Google Scholar]
- 9. Goldenberg S, Liyanage R, Braschel M, Shannon K. Structural barriers to condom access in a community-based cohort of sex workers in Vancouver, Canada: influence of policing, violence and end-demand criminalisation. BMJ Sex Reprod Health 2020; 46:301–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Shirley-Beavan S, Roig A, Burke-Shyne N, Daniels C, Csak R. Women and barriers to harm reduction services: a literature review and initial findings from a qualitative study in Barcelona, Spain. Harm Reduct J 2020; 17:78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Strike C, Watson TM. Losing the uphill battle? Emergent harm reduction interventions and barriers during the opioid overdose crisis in Canada. Int J Drug Policy 2019; 71:178–82. [DOI] [PubMed] [Google Scholar]
- 12. Thakarar K, Kohut M, Hutchinson R, et al. The impact of the COVID-19 pandemic on people who inject drugs accessing harm reduction services in an rural American state. Harm Reduct J 2022; 19:80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ellis K, Walters S, Friedman SR, et al. Breaching trust: a qualitative study of healthcare experiences of people who use drugs in a rural setting. Front Sociol 2020; 5:593925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Stone R. Pregnant women and substance use: fear, stigma, and barriers to care. Health Justice 2015; 3:1–15. [Google Scholar]
- 15. McNeil R, Small W, Wood E, Kerr T. Hospitals as a “risk environment” an ethno-epidemiological study of voluntary and involuntary discharge from hospital against medical advice among people who inject drugs. Soc Sci Med 2014; 105:59–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Simon R, Snow R, Wakeman S. Understanding why patients with substance use disorders leave the hospital against medical advice: a qualitative study. Subst Abus 2020; 41:519–25. [DOI] [PubMed] [Google Scholar]
- 17. Mayer KH, Agwu A, Malebranche D. Barriers to the wider use of pre-exposure prophylaxis in the United States: a narrative review. Adv Ther 2020; 37:1778–811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hoff L, Scheidell JD, Mazumdar M, et al. The associations of incarceration and depression with healthcare experiences and utilization among Black men who have sex with men in HPTN 061. AIDS Care 2022; 34:1169–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Dennis AC, Chung EO, Lodge EK, Martinez RA, Wilbur RE. Looking back to leap forward: a framework for operationalizing the structural racism construct in minority health research. Ethn Dis 2021; 31(Suppl 1):301–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Holden TM, Simon MA, Arnold DT, Halloway V, Gerardin J. Structural racism and COVID-19 response: higher risk of exposure drives disparate COVID-19 deaths among Black and Hispanic/Latinx residents of Illinois, USA. BMC Public Health 2022; 22:312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Wurcel AG, Essien UR, Ortiz C, et al. Variation by race in antibiotics prescribed for hospitalized patients with skin and soft tissue infections. JAMA Netw Open 2021; 4:e2140798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Beer L, Bradley H, Mattson CL, Johnson CH, Hoots B, Shouse RL. Trends in racial and ethnic disparities in antiretroviral therapy prescription and viral suppression in the United States, 2009–2013. J Acquir Immune Defic Syndr 2016; 73:446–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Infectious Diseases Society of America . Toolkit to address health inequities. 2023. Available at: https://www.idsociety.org/globalassets/covid-19-real-time-learning-network/covid-health-equity-resources/toolkit-to-address-health-inequities-pdf.pdf. Accessed 10 November 2023.
- 24. Toward the Heart . Safer sex and safer drug use. 2023. Available at: https://towardtheheart.com/safer-use. Accessed 10 November 2023.
- 25. Bomhof-Roordink H, Gärtner FR, Stiggelbout AM, Pieterse AH. Key components of shared decision making models: a systematic review. BMJ Open 2019; 9:e031763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Rabi DM, Kunneman M, Montori VM. When guidelines recommend shared decision-making. JAMA 2020; 323:1345–6. [DOI] [PubMed] [Google Scholar]
- 27. Gilbert AR, Hellman JL, Wilkes MS, Rees VW, Summers PJ. Self-care habits among people who inject drugs with skin and soft tissue infections: a qualitative analysis. Harm Reduct J 2019; 16:69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Grigoryan A, Hall HI, Durant T, Wei X. Late HIV diagnosis and determinants of progression to AIDS or death after HIV diagnosis among injection drug users, 33 US states, 1996–2004. PLoS One 2009; 4:e4445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Giacobbe DR, Salsano A, Santini F, Bassetti M. Antibiotics and missed etiological diagnosis of infective endocarditis: a dangerous duo. J Clin Med 2022; 11:4533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Suzuki J, Johnson JA, Montgomery MW, et al. Long-term outcomes of injection drug-related infective endocarditis among people who inject drugs. J Addict Med 2020; 14:282–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Sikka MK, Gore S, Vega T, Strnad L, Gregg J, Englander H. “OPTIONS-DC”, a feasible discharge planning conference to expand infection treatment options for people with substance use disorder. BMC Infect Dis 2021; 21:772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Fanucchi LC, Walsh SL, Thornton AC, Nuzzo PA, Lofwall MR. Outpatient parenteral antimicrobial therapy plus buprenorphine for opioid use disorder and severe injection-related infections. Clin Infect Dis 2020; 70:1226–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Kimmel SD, Rosenmoss S, Bearnot B, et al. Northeast postacute medical facilities disproportionately reject referrals for patients with opioid use disorder. Health Aff (Millwood) 2022; 41:434–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Civil Rights Division . The Americans with Disabilities Act and the opioid crisis: combating discrimination against people in treatment or recovery. 2022. Available at: https://archive.ada.gov/opioid_guidance.pdf. Accessed 10 November 2023.
- 35. Wakeman SE, Rich JD. Barriers to medications for addiction treatment: how stigma kills. Subst Use Misuse 2018; 53:330–3. [DOI] [PubMed] [Google Scholar]
- 36. McNeil R, Small W. “Safer environment interventions”: a qualitative synthesis of the experiences and perceptions of people who inject drugs. Soc Sci Med 2014; 106:151–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Eckland A, Kohut M, Stoddard H, et al. “I know my body better than anyone else”: a qualitative study of perspectives of people with lived experience on antimicrobial treatment decisions for injection drug use-associated infections. Therapeutic Advances in Infectious Disease 2023; 10. doi: 10.1177/20499361231197065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Stacey D, Hill S, McCaffery K, Boland L, Lewis KB, Horvat L. Shared decision making interventions: theoretical and empirical evidence with implications for health literacy. Stud Health Technol Inform 2017; 240:263–83. [PubMed] [Google Scholar]
- 39. Coronado-Vazquez V, Canet-Fajas C, Delgado-Marroquín MT, Magallón-Botaya R, Romero-Martín M, Gómez-Salgado J. Interventions to facilitate shared decision-making using decision aids with patients in primary health care: a systematic review. Medicine (Baltimore) 2020; 99:e21389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Labs A. Serious illness conversation guide. [cited 2022 September 11]. Available at https://www.ariadnelabs.org/tools-and-downloads/. Accessed 10 November 2023.
- 41. Bernacki RE, Block SD; American College of Physicians High Value Care Task Force . Communication about serious illness care goals: a review and synthesis of best practices. JAMA Intern Med 2014; 174:1994–2003. [DOI] [PubMed] [Google Scholar]
- 42. Botelho-Nevers E, Thuny F, Casalta JP, et al. Dramatic reduction in infective endocarditis-related mortality with a management-based approach. Arch Intern Med 2009; 169:1290–8. [DOI] [PubMed] [Google Scholar]
- 43. Habib G. Infective endocarditis in Portugal: changing epidemiology but still a deadly disease. Rev Port Cardiol (Engl Ed) 2021; 40:219–20. [DOI] [PubMed] [Google Scholar]
- 44. Mori M, Amabile A, Weimer MB, Geirsson A. The opioid epidemic and endocarditis: frontiers in the management of injection drug use-related endocarditis. JTCVS Open 2021; 8:315–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. El-Dalati S, Cronin D, Riddell J, et al. A step-by-step guide to implementing a multidisciplinary endocarditis team. Ther Adv Infect Dis 2021; 8:20499361211065596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Conte M, Schneider B, Varley CD, Streifel AC, Sikka MK. Description and outcomes of patients with substance use disorder with serious bacterial infections who had a multidisciplinary care conference. Ther Adv Infect Dis 2022; 9:20499361221117974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Larochelle MR, Bernstein R, Bernson D, et al. Touchpoints—opportunities to predict and prevent opioid overdose: a cohort study. Drug Alcohol Depend 2019; 204:107537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Serota DP, Tookes HE, Hervera B, et al. Harm reduction for the treatment of patients with severe injection-related infections: description of the Jackson SIRI Team. Ann Med 2021; 53:1960–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Englander H, Jones A, Krawczyk N, et al. A taxonomy of hospital-based addiction care models: a scoping review and key informant interviews. J Gen Intern Med 2022; 37:2821–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Wilson JD, Altieri Dunn SC, Roy P, Joseph E, Klipp S, Liebschutz J. Inpatient addiction medicine consultation service impact on post-discharge patient mortality: a propensity-matched analysis. J Gen Intern Med 2022; 37:2521–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Serota DP, Barocas JA, Springer SA. Infectious complications of addiction: a call for a new subspecialty within infectious diseases. Clin Infect Dis 2020; 70:968–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Krawczyk N, Mojtabai R, Stuart EA, et al. Opioid agonist treatment and fatal overdose risk in a state-wide US population receiving opioid use disorder services. Addiction 2020; 115:1683–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Lofwall MR, Walsh SL, Nunes EV, et al. Weekly and monthly subcutaneous buprenorphine depot formulations vs daily sublingual buprenorphine with naloxone for treatment of opioid use disorder: a randomized clinical trial. JAMA Intern Med 2018; 178:764–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Herring AA, Vosooghi AA, Luftig J, et al. High-dose buprenorphine induction in the emergency department for treatment of opioid use disorder. JAMA Netw Open 2021; 4:e2117128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Sokolski E, Skogrand E, Goff A, Englander H. Rapid low-dose buprenorphine initiation for hospitalized patients with opioid use disorder. J Addict Med 2023; 17:e278–80. [DOI] [PubMed] [Google Scholar]
- 56. PCSS Mentoring Program . [cited 2023 July 7]. Available at https://pcssnow.org/mentoring/. Accessed 10 November 2023.
- 57. AIDS Education and Training Centers Program . National Clinician Consultation Center substance use warmline [cited 2023 July 27]. Available at: https://aidetc.org/aetc-program/nccc#:~:text=Substance%20Use%20Management%20(Substance%20Use,for%20substance%20use%20disorder%20treatment. Accessed 31 July 2023.
- 58. Berg KM, Litwin A, Li X, Heo M, Arnsten JH. Directly observed antiretroviral therapy improves adherence and viral load in drug users attending methadone maintenance clinics: a randomized controlled trial. Drug Alcohol Depend 2011; 113:192–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Sorensen JL, Haug NA, Larios S, et al. Directly administered antiretroviral therapy: pilot study of a structural intervention in methadone maintenance. J Subst Abuse Treat 2012; 43:418–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Norton BL, Akiyama MJ, Zamor PJ, Litwin AH. Treatment of chronic hepatitis C in patients receiving opioid agonist therapy: a review of best practice. Infect Dis Clin North Am 2018; 32:347–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Ndegwa S, Pant S, Pohar S, Mierzwinski-Urban M. Injectable extended-release naltrexone to treat opioid use disorder. Ottawa, ON: CADTH Issues in Emerging Health Technologies, 2017. [PubMed] [Google Scholar]
- 62. Han B, Compton WM, Jones CM, Einstein EB, Volkow ND. Methamphetamine use, methamphetamine use disorder, and associated overdose deaths among US adults. JAMA Psychiatry 2021; 78:1329–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Coffin PO, Santos G-M, Hern J, et al. Effects of mirtazapine for methamphetamine use disorder among cisgender men and transgender women who have sex with men: a placebo-controlled randomized clinical trial. JAMA Psychiatry 2020; 77:246–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Brown HD, DeFulio A. Contingency management for the treatment of methamphetamine use disorder: a systematic review. Drug Alcohol Depend 2020; 216:108307. [DOI] [PubMed] [Google Scholar]
- 65. Lussier JP, Heil SH, Mongeon JA, Badger GJ, Higgins ST. A meta-analysis of voucher-based reinforcement therapy for substance use disorders. Addiction 2006; 101:192–203. [DOI] [PubMed] [Google Scholar]
- 66. Prendergast M, Podus D, Finney J, Greenwell L, Roll J. Contingency management for treatment of substance use disorders: a meta-analysis. Addiction 2006; 101:1546–60. [DOI] [PubMed] [Google Scholar]
- 67. Dutra L, Stathopoulou G, Basden SL, Leyro TM, Powers MB, Otto MW. A meta-analytic review of psychosocial interventions for substance use disorders. Am J Psychiatry 2008; 165:179–87. [DOI] [PubMed] [Google Scholar]
- 68. Bolivar HA, Klemperer EM, Coleman SRM, DeSarno M, Skelly JM, Higgins ST. Contingency management for patients receiving medication for opioid use disorder: a systematic review and meta-analysis. JAMA Psychiatry 2021; 78:1092–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Bentzley BS, Han SS, Neuner S, Humphreys K, Kampman KM, Halpern CH. Comparison of treatments for cocaine use disorder among adults: a systematic review and meta-analysis. JAMA Netw Open 2021; 4:e218049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Ronsley C, Nolan S, Knight R, et al. Treatment of stimulant use disorder: a systematic review of reviews. PLoS One 2020; 15:e0234809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Herrmann ES, Matusiewicz AK, Stitzer ML, Higgins ST, Sigmon SC, Heil SH. Contingency management interventions for HIV, tuberculosis, and hepatitis control among individuals with substance use disorders: a systematized review. J Subst Abuse Treat 2017; 72:117–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Volkow N. Five areas where “more research” isn’t needed to curb the overdose crisis. 2022 [cited 2023 March 4]. Available at: https://nida.nih.gov/about-nida/noras-blog/2022/08/five-areas-where-more-research-isnt-needed-to-curb-overdose-crisis. Accessed 10 November 2023.
- 73. Priest KC, Englander H, McCarty D. Hospital policies for opioid use disorder treatment: a policy content analysis and environmental scan checklist. Gen Hosp Psychiatry 2021; 70:18–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Martin M, Snyder HR, Otway G, Holpit L, Day LW, Seidman D. In-hospital substance use policies: an opportunity to advance equity, reduce stigma, and offer evidence-based addiction care. J Addict Med 2023; 17:10–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Baddour LM, Weimer MB, Wurcel AG, et al. Management of infective endocarditis in people who inject drugs: a scientific statement from the American Heart Association. Circulation 2022; 146:e187–201. [DOI] [PubMed] [Google Scholar]
- 76. Fraimow HS. Systemic antimicrobial therapy in osteomyelitis. Semin Plast Surg 2009; 23:90–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Dunne MW, Puttagunta S, Sprenger CR, Rubino C, Van Wart S, Baldassarre J. Extended-duration dosing and distribution of dalbavancin into bone and articular tissue. Antimicrob Agents Chemother 2015; 59:1849–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Bryson-Cahn C, Beieler AM, Chan JD, Harrington RD, Dhanireddy S. Dalbavancin as secondary therapy for serious Staphylococcus aureus infections in a vulnerable patient population. Open Forum Infect Dis 2019; 6:ofz028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Ajaka L, Heil E, Schmalzle S. Dalbavancin in the treatment of bacteremia and endocarditis in people with barriers to standard care. Antibiotics (Basel) 2020; 9:700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. National Institutes of Health, US National Library of Medicine . DOTS: Dalbavancin as an option for treatment of Staphylococcus aureus bacteremia. 2023 [cited 2023 March 20]. Available at https://clinicaltrials.gov/study/NCT04775953. Accessed 10 November 2023.
- 81. Marks LR, Liang SY, Muthulingam D, et al. Evaluation of partial oral antibiotic treatment for persons who inject drugs and are hospitalized with invasive infections. Clin Infect Dis 2020; 71:e650–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Serrano-Perez P, Rivero-Santana A, Daigre-Blanco C, et al. Shared decision making in patients with substance use disorders: A one-year follow-up study. Psychiatry Research 2023; 329. doi: 10.1016/j.psychres.2023.115540 [DOI] [PubMed] [Google Scholar]
- 83. Yang W-T, Dombrowski JC, Glick SN, et al. Partial-oral antibiotic therapy for bone and joint infections in people with recent injection drug use. Open Forum Infect Dis 2023; 10:ofad005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Iversen K, Ihlemann N, Gill SU, et al. Partial oral versus intravenous antibiotic treatment of endocarditis. N Engl J Med 2019; 380:415–24. [DOI] [PubMed] [Google Scholar]
- 85. McCance-Katz EF, Sullivan LE, Nallani S. Drug interactions of clinical importance among the opioids, methadone and buprenorphine, and other frequently prescribed medications: a review. Am J Addict 2010; 19:4–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Brown LS, Sawyer RC, Li R, Cobb MN, Colborn DC, Narang PK. Lack of a pharmacologic interaction between rifabutin and methadone in HIV-infected former injecting drug users. Drug Alcohol Depend 1996; 43:71–7. [DOI] [PubMed] [Google Scholar]
- 87. McCance-Katz EF, Moody DE, Prathikanti S, Friedland G, Rainey PM. Rifampin, but not rifabutin, may produce opiate withdrawal in buprenorphine-maintained patients. Drug Alcohol Depend 2011; 118:326–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Rossen LM, Khan D, Warner M. Trends and geographic patterns in drug-poisoning death rates in the U.S., 1999–2009. Am J Prev Med 2013; 45:e19–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Nolte K, Drew AL, Friedmann PD, Romo E, Kinney LM, Stopka TJ. Opioid initiation and injection transition in rural northern New England: a mixed-methods approach. Drug Alcohol Depend 2020; 217:108256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Hirchak KA, Murphy SM. Assessing differences in the availability of opioid addiction therapy options: rural versus urban and American Indian reservation versus nonreservation. J Rural Health 2017; 33:102–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Sigmon SC, Kennedy AG. The prescription opioid abuse epidemic in rural America: a look at how it begins. J Opioid Manag 2014; 10:225–6. [DOI] [PubMed] [Google Scholar]
- 92. Triffault-Fillit C, Ferry T, Perpoint T, et al. Outpatient parenteral antibiotic therapy: evaluation of practices and limits of use in rural areas in France. Med Mal Infect 2018; 48:130–5. [DOI] [PubMed] [Google Scholar]
- 93. Kaiser Family Foundation . Status of state Medicaid expansion decisions: interactive map. 2023. Available at: https://www.kff.org/medicaid/issue-brief/status-of-state-medicaid-expansion-decisions-interactive-map. Accessed 10 November 2023.
- 94. Young JD, Abdel-Massih R, Herchline T, et al. Infectious Diseases Society of America position statement on telehealth and telemedicine as applied to the practice of infectious diseases. Clin Infect Dis 2019; 68:1437–43. [DOI] [PubMed] [Google Scholar]
- 95. Suarez E Jr, Bartholomew TS, Plesons M, et al. Adaptation of the tele-harm reduction intervention to promote initiation and retention in buprenorphine treatment among people who inject drugs: a retrospective cohort study. Ann Med 2023; 55:733–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Drake C, Yu J, Lurie N, Kraemer K, Polsky D, Chaiyachati KH. Policies to improve substance use disorder treatment with telehealth during the COVID-19 pandemic and beyond. J Addict Med 2020; 14:e139–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Duarte C, Cameron DB, Kwan AT, Bertozzi SM, Williams BA, McCoy SI. COVID-19 outbreak in a state prison: a case study on the implementation of key public health recommendations for containment and prevention. BMC Public Health 2022; 22:977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Kufel WD, Blaine B, Sepiol R, et al. Evaluation of serotonin syndrome with linezolid and serotonergic agents at an academic medical center. Open Forum Infect Dis 2022; 9:ofac492.032. [Google Scholar]
- 99. Lewis S, Liang SY, Schwarz ES, et al. Patients with serious injection drug use-related infections who experience patient-directed discharges on oral antibiotics have high rates of antibiotic adherence but require multidisciplinary outpatient support for retention in care. Open Forum Infect Dis 2022; 9:ofab633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Centers for Disease Control and Prevention . Screening recommendations and considerations referenced in treatment guidelines and original sources, 2021 [cited 2023 January 5]. Available at https://www.cdc.gov/std/treatment-guidelines/screening-recommendations.htm. Accessed 10 November 2023.
- 101. Poteat T, Reisner SL, Radix A. HIV epidemics among transgender women. Curr Opin HIV AIDS 2014; 9:168–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Thakarar K, Weinstein ZM, Walley AY. Optimising health and safety of people who inject drugs during transition from acute to outpatient care: narrative review with clinical checklist. Postgrad Med J 2016; 92:356–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Beieler AM, Klein JW, Bhatraju E, Iles-Shih M, Enzian L, Dhanireddy S. Evaluation of bundled interventions for patients with opioid use disorder experiencing homelessness receiving extended antibiotics for severe infection. Open Forum Infect Dis 2021; 8:ofab285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Williams DR, Rucker TD. Understanding and addressing racial disparities in health care. Health Care Financ Rev 2000; 21:75–90. [PMC free article] [PubMed] [Google Scholar]
- 105. Salcedo-Betancourt JD, Farouk SS, Reddy YNV. Ensuring health equity for sexual and/or gender minority individuals. Nat Rev Nephrol 2022; 18:341–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Stewart J, Stadeli KM, Green ML, et al. A co-located continuity clinic model to address healthcare needs of women living unhoused with opioid use disorder, who engage in transactional sex in north Seattle. Sex Transm Dis 2020; 47:e5–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Perera R, Stephan L, Appa A, et al. Meeting people where they are: implementing hospital-based substance use harm reduction. Harm Reduct J 2022; 19:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Abdul-Mutakabbir JC, Casey S, Jews V, et al. A three-tiered approach to address barriers to COVID-19 vaccine delivery in the Black community. Lancet Glob Health 2021; 9:e749–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Green TC, Olson R, Jarczyk C, et al. Implementation and uptake of the Massachusetts drug supply data stream: a statewide public health-public safety partnership drug checking program. J Public Health Manag Pract 2022; 28(Suppl 6):S347–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Fanucchi LC, Walsh SL, Thornton AC, Lofwall MR. Integrated outpatient treatment of opioid use disorder and injection-related infections: a description of a new care model. Prev Med 2019; 128:105760. [DOI] [PubMed] [Google Scholar]
- 111. Appa A, Barocas JA. Can I safely discharge a patient with a substance use disorder home with a peripherally inserted central catheter? NEJM Evidence 2022; 1:EVIDccon2100012. [DOI] [PubMed] [Google Scholar]
- 112. McNeil R, Kerr T, Pauly B, Wood E, Small W. Advancing patient-centered care for structurally vulnerable drug-using populations: a qualitative study of the perspectives of people who use drugs regarding the potential integration of harm reduction interventions into hospitals. Addiction 2016; 111:685–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Dong KA, Brouwer J, Johnston C, Hyshka E. Supervised consumption services for acute care hospital patients. CMAJ 2020; 192:E476–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Ti L, Milloy M-J, Turje RB, Montaner J, Wood E, Kerr T. The impact of an HIV/AIDS adult integrated health program on leaving hospital against medical advice among HIV-positive people who use illicit drugs. J Public Health (Oxf) 2017; 39:e33–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. AASLD-IDSA . Unique and key populations: Identification and Management of HCV among PWID. [cited 2023 January 20]. Available at: https://www.hcvguidelines.org/unique-populations/pwid. Accessed 10 November 2023.
- 116. Norton BL, Fleming J, Bachhuber MA, et al. High HCV cure rates for people who use drugs treated with direct acting antiviral therapy at an urban primary care clinic. Int J Drug Policy 2017; 47:196–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Dore GJ, Altice F, Litwin AH, et al. Elbasvir-grazoprevir to treat hepatitis C virus infection in persons receiving opioid agonist therapy: a randomized trial. Ann Intern Med 2016; 165:625–34. [DOI] [PubMed] [Google Scholar]
- 118. Grady BP, Schinkel J, Thomas XV, Dalgard O. Hepatitis C virus reinfection following treatment among people who use drugs. Clin Infect Dis 2013; 57(Suppl 2):S105–10. [DOI] [PubMed] [Google Scholar]
- 119. McCrary LM, Roberts K, Jordan R, Bowman MC, Schranz AJ. Inpatient vs. outpatient initiation of hepatitis C treatment among hospitalized patients who inject drugs: lessons from a quality improvement project. Open Forum Infect Dis 2022; 9:ofac492.1075. [Google Scholar]