Abstract
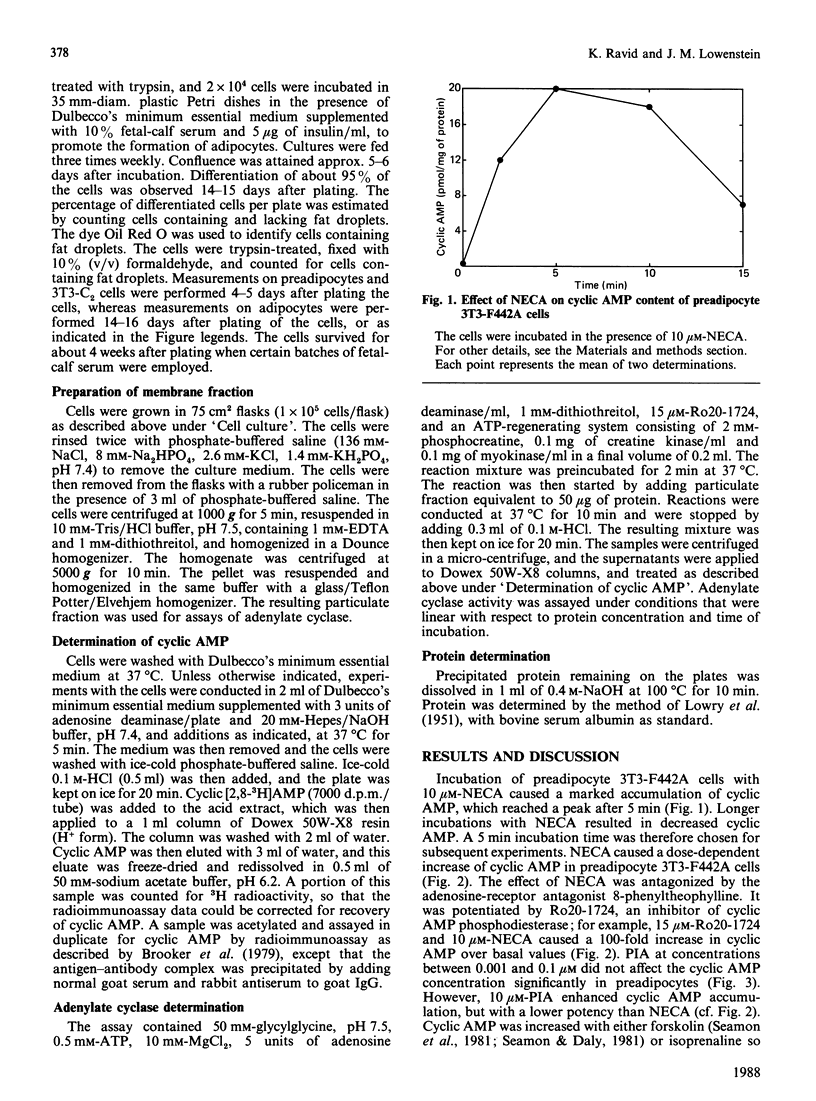
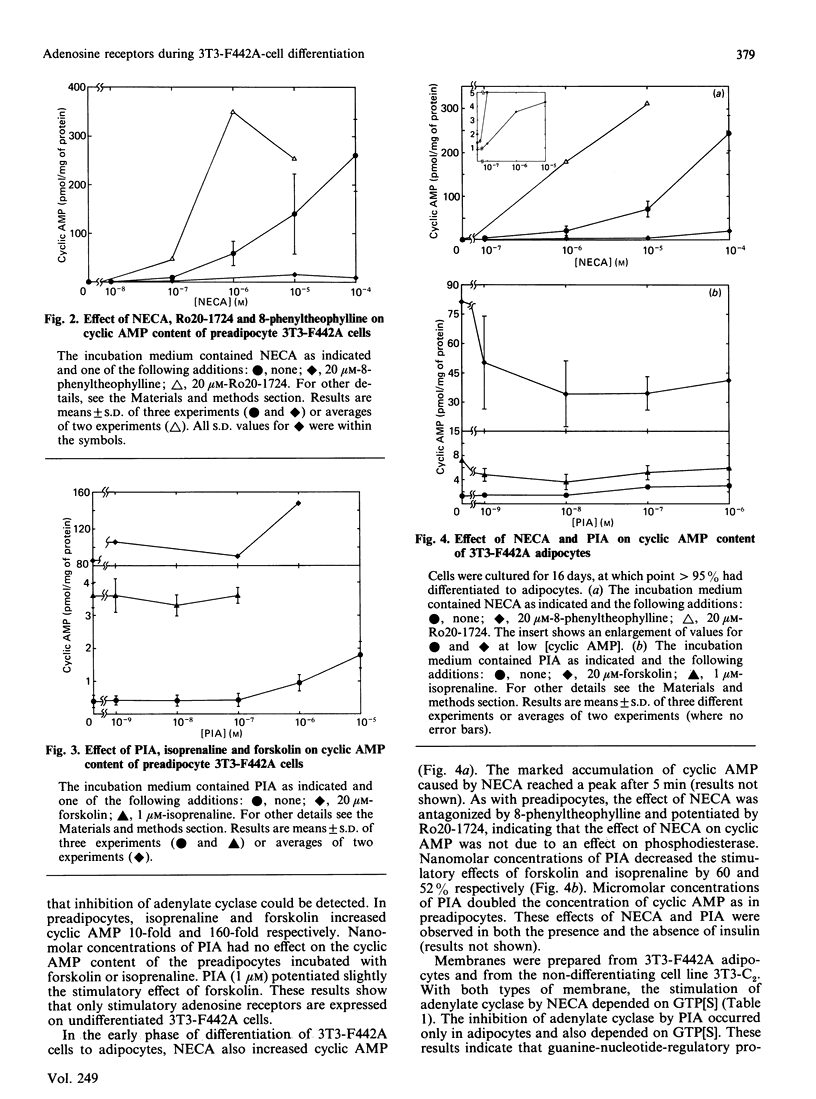
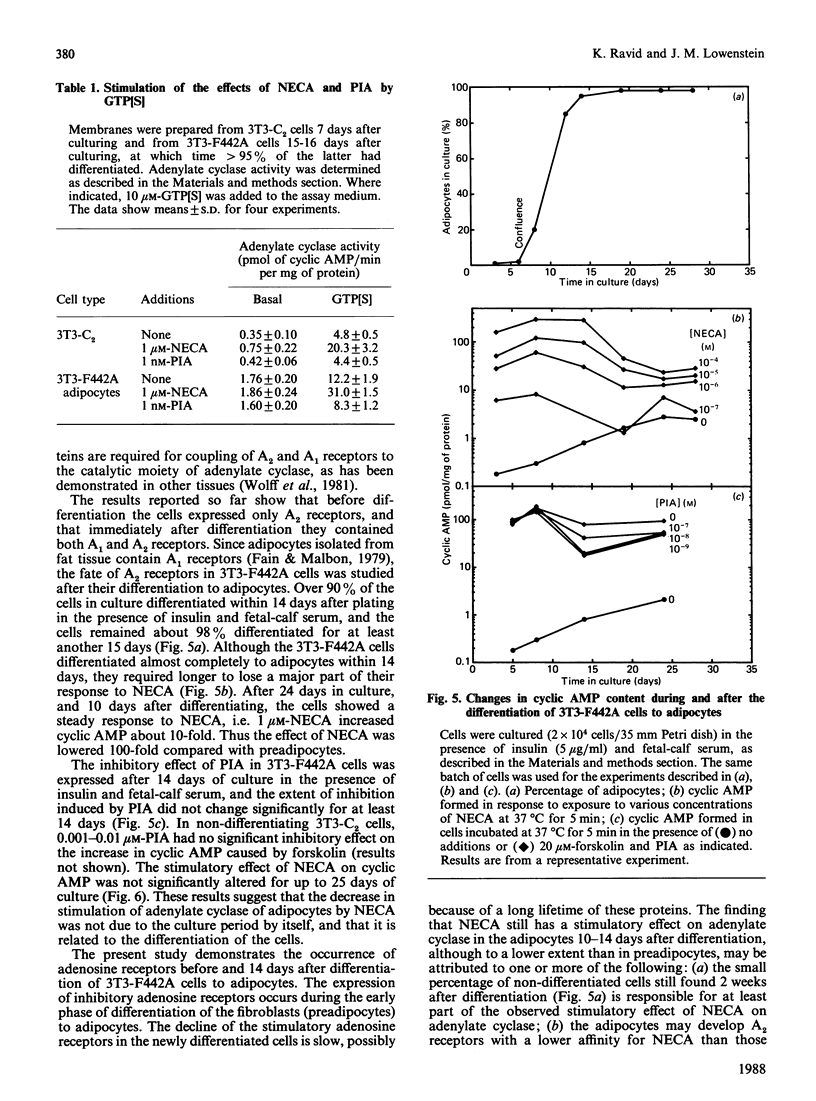
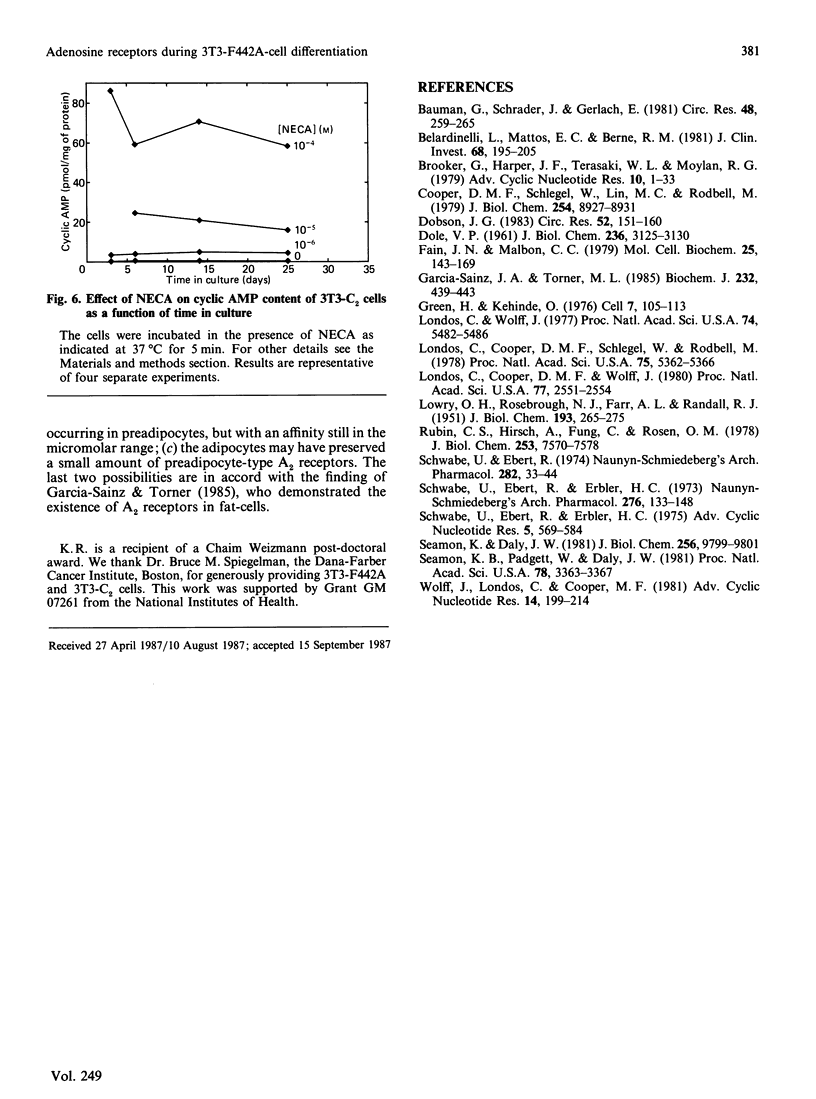
Incubation of undifferentiated 3T3-F442A cells (preadipocytes) with 5'-N-ethylcarboxamidoadenosine (NECA) increases intracellular cyclic AMP in a dose-dependent manner. The effect of NECA is antagonized by 8-phenyltheophylline, but potentiated by 4-(3-butoxy-4-methoxybenzyl)-2-imidazolidine, an inhibitor of cyclic AMP phosphodiesterase. Incubation of preadipocytes with (-)-N6-(R-phenylisopropyl)adenosine (PIA) has no inhibitory effect on the basal concentration of cyclic AMP or on the stimulation of adenylate cyclase by isoprenaline or forskolin. Micromolar concentrations of PIA increase intracellular cyclic AMP, but with a lower potency than NECA. Similar findings are obtained with the non-differentiating cell line 3T3-C2. Thus preadipocyte 3T3-F442A cells and 3T3-C2 cells appear to express only stimulatory adenosine receptors. For some time after 3T3-F442A cells have differentiated to adipocytes, micromolar concentrations of NECA and PIA continue to increase cyclic AMP to a similar extent to that in preadipocytes, whereas nanomolar concentrations of PIA decrease the stimulatory effects of isoprenaline and forskolin on adenylate cyclase by 50%. However, several days after differentiation, the adipocytes gradually lose the major part of their positive response to NECA and reach a steady response to NECA 10 days after differentiation. The inhibition of adenylate cyclase caused by PIA remains constant for at least 2 weeks after differentiation. With membranes derived from the cells, the effects of NECA and PIA depend on GTP. These results indicate that, during the differentiation of 3T3-F442A cells to adipocytes, new inhibitory adenosine receptors are expressed, whereas the stimulatory receptors become attenuated.
Full text
PDF
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baumann G., Schrader J., Gerlach E. Inhibitory action of adenosine on histamine- and dopamine-stimulated cardiac contractility and adenylate cyclase in guinea pigs. Circ Res. 1981 Feb;48(2):259–266. doi: 10.1161/01.res.48.2.259. [DOI] [PubMed] [Google Scholar]
- Belardinelli L., Mattos E. C., Berne R. M. Evidence for adenosine mediation of atrioventricular block in the ischemic canine myocardium. J Clin Invest. 1981 Jul;68(1):195–205. doi: 10.1172/JCI110235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooker G., Harper J. F., Terasaki W. L., Moylan R. D. Radioimmunoassay of cyclic AMP and cyclic GMP. Adv Cyclic Nucleotide Res. 1979;10:1–33. [PubMed] [Google Scholar]
- Cooper D. M., Schlegel W., Lin M. C., Rodbell M. The fat cell adenylate cyclase system. Characterization and manipulation of its bimodal regulation by GTP. J Biol Chem. 1979 Sep 25;254(18):8927–8931. [PubMed] [Google Scholar]
- DOLE V. P. Effect of nucleic acid metabolites on lipolysis in adipose tissue. J Biol Chem. 1961 Dec;236:3125–3130. [PubMed] [Google Scholar]
- Dobson J. G., Jr Mechanism of adenosine inhibition of catecholamine-induced responses in heart. Circ Res. 1983 Feb;52(2):151–160. doi: 10.1161/01.res.52.2.151. [DOI] [PubMed] [Google Scholar]
- Fain J. N., Malbon C. C. Regulation of adenylate cyclase by adenosine. Mol Cell Biochem. 1979 Jun 15;25(3):143–169. doi: 10.1007/BF00235364. [DOI] [PubMed] [Google Scholar]
- García-Sáinz J. A., Torner M. L. Rat fat-cells have three types of adenosine receptors (Ra, Ri and P). Differential effects of pertussis toxin. Biochem J. 1985 Dec 1;232(2):439–443. doi: 10.1042/bj2320439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green H., Kehinde O. Spontaneous heritable changes leading to increased adipose conversion in 3T3 cells. Cell. 1976 Jan;7(1):105–113. doi: 10.1016/0092-8674(76)90260-9. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Londos C., Cooper D. M., Schlegel W., Rodbell M. Adenosine analogs inhibit adipocyte adenylate cyclase by a GTP-dependent process: basis for actions of adenosine and methylxanthines on cyclic AMP production and lipolysis. Proc Natl Acad Sci U S A. 1978 Nov;75(11):5362–5366. doi: 10.1073/pnas.75.11.5362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Londos C., Cooper D. M., Wolff J. Subclasses of external adenosine receptors. Proc Natl Acad Sci U S A. 1980 May;77(5):2551–2554. doi: 10.1073/pnas.77.5.2551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Londos C., Wolff J. Two distinct adenosine-sensitive sites on adenylate cyclase. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5482–5486. doi: 10.1073/pnas.74.12.5482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin C. S., Hirsch A., Fung C., Rosen O. M. Development of hormone receptors and hormonal responsiveness in vitro. Insulin receptors and insulin sensitivity in the preadipocyte and adipocyte forms of 3T3-L1 cells. J Biol Chem. 1978 Oct 25;253(20):7570–7578. [PubMed] [Google Scholar]
- Schwabe U., Ebert R., Erbler H. C. Adenosine release from fat cells: effect on cyclic AMP levels and hormone actions. Adv Cyclic Nucleotide Res. 1975;5:569–584. [PubMed] [Google Scholar]
- Schwabe U., Ebert R., Erbler H. C. Adenosine release from isolated fat cells and its significance for the effects of hormones on cyclic 3',5'-AMP levels and lipolysis. Naunyn Schmiedebergs Arch Pharmacol. 1973;276(2):133–148. doi: 10.1007/BF00501186. [DOI] [PubMed] [Google Scholar]
- Schwabe U., Ebert R. Stimulation of cyclic adenosine 3',5'-monophosphate accumulation and lipolysis in fat cells by adenosine deaminase. Naunyn Schmiedebergs Arch Pharmacol. 1974;282(1):33–44. doi: 10.1007/BF00647401. [DOI] [PubMed] [Google Scholar]
- Seamon K. B., Padgett W., Daly J. W. Forskolin: unique diterpene activator of adenylate cyclase in membranes and in intact cells. Proc Natl Acad Sci U S A. 1981 Jun;78(6):3363–3367. doi: 10.1073/pnas.78.6.3363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seamon K., Daly J. W. Activation of adenylate cyclase by the diterpene forskolin does not require the guanine nucleotide regulatory protein. J Biol Chem. 1981 Oct 10;256(19):9799–9801. [PubMed] [Google Scholar]
- Wolff J., Londos C., Cooper D. M. Adenosine receptors and the regulation of adenylate cyclase. Adv Cyclic Nucleotide Res. 1981;14:199–214. [PubMed] [Google Scholar]


