Abstract
Induction of metallothionein-I (MT-I) and metallothionein-II (MT-II) by glucocorticoids was determined by h.p.l.c. analysis of proteins and Northern-blot analysis of MT mRNAs. Rats were injected with dexamethasone (0.03-10 mumol/kg) and hepatic concentrations of MTs were determined 24 h later. In control rats, only MT-II was detected (9.4 +/- 2.5 micrograms/g of liver), whereas the hepatic concentration of MT-I was below the detection limit (5 micrograms of MT/g). Dexamethasone did not increase MT-I above the detection limit at any dosage tested, but MT-II increased to 2.5 times control values at dosages of 0.30 mumol/kg and higher. Time-course experiments indicated that MT-II reached a maximum at 24 h after a single dosage of dexamethasone and returned to control values by 48 h. To determine whether dexamethasone increased MT-I in liver, samples were saturated with 109Cd, after which the amount of 109Cd in MT-I and MT-II was determined. Results indicated that, by this approach, MT-I and MT-II could be detected in control rats, and there was approx. 1.8 times more 109Cd in MT-II than in MT-I. At 24 h after administration of dexamethasone (1 mumol/kg), there was a small increase in the amount of 109Cd bound to MT-I, whereas the amount of 109Cd bound to MT-II increased to more than 2 times control values. Northern-blot hybridization with mouse cRNA probes indicated that MT-I and MT-II mRNAs increased co-ordinately after administration of dexamethasone. Thus, although glucocorticoids increase both MT-I and MT-II mRNAs, MT-II preferentially accumulates after administration of dexamethasone.
Full text
PDF

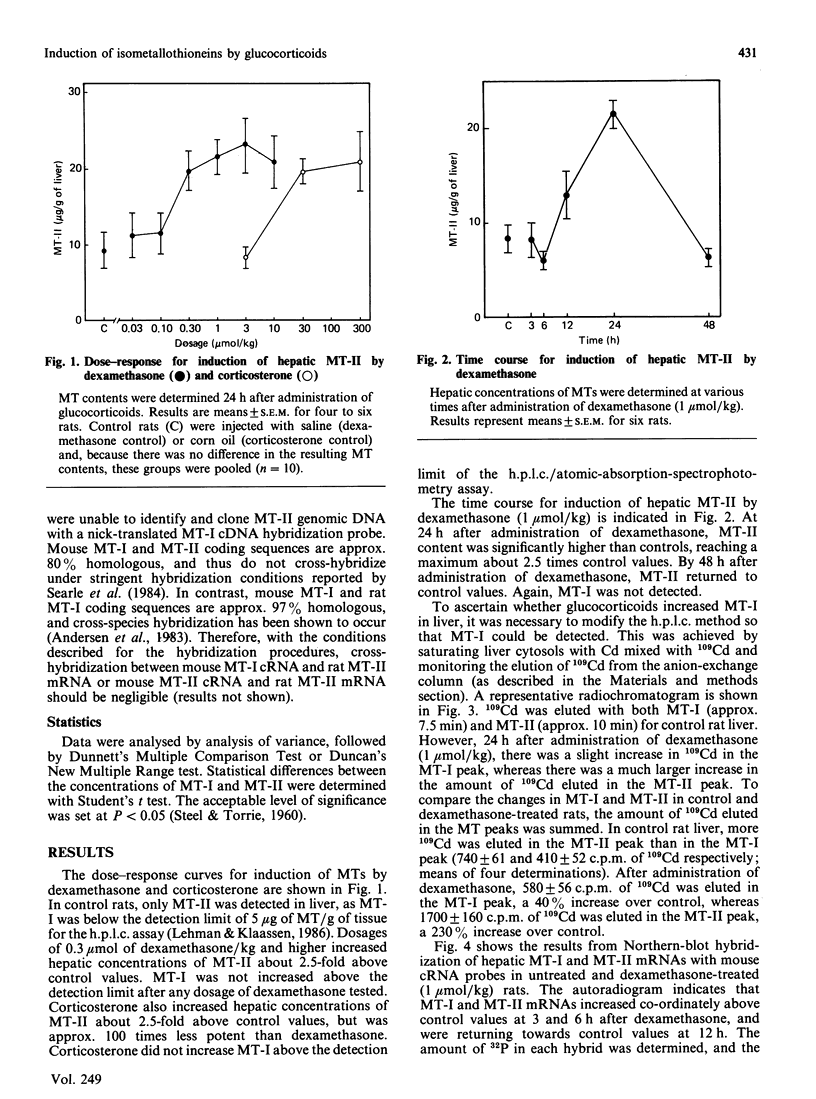
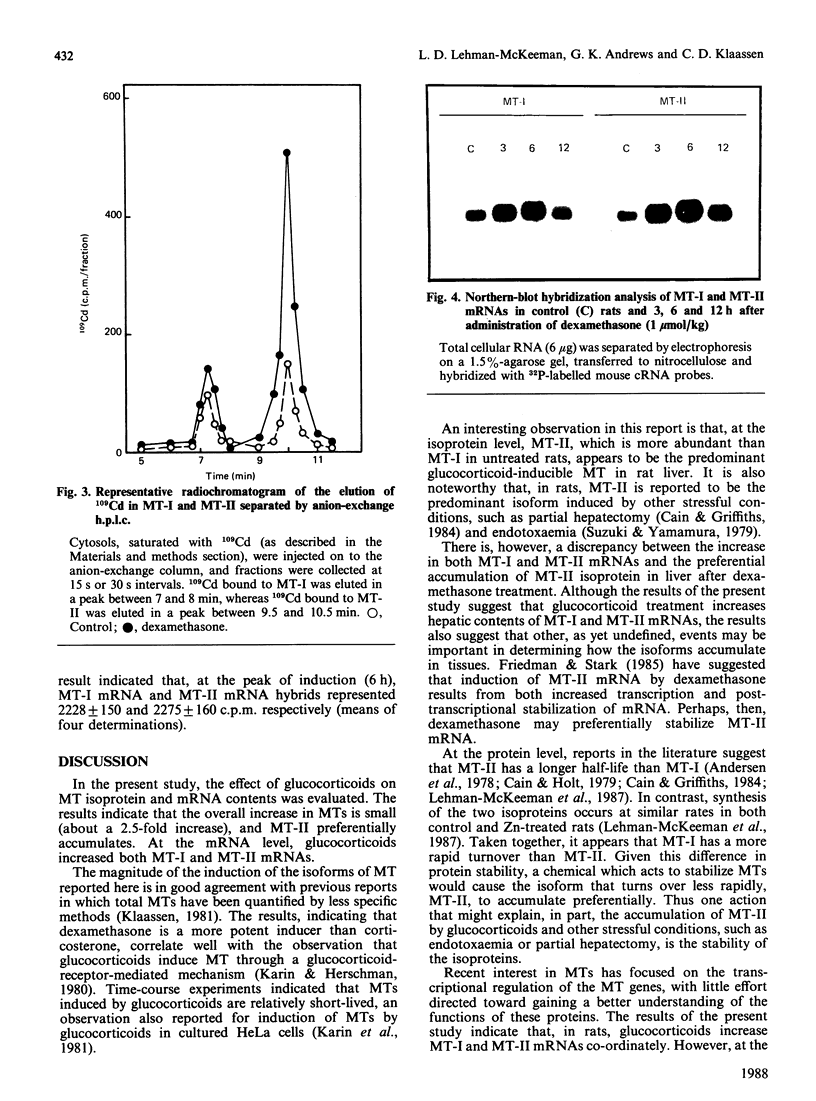

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andersen R. D., Birren B. W., Ganz T., Piletz J. E., Herschman H. R. Molecular cloning of the rat metallothionein 1 (MT-1) mRNA sequence. DNA. 1983;2(1):15–22. doi: 10.1089/dna.1.1983.2.15. [DOI] [PubMed] [Google Scholar]
- Andersen R. D., Winter W. P., Maher J. J., Bernstein I. A. Turnover of metallothioneins in rat liver. Biochem J. 1978 Jul 15;174(1):327–338. doi: 10.1042/bj1740327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews G. K., Adamson E. D., Gedamu L. The ontogeny of expression of murine metallothionein: comparison with the alpha-fetoprotein gene. Dev Biol. 1984 Jun;103(2):294–303. doi: 10.1016/0012-1606(84)90317-8. [DOI] [PubMed] [Google Scholar]
- Andrews G. K., Teng C. S. Studies on sex-organ development. Prenatal effect of oestrogenic hormone on tubular-gland cell morphogenesis and ovalbumin-gene expression in the chick Müllerian duct. Biochem J. 1979 Aug 15;182(2):271–286. doi: 10.1042/bj1820271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bremner I., Davies N. T. The induction of metallothionein in rat liver by zinc injection and restriction of food intake. Biochem J. 1975 Sep;149(3):733–738. doi: 10.1042/bj1490733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cain K., Griffiths B. L. A comparison of isometallothionein synthesis in rat liver after partial hepatectomy and parenteral zinc injection. Biochem J. 1984 Jan 1;217(1):85–92. doi: 10.1042/bj2170085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cain K., Holt D. E. Metallothionein degradation: metal composition as a controlling factor. Chem Biol Interact. 1979;28(1):91–106. doi: 10.1016/0009-2797(79)90117-0. [DOI] [PubMed] [Google Scholar]
- Cherian M. G. Isolation and purification of cadmium binding proteins from rat liver. Biochem Biophys Res Commun. 1974 Dec 11;61(3):920–926. doi: 10.1016/0006-291x(74)90243-5. [DOI] [PubMed] [Google Scholar]
- Fell G. S. Analytical procedures for diagnosis of trace element disorders. J Inherit Metab Dis. 1983;6 (Suppl 1):5–8. doi: 10.1007/BF01811316. [DOI] [PubMed] [Google Scholar]
- Friedman R. L., Stark G. R. alpha-Interferon-induced transcription of HLA and metallothionein genes containing homologous upstream sequences. Nature. 1985 Apr 18;314(6012):637–639. doi: 10.1038/314637a0. [DOI] [PubMed] [Google Scholar]
- Green M. R., Maniatis T., Melton D. A. Human beta-globin pre-mRNA synthesized in vitro is accurately spliced in Xenopus oocyte nuclei. Cell. 1983 Mar;32(3):681–694. doi: 10.1016/0092-8674(83)90054-5. [DOI] [PubMed] [Google Scholar]
- Karin M., Andersen R. D., Slater E., Smith K., Herschman H. R. Metallothionein mRNA induction in HeLa cells in response to zinc or dexamethasone is a primary induction response. Nature. 1980 Jul 17;286(5770):295–297. doi: 10.1038/286295a0. [DOI] [PubMed] [Google Scholar]
- Karin M., Eddy R. L., Henry W. M., Haley L. L., Byers M. G., Shows T. B. Human metallothionein genes are clustered on chromosome 16. Proc Natl Acad Sci U S A. 1984 Sep;81(17):5494–5498. doi: 10.1073/pnas.81.17.5494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karin M., Herschman H. R. Glucocorticoid hormone receptor mediated induction of metallothionein synthesis in HeLa cells. J Cell Physiol. 1980 Apr;103(1):35–40. doi: 10.1002/jcp.1041030106. [DOI] [PubMed] [Google Scholar]
- Karin M., Herschman H. R., Weinstein D. Primary induction of metallothionein by dexamethasone in culture rat hepatocytes. Biochem Biophys Res Commun. 1980 Feb 12;92(3):1052–1069. doi: 10.1016/0006-291x(80)90808-6. [DOI] [PubMed] [Google Scholar]
- Karin M., Slater E. P., Herschman H. R. Regulation of metallothionein synthesis in HeLa cells by heavy metals and glucocorticoids. J Cell Physiol. 1981 Jan;106(1):63–74. doi: 10.1002/jcp.1041060108. [DOI] [PubMed] [Google Scholar]
- Klaassen C. D. Induction of metallothionein by adrenocortical steroids. Toxicology. 1981;20(4):275–279. doi: 10.1016/0300-483x(81)90034-2. [DOI] [PubMed] [Google Scholar]
- Kotsonis F. N., Klaassen C. D. Increase in hepatic metallothionein in rats treated with alkylating agents. Toxicol Appl Pharmacol. 1979 Oct;51(1):19–27. doi: 10.1016/0041-008x(79)90004-8. [DOI] [PubMed] [Google Scholar]
- Kägi J. H., Vasák M., Lerch K., Gilg D. E., Hunziker P., Bernhard W. R., Good M. Structure of mammalian metallothionein. Environ Health Perspect. 1984 Mar;54:93–103. doi: 10.1289/ehp.54-1568188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leber A. P., Miya T. S. A mechanism for cadmium- and zinc-induced tolerance to cadmium toxicity: involvement of metallothionein. Toxicol Appl Pharmacol. 1976 Sep;37(3):403–414. doi: 10.1016/0041-008x(76)90202-7. [DOI] [PubMed] [Google Scholar]
- Lehman L. D., Klaassen C. D. Separation and quantitation of metallothioneins by high-performance liquid chromatography coupled with atomic absorption spectrophotometry. Anal Biochem. 1986 Mar;153(2):305–314. doi: 10.1016/0003-2697(86)90097-7. [DOI] [PubMed] [Google Scholar]
- Lehrach H., Diamond D., Wozney J. M., Boedtker H. RNA molecular weight determinations by gel electrophoresis under denaturing conditions, a critical reexamination. Biochemistry. 1977 Oct 18;16(21):4743–4751. doi: 10.1021/bi00640a033. [DOI] [PubMed] [Google Scholar]
- Nordberg G. F., Nordberg M., Piscator M., Vesterberg O. Separation of two forms of rabbit metallothionein by isoelectric focusing. Biochem J. 1972 Feb;126(3):491–498. doi: 10.1042/bj1260491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh S. H., Deagen J. T., Whanger P. D., Weswig P. H. Biological function of metallothionein. V. Its induction in rats by various stresses. Am J Physiol. 1978 Mar;234(3):E282–E285. doi: 10.1152/ajpendo.1978.234.3.E282. [DOI] [PubMed] [Google Scholar]
- Richards R. I., Heguy A., Karin M. Structural and functional analysis of the human metallothionein-IA gene: differential induction by metal ions and glucocorticoids. Cell. 1984 May;37(1):263–272. doi: 10.1016/0092-8674(84)90322-2. [DOI] [PubMed] [Google Scholar]
- Searle P. F., Davison B. L., Stuart G. W., Wilkie T. M., Norstedt G., Palmiter R. D. Regulation, linkage, and sequence of mouse metallothionein I and II genes. Mol Cell Biol. 1984 Jul;4(7):1221–1230. doi: 10.1128/mcb.4.7.1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobocinski P. Z., Canterbury W. J., Jr, Mapes C. A., Dinterman R. E. Involvement of hepatic metallothioneins in hypozincemia associated with bacterial infection. Am J Physiol. 1978 Apr;234(4):E399–E406. doi: 10.1152/ajpendo.1978.234.4.E399. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Suzuki K. T., Yamamura M. Induction of hepatic zinc-thionein in rat by endotoxin. Biochem Pharmacol. 1980 Aug 15;29(16):2260–2260. doi: 10.1016/0006-2952(80)90210-5. [DOI] [PubMed] [Google Scholar]
- Thomas P. S. Hybridization of denatured RNA and small DNA fragments transferred to nitrocellulose. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5201–5205. doi: 10.1073/pnas.77.9.5201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varshney U., Jahroudi N., Foster R., Gedamu L. Structure, organization, and regulation of human metallothionein IF gene: differential and cell-type-specific expression in response to heavy metals and glucocorticoids. Mol Cell Biol. 1986 Jan;6(1):26–37. doi: 10.1128/mcb.6.1.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong K. L., Klaassen C. D. Isolation and characterization of metallothionein which is highly concentrated in newborn rat liver. J Biol Chem. 1979 Dec 25;254(24):12399–12403. [PubMed] [Google Scholar]
- Yagle M. K., Palmiter R. D. Coordinate regulation of mouse metallothionein I and II genes by heavy metals and glucocorticoids. Mol Cell Biol. 1985 Feb;5(2):291–294. doi: 10.1128/mcb.5.2.291. [DOI] [PMC free article] [PubMed] [Google Scholar]



