Abstract
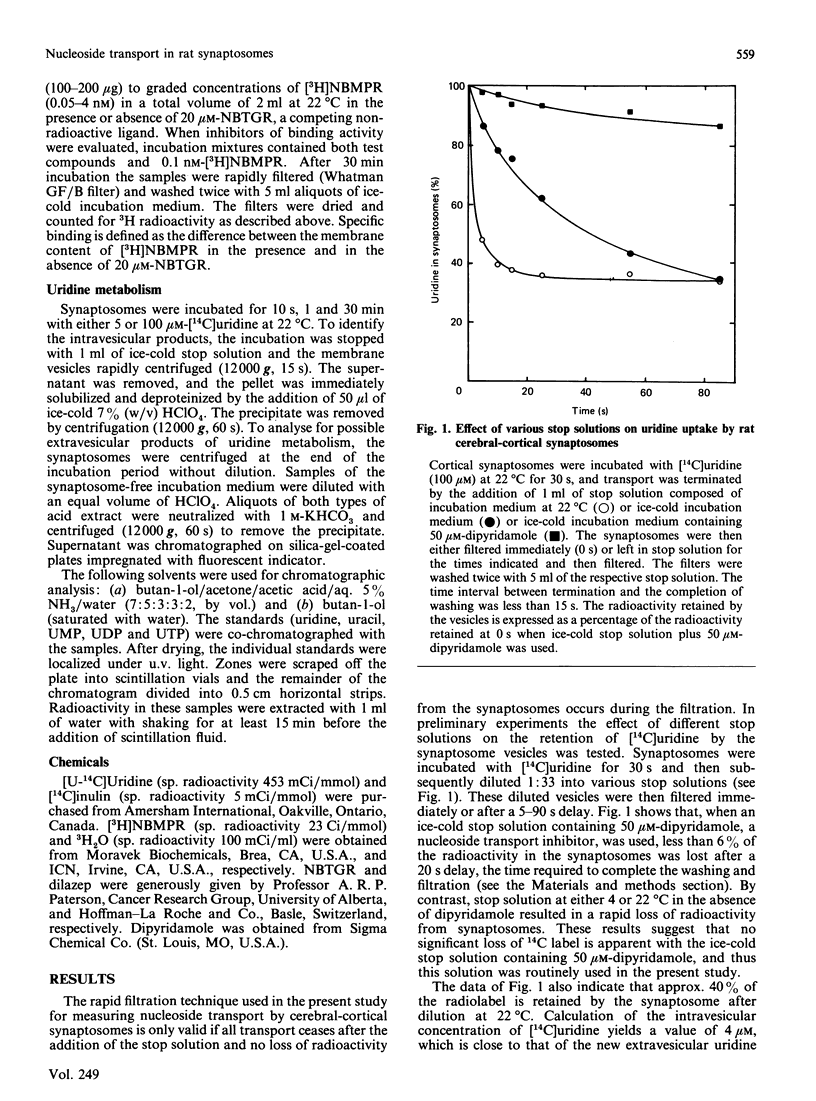
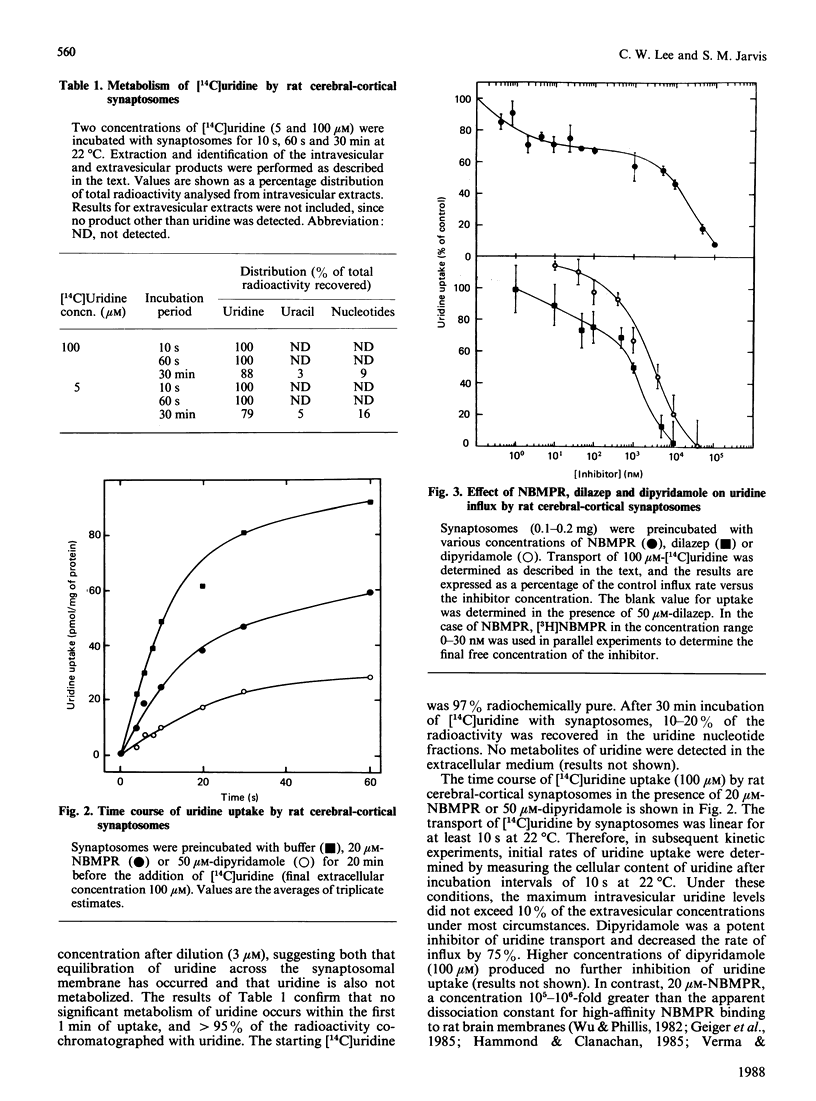
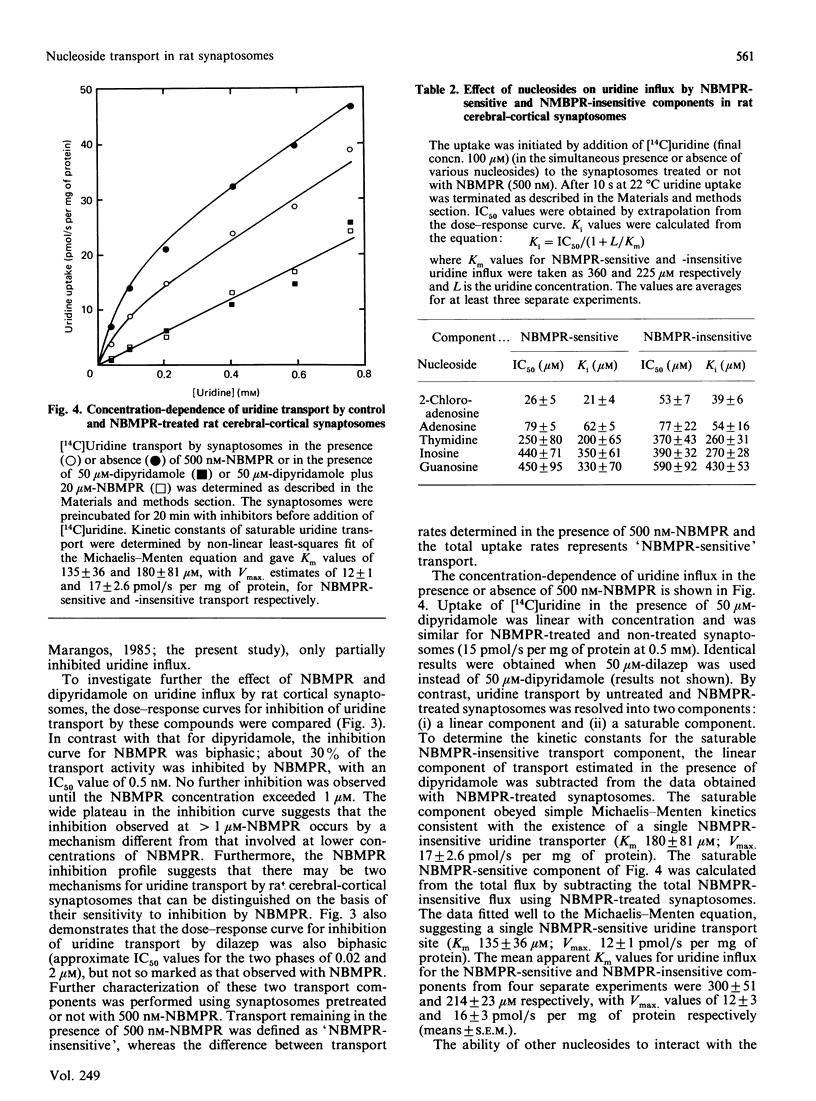
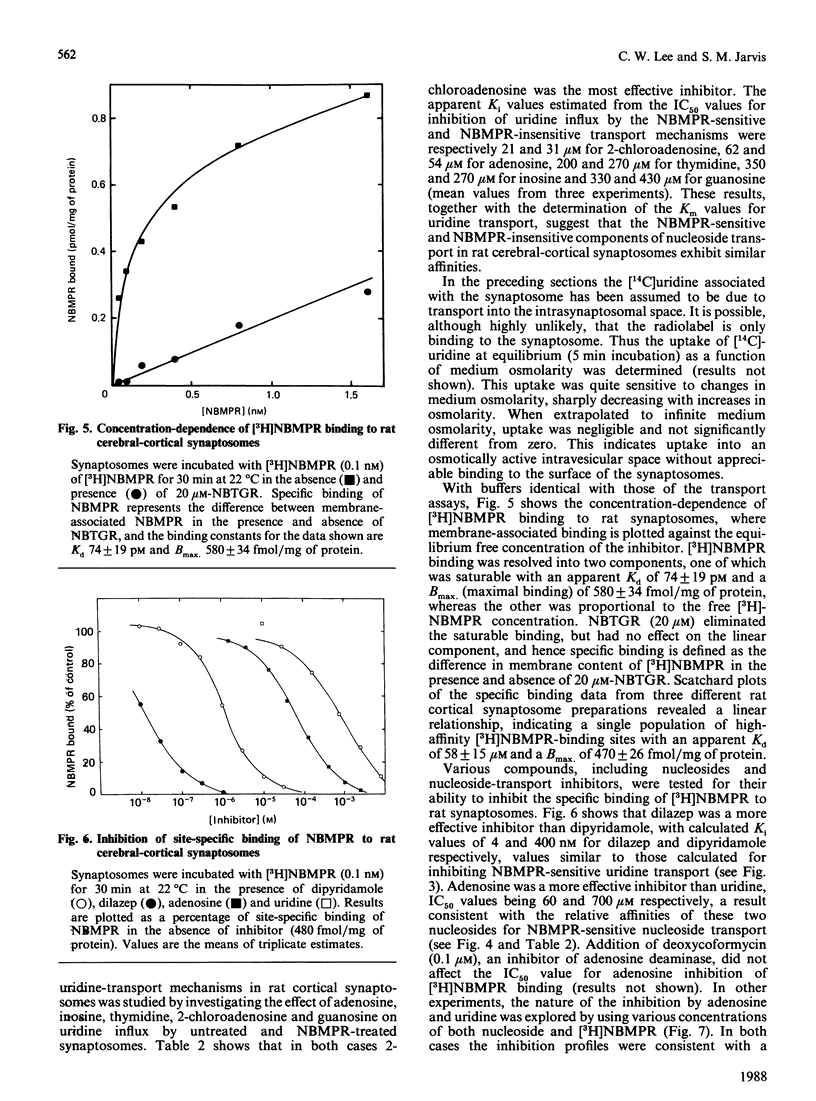
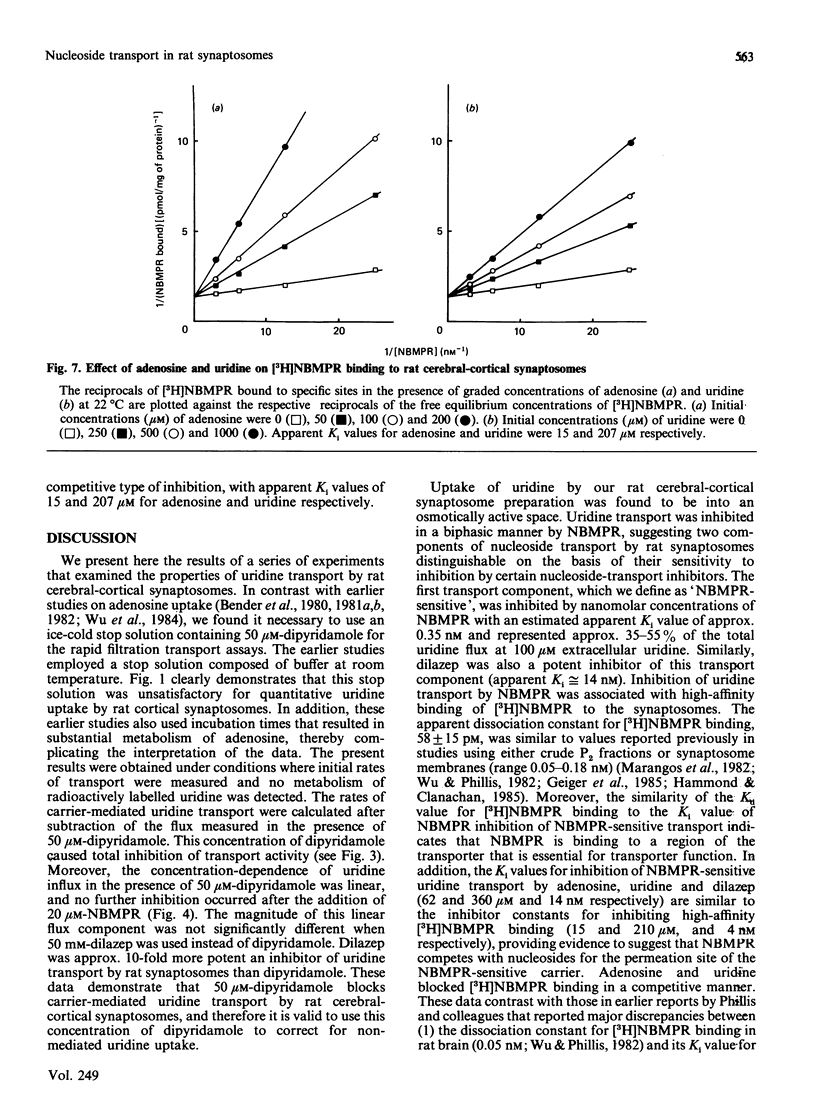
The transport of [U-14C]uridine was investigated in rat cerebral-cortical synaptosomes using an inhibitor-stop filtration method. Under these conditions the rapid efflux of uridine from the synaptosomes is prevented and uridine is not significantly metabolized in the synaptosome during the first 1 min of uptake. The dose-response curve for the inhibition of uridine transport by nitrobenzylthioinosine (NBMPR) was biphasic: approx. 40% of the transport activity was inhibited with an IC50 (concentration causing half-maximal inhibition) value of 0.5 nM, but the remaining activity was insensitive to concentrations as high as 1 microM. Similar biphasic dose-response curves were observed for dilazep inhibition, but both transport components were equally sensitive to dipyridamole inhibition. Uridine influx by both components was saturable (Km 300 +/- 51 and 214 +/- 23 microM, and Vmax. 12 +/- 3 and 16 +/- 3 pmol/s per mg of protein, for NBMPR-sensitive and NBMPR-insensitive components respectively), and inhibited by other nucleosides such as 2-chloroadenosine, adenosine, inosine, thymidine and guanosine with similar IC50 values for the two components. Inhibition of uridine transport by NBMPR was associated with high-affinity binding of NBMPR to the synaptosome membrane (Kd 58 +/- 15 pM). Binding of NBMPR to these sites was competitively blocked by uridine and adenosine and inhibited by dilazep and dipyridamole, with Ki values similar to those measured for inhibiting NBMPR-sensitive uridine influx. These results demonstrate that there are two components of nucleoside transport in our rat synaptosomal preparation that differ in their sensitivity to inhibition by NBMPR. Thus conclusions regarding nucleoside transport in rat brain based only on NBMPR-binding activity must be viewed with caution.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barberis C., Minn A., Gayet J. Adenosine transport into guinea-pig synaptosomes. J Neurochem. 1981 Feb;36(2):347–354. doi: 10.1111/j.1471-4159.1981.tb01601.x. [DOI] [PubMed] [Google Scholar]
- Belt J. A. Heterogeneity of nucleoside transport in mammalian cells. Two types of transport activity in L1210 and other cultured neoplastic cells. Mol Pharmacol. 1983 Nov;24(3):479–484. [PubMed] [Google Scholar]
- Bender A. S., Wu P. H., Phillis J. W. Competitive inhibition of the uptake of adenosine into rat brain synaptosomes by prostaglandins. Pharmacol Res Commun. 1982 May;14(5):409–416. doi: 10.1016/s0031-6989(82)80069-6. [DOI] [PubMed] [Google Scholar]
- Bender A. S., Wu P. H., Phillis J. W. The characterization of [3H] adenosine uptake into rat cerebral cortical synaptosomes. J Neurochem. 1980 Sep;35(3):629–640. doi: 10.1111/j.1471-4159.1980.tb03702.x. [DOI] [PubMed] [Google Scholar]
- Bender A. S., Wu P. H., Phillis J. W. The rapid uptake and release of [3H]adenosine by rat cerebral cortical synaptosomes. J Neurochem. 1981 Feb;36(2):651–660. doi: 10.1111/j.1471-4159.1981.tb01638.x. [DOI] [PubMed] [Google Scholar]
- Bisserbe J. C., Deckert J., Marangos P. Autoradiographic localization of adenosine uptake sites in guinea pig brain using [3H]dipyridamole. Neurosci Lett. 1986 May 23;66(3):341–345. doi: 10.1016/0304-3940(86)90043-1. [DOI] [PubMed] [Google Scholar]
- Bisserbe J. C., Patel J., Marangos P. J. Autoradiographic localization of adenosine uptake sites in rat brain using [3H]nitrobenzylthioinosine. J Neurosci. 1985 Feb;5(2):544–550. doi: 10.1523/JNEUROSCI.05-02-00544.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GRAY E. G., WHITTAKER V. P. The isolation of nerve endings from brain: an electron-microscopic study of cell fragments derived by homogenization and centrifugation. J Anat. 1962 Jan;96:79–88. [PMC free article] [PubMed] [Google Scholar]
- Geiger J. D., LaBella F. S., Nagy J. I. Characterization of nitrobenzylthioinosine binding to nucleoside transport sites selective for adenosine in rat brain. J Neurosci. 1985 Mar;5(3):735–740. doi: 10.1523/JNEUROSCI.05-03-00735.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geiger J. D., Nagy J. I. Heterogeneous distribution of adenosine transport sites labelled by [3H]nitrobenzylthioinosine in rat brain: an autoradiographic and membrane binding study. Brain Res Bull. 1984 Nov;13(5):657–666. doi: 10.1016/0361-9230(84)90198-9. [DOI] [PubMed] [Google Scholar]
- Hammond J. R., Clanachan A. S. Species differences in the binding of [3H]nitrobenzylthioinosine to the nucleoside transport system in mammalian central nervous system membranes: evidence for interconvertible conformations of the binding site/transporter complex. J Neurochem. 1985 Aug;45(2):527–535. doi: 10.1111/j.1471-4159.1985.tb04020.x. [DOI] [PubMed] [Google Scholar]
- Jarvis S. M., Young J. D. Nucleoside transport in rat erythrocytes: two components with differences in sensitivity to inhibition by nitrobenzylthioinosine and p-chloromercuriphenyl sulfonate. J Membr Biol. 1986;93(1):1–10. doi: 10.1007/BF01871013. [DOI] [PubMed] [Google Scholar]
- Marangos P. J., Houston M., Montgomery P. [3H]dipyridamole: a new ligand probe for brain adenosine uptake sites. Eur J Pharmacol. 1985 Nov 19;117(3):393–394. doi: 10.1016/0014-2999(85)90017-2. [DOI] [PubMed] [Google Scholar]
- Marangos P. J., Patel J., Clark-Rosenberg R., Martino A. M. [3H]nitrobenzylthioinosine binding as a probe for the study of adenosine uptake sites in brain. J Neurochem. 1982 Jul;39(1):184–191. doi: 10.1111/j.1471-4159.1982.tb04717.x. [DOI] [PubMed] [Google Scholar]
- Marchbanks R. M. The chloride content, anion deficit and volume of synaptosomes. J Neurochem. 1975 Oct;25(4):463–470. doi: 10.1111/j.1471-4159.1975.tb04351.x. [DOI] [PubMed] [Google Scholar]
- Minn A., Gayet J. Kinetic study of glutamate transport in rat brain mitochondria. J Neurochem. 1977 Nov;29(5):873–881. doi: 10.1111/j.1471-4159.1977.tb10731.x. [DOI] [PubMed] [Google Scholar]
- Olafson R. W., Drummond G. I., Lee J. F. Studies on 2',3'-cyclic nucleotide-3'-phosphohydrolase from brain. Can J Biochem. 1969 Oct;47(10):961–966. doi: 10.1139/o69-151. [DOI] [PubMed] [Google Scholar]
- Phillis J. W., Wu P. H., Bender A. S. Inhibition of adenosine uptake into rat brain synaptosomes by the benzodiazepines. Gen Pharmacol. 1981;12(1):67–70. doi: 10.1016/0306-3623(81)90030-6. [DOI] [PubMed] [Google Scholar]
- Phillis J. W., Wu P. H., Coffin V. L. Inhibition of adenosine uptake into rat brain synaptosomes by prostaglandins, benzodiazepines and other centrally active compounds. Gen Pharmacol. 1983;14(5):475–479. doi: 10.1016/0306-3623(83)90106-4. [DOI] [PubMed] [Google Scholar]
- Phillis J. W., Wu P. H. Nitrobenzylthioinosine inhibition of adenosine uptake in guinea-pig brain. J Pharm Pharmacol. 1983 Aug;35(8):540–540. doi: 10.1111/j.2042-7158.1983.tb04832.x. [DOI] [PubMed] [Google Scholar]
- Phillis J. W., Wu P. H. The role of adenosine and its nucleotides in central synaptic transmission. Prog Neurobiol. 1981;16(3-4):187–239. doi: 10.1016/0301-0082(81)90014-9. [DOI] [PubMed] [Google Scholar]
- Plagemann P. G., Wohlhueter R. M. Nitrobenzylthioinosine-sensitive and -resistant nucleoside transport in normal and transformed rat cells. Biochim Biophys Acta. 1985 Jun 27;816(2):387–395. doi: 10.1016/0005-2736(85)90506-1. [DOI] [PubMed] [Google Scholar]
- Stone T. W. Physiological roles for adenosine and adenosine 5'-triphosphate in the nervous system. Neuroscience. 1981;6(4):523–555. doi: 10.1016/0306-4522(81)90145-7. [DOI] [PubMed] [Google Scholar]
- Verma A., Marangos P. J. Nitrobenzylthioinosine binding in brain: an interspecies study. Life Sci. 1985 Jan 21;36(3):283–290. doi: 10.1016/0024-3205(85)90071-2. [DOI] [PubMed] [Google Scholar]
- White T. D. A role for divalent cations in the uptake of noradrenaline by synaptosomes. J Neurochem. 1975 May;24(5):1037–1042. doi: 10.1111/j.1471-4159.1975.tb03674.x. [DOI] [PubMed] [Google Scholar]
- Wu P. H., Barraco R. A., Phillis J. W. Further studies on the inhibition of adenosine uptake into rat brain synaptosomes by adenosine derivatives and methylxanthines. Gen Pharmacol. 1984;15(3):251–254. doi: 10.1016/0306-3623(84)90169-1. [DOI] [PubMed] [Google Scholar]
- Wu P. H., Phillis J. W. Nucleoside transport in rat cerebral cortical synaptosomal membrane: a high affinity probe study. Int J Biochem. 1982;14(12):1101–1105. doi: 10.1016/0020-711x(82)90167-7. [DOI] [PubMed] [Google Scholar]
- Young J. D., Jarvis S. M. Nucleoside transport in animal cells. Biosci Rep. 1983 Apr;3(4):309–322. doi: 10.1007/BF01122895. [DOI] [PubMed] [Google Scholar]


