Abstract
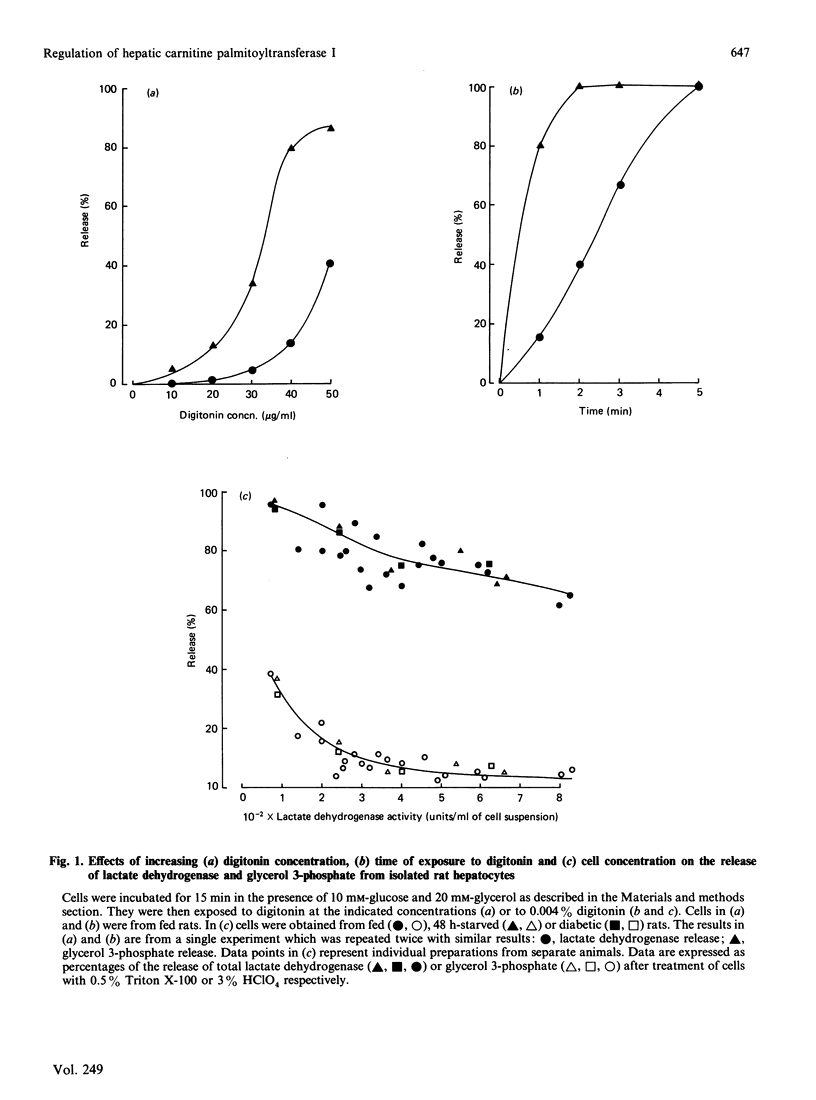
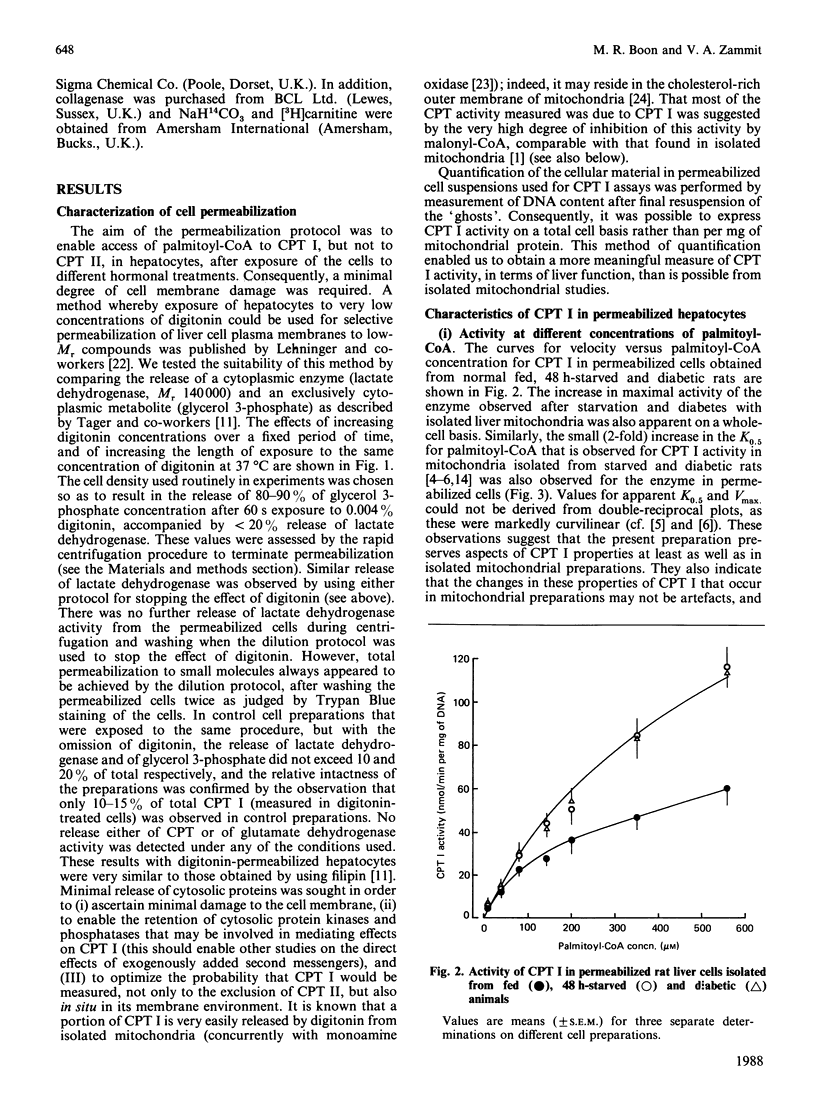
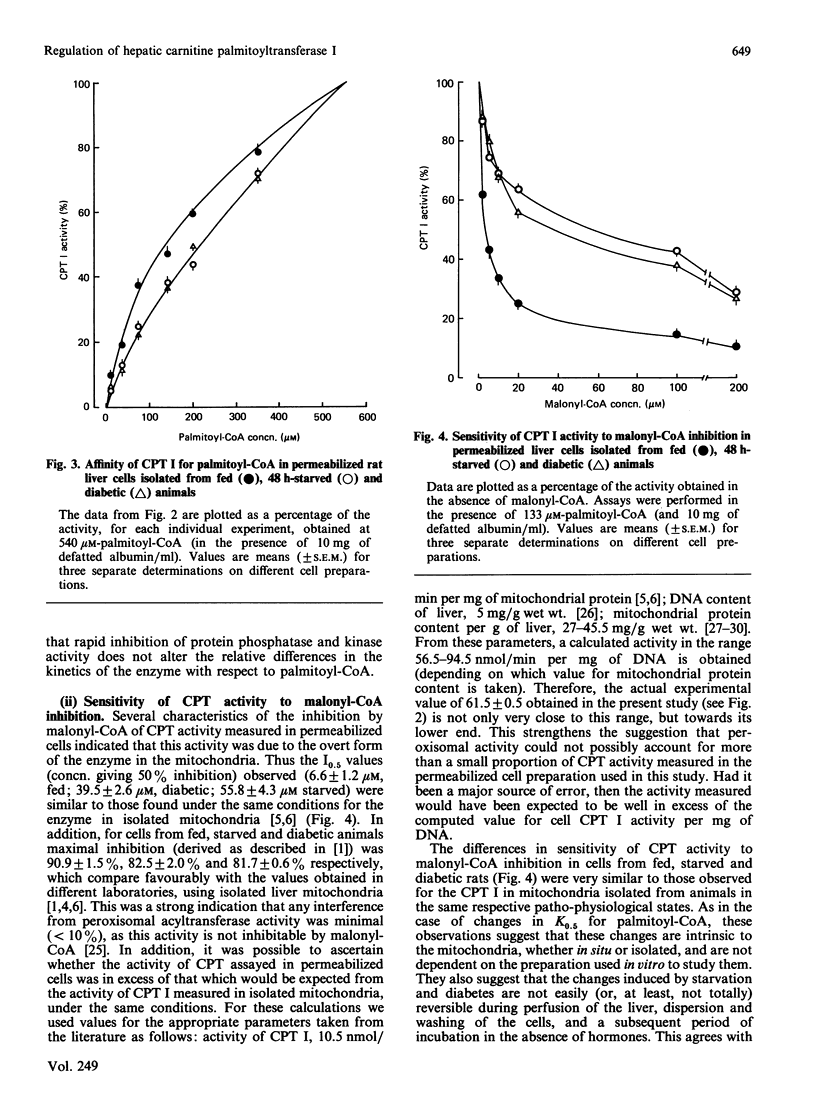
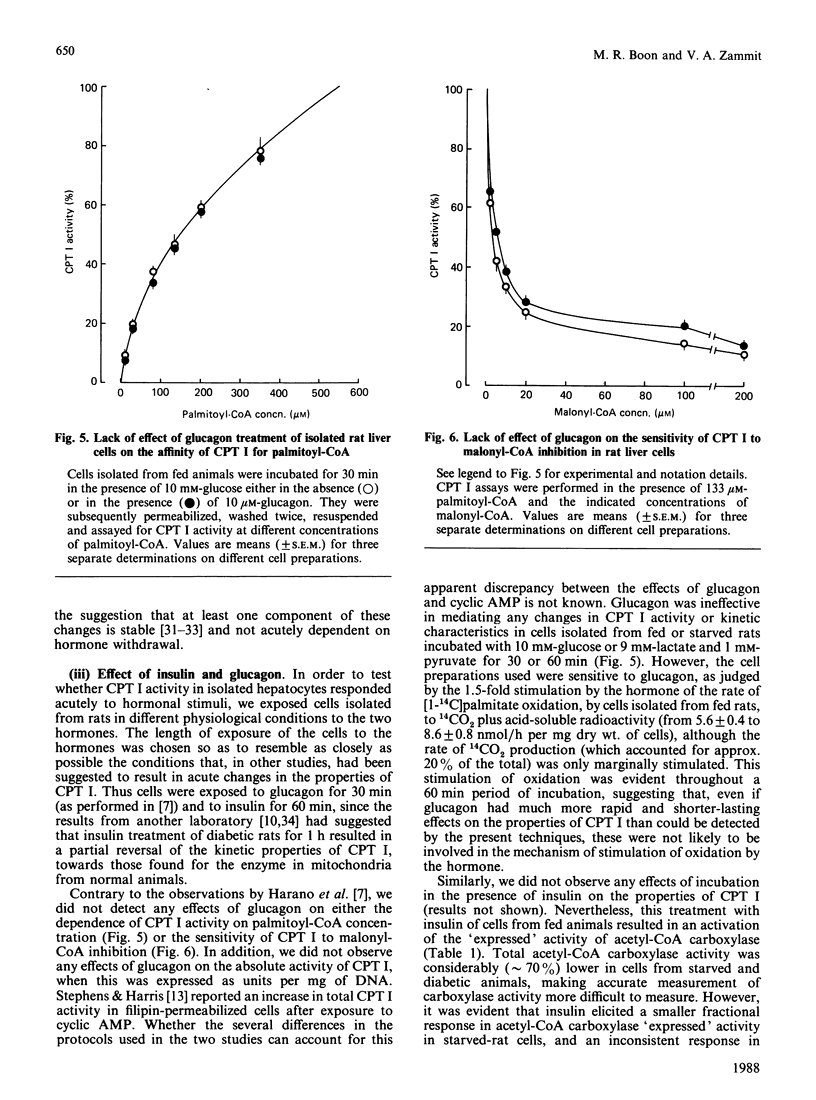
1. A permeabilized isolated rat liver cell preparation was developed to achieve selective permeabilization of the cell membrane to metabolites and to allow the assay of mitochondrial overt carnitine palmitoyltransferase (CPT I) activity in situ. By performing the digitonin-induced permeabilization in the presence of fluoride and bivalent-metal-cation sequestrants, it was possible to demonstrate that the activity of other enzymes, which are regulated by reversible phosphorylation, was preserved during the procedure and subsequent washing of cells before assay. 2. CPT activity at a sub-optimal palmitoyl-CoA concentration was almost totally (approximately 90%) inhibited by malonyl-CoA, indicating that mitochondrial CPT I was largely measured in this preparation. 3. The palmitoyl-CoA-saturation and malonyl-CoA-inhibition curves for CPT activity in permeabilized cells were very similar to those obtained previously for the enzyme in isolated liver mitochondria. Moreover, starvation and diabetes had the same effects on enzyme activity, affinity for palmitoyl-CoA and malonyl-CoA sensitivity of CPT I in isolated cells as found in isolated mitochondria. These physiologically induced changes persisted through the cell preparation and incubation period. 4. Neither incubation of cells with glucagon or insulin nor incubation with pyruvate and lactate before permeabilization resulted in alterations of these parameters of CPT I in isolated cells. 5. The results are discussed in relation to the temporal relationships of changes in the activity and properties of CPT I in vivo in relation to the effects of insulin and glucagon on fatty acid metabolism in vivo.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bijleveld C., Geelen M. J. Measurement of acetyl-CoA carboxylase activity in isolated hepatocytes. Biochim Biophys Acta. 1987 Apr 24;918(3):274–283. doi: 10.1016/0005-2760(87)90231-1. [DOI] [PubMed] [Google Scholar]
- Blackshear P. J., Alberti K. G. Experimental diabetic ketoacidosis. Sequential changes of metabolic intermediates in blood, liver, cerebrospinal fluid and brain after acute insulin deprivation in the streptozotocin-diabetic rat. Biochem J. 1974 Jan;138(1):107–117. doi: 10.1042/bj1380107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bustamante E., Soper J. W., Pedersen P. L. A high-yield preparative method for isolation of rat liver mitochondria. Anal Biochem. 1977 Jun;80(2):401–408. doi: 10.1016/0003-2697(77)90661-3. [DOI] [PubMed] [Google Scholar]
- Cook G. A. Differences in the sensitivity of carnitine palmitoyltransferase to inhibition by malonyl-CoA are due to differences in Ki values. J Biol Chem. 1984 Oct 10;259(19):12030–12033. [PubMed] [Google Scholar]
- Cook G. A., Gamble M. S. Regulation of carnitine palmitoyltransferase by insulin results in decreased activity and decreased apparent Ki values for malonyl-CoA. J Biol Chem. 1987 Feb 15;262(5):2050–2055. [PubMed] [Google Scholar]
- Cook G. A., Stephens T. W., Harris R. A. Altered sensitivity of carnitine palmitoyltransferase to inhibition by malonyl-CoA in ketotic diabetic rats. Biochem J. 1984 Apr 1;219(1):337–339. doi: 10.1042/bj2190337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Easom R. A., Zammit V. A. A cold-clamping technique for the rapid sampling of rat liver for studies on enzymes in separate cell fractions. Suitability for the study of enzymes regulated by reversible phosphorylation-dephosphorylation. Biochem J. 1984 Jun 15;220(3):733–738. doi: 10.1042/bj2200733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiskum G., Craig S. W., Decker G. L., Lehninger A. L. The cytoskeleton of digitonin-treated rat hepatocytes. Proc Natl Acad Sci U S A. 1980 Jun;77(6):3430–3434. doi: 10.1073/pnas.77.6.3430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gamble M. S., Cook G. A. Alteration of the apparent Ki of carnitine palmitoyltransferase for malonyl-CoA by the diabetic state and reversal by insulin. J Biol Chem. 1985 Aug 15;260(17):9516–9519. [PubMed] [Google Scholar]
- Gankema H. S., Laanen E., Groen A. K., Tager J. M. Characterization of isolated rat-liver cells made permeable with filipin. Eur J Biochem. 1981 Oct;119(2):409–414. doi: 10.1111/j.1432-1033.1981.tb05623.x. [DOI] [PubMed] [Google Scholar]
- Grantham B. D., Zammit V. A. Restoration of the properties of carnitine palmitoyltransferase I in liver mitochondria during re-feeding of starved rats. Biochem J. 1986 Oct 15;239(2):485–488. doi: 10.1042/bj2390485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grantham B. D., Zammit V. A. Role of carnitine palmitoyltransferase I in the regulation of hepatic ketogenesis during the onset and reversal of chronic diabetes. Biochem J. 1988 Jan 15;249(2):409–414. doi: 10.1042/bj2490409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grantham B. D., Zammit V. A. Studies on the activation in vitro of carnitine palmitoyltransferase I in liver mitochondria from normal, diabetic and glucagon-treated rats. Biochem J. 1987 Apr 1;243(1):261–265. doi: 10.1042/bj2430261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harano Y., Kashiwagi A., Kojima H., Suzuki M., Hashimoto T., Shigeta Y. Phosphorylation of carnitine palmitoyltransferase and activation by glucagon in isolated rat hepatocytes. FEBS Lett. 1985 Sep 2;188(2):267–272. doi: 10.1016/0014-5793(85)80385-9. [DOI] [PubMed] [Google Scholar]
- Hopewell R., Martin-Sanz P., Martin A., Saxton J., Brindley D. N. Regulation of the translocation of phosphatidate phosphohydrolase between the cytosol and the endoplasmic reticulum of rat liver. Effects of unsaturated fatty acids, spermine, nucleotides, albumin and chlorpromazine. Biochem J. 1985 Dec 1;232(2):485–491. doi: 10.1042/bj2320485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingebritsen T. S., Parker R. A., Gibson D. M. Regulation of liver hydroxymethylglutaryl-CoA reductase by a bicyclic phosphorylation system. J Biol Chem. 1981 Feb 10;256(3):1138–1144. [PubMed] [Google Scholar]
- Kojima H., Harano Y., Kosugi K., Nakano T., Shigeta Y. A suppressive role of c-kinase for the stimulation of hepatic ketogenesis by glucagon and epinephrine. FEBS Lett. 1986 Jun 9;201(2):271–276. doi: 10.1016/0014-5793(86)80622-6. [DOI] [PubMed] [Google Scholar]
- Labarca C., Paigen K. A simple, rapid, and sensitive DNA assay procedure. Anal Biochem. 1980 Mar 1;102(2):344–352. doi: 10.1016/0003-2697(80)90165-7. [DOI] [PubMed] [Google Scholar]
- Lahav M., Schoenfeld N., Epstein O., Atsmon A. A method for obtaining high recovery of purified subcellular fractions of rat liver homogenate. Anal Biochem. 1982 Mar 15;121(1):114–122. doi: 10.1016/0003-2697(82)90563-2. [DOI] [PubMed] [Google Scholar]
- McGarry J. D., Foster D. W. In support of the roles of malonyl-CoA and carnitine acyltransferase I in the regulation of hepatic fatty acid oxidation and ketogenesis. J Biol Chem. 1979 Sep 10;254(17):8163–8168. [PubMed] [Google Scholar]
- McNeillie E. M., Zammit V. A. Regulation of acetyl-CoA carboxylase in rat mammary gland. Effects of starvation and of insulin and prolactin deficiency on the fraction of the enzyme in the active form in vivo. Biochem J. 1982 Apr 15;204(1):273–280. doi: 10.1042/bj2040273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller T. B., Jr, Garnache A. K., Cruz J., McPherson R. K., Wolleben C. Regulation of glycogen metabolism in primary cultures of rat hepatocytes. Restoration of acute effects of insulin and glucose in cells from diabetic rats. J Biol Chem. 1986 Jan 15;261(2):785–790. [PubMed] [Google Scholar]
- Miyazawa S., Ozasa H., Osumi T., Hashimoto T. Purification and properties of carnitine octanoyltransferase and carnitine palmitoyltransferase from rat liver. J Biochem. 1983 Aug;94(2):529–542. doi: 10.1093/oxfordjournals.jbchem.a134384. [DOI] [PubMed] [Google Scholar]
- Murthy M. S., Pande S. V. Malonyl-CoA binding site and the overt carnitine palmitoyltransferase activity reside on the opposite sides of the outer mitochondrial membrane. Proc Natl Acad Sci U S A. 1987 Jan;84(2):378–382. doi: 10.1073/pnas.84.2.378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reeves R. E., Sols A. Regulation of Escherichia coli phosphofructokinase in situ. Biochem Biophys Res Commun. 1973 Jan 23;50(2):459–466. doi: 10.1016/0006-291x(73)90862-0. [DOI] [PubMed] [Google Scholar]
- Reinhart P. H., Taylor W. M., Bygrave F. L. A procedure for the rapid preparation of mitochondria from rat liver. Biochem J. 1982 Jun 15;204(3):731–735. doi: 10.1042/bj2040731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson I. N., Zammit V. A. Sensitivity of carnitine acyltransferase I to malonly-CoA inhibition in isolated rat liver mitochondria is quantitatively related to hepatic malonyl-CoA concentration in vivo. Biochem J. 1982 Jul 15;206(1):177–179. doi: 10.1042/bj2060177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saggerson E. D., Carpenter C. A. Effects of fasting, adrenalectomy and streptozotocin-diabetes on sensitivity of hepatic carnitine acyltransferase to malonyl CoA. FEBS Lett. 1981 Jul 6;129(2):225–228. doi: 10.1016/0014-5793(81)80170-6. [DOI] [PubMed] [Google Scholar]
- Seglen P. O. Preparation of rat liver cells. 3. Enzymatic requirements for tissue dispersion. Exp Cell Res. 1973 Dec;82(2):391–398. doi: 10.1016/0014-4827(73)90357-1. [DOI] [PubMed] [Google Scholar]
- Stephens T. W., Harris R. A. Effect of starvation and diabetes on the sensitivity of carnitine palmitoyltransferase I to inhibition by 4-hydroxyphenylglyoxylate. Biochem J. 1987 Apr 15;243(2):405–412. doi: 10.1042/bj2430405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson M. A., Cascarano J., Wooten W. L., Pickett C. B. Quantitative isolation of liver mitochondria by zonal centrifugation. Anal Biochem. 1978 Mar;85(1):255–264. doi: 10.1016/0003-2697(78)90297-x. [DOI] [PubMed] [Google Scholar]
- Woodside W. F., Heimberg M. Effects of anti-insulin serum, insulin, and glucose on output of triglycerides and on ketogenesis by the perfused rat liver. J Biol Chem. 1976 Jan 10;251(1):13–23. [PubMed] [Google Scholar]
- Zammit V. A., Corstorphine C. G. Altered release of carnitine palmitoyltransferase activity by digitonin from liver mitochondria of rats in different physiological states. Biochem J. 1985 Sep 1;230(2):389–394. doi: 10.1042/bj2300389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zammit V. A., Corstorphine C. G. Changes in the proportion of acetyl-CoA carboxylase in the active form in rat liver. Effect of starvation, lactation and weaning. Biochem J. 1982 Jun 15;204(3):757–764. doi: 10.1042/bj2040757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zammit V. A. Mechanisms of regulation of the partition of fatty acids between oxidation and esterification in the liver. Prog Lipid Res. 1984;23(1):39–67. doi: 10.1016/0163-7827(84)90005-5. [DOI] [PubMed] [Google Scholar]
- Zammit V. A. Time-dependence of inhibition of carnitine palmitoyltransferase I by malonyl-CoA in mitochondria isolated from livers of fed or starved rats. Evidence for transition of the enzyme between states of low and high affinity for malonyl-CoA. Biochem J. 1984 Mar 1;218(2):379–386. doi: 10.1042/bj2180379. [DOI] [PMC free article] [PubMed] [Google Scholar]


