Abstract
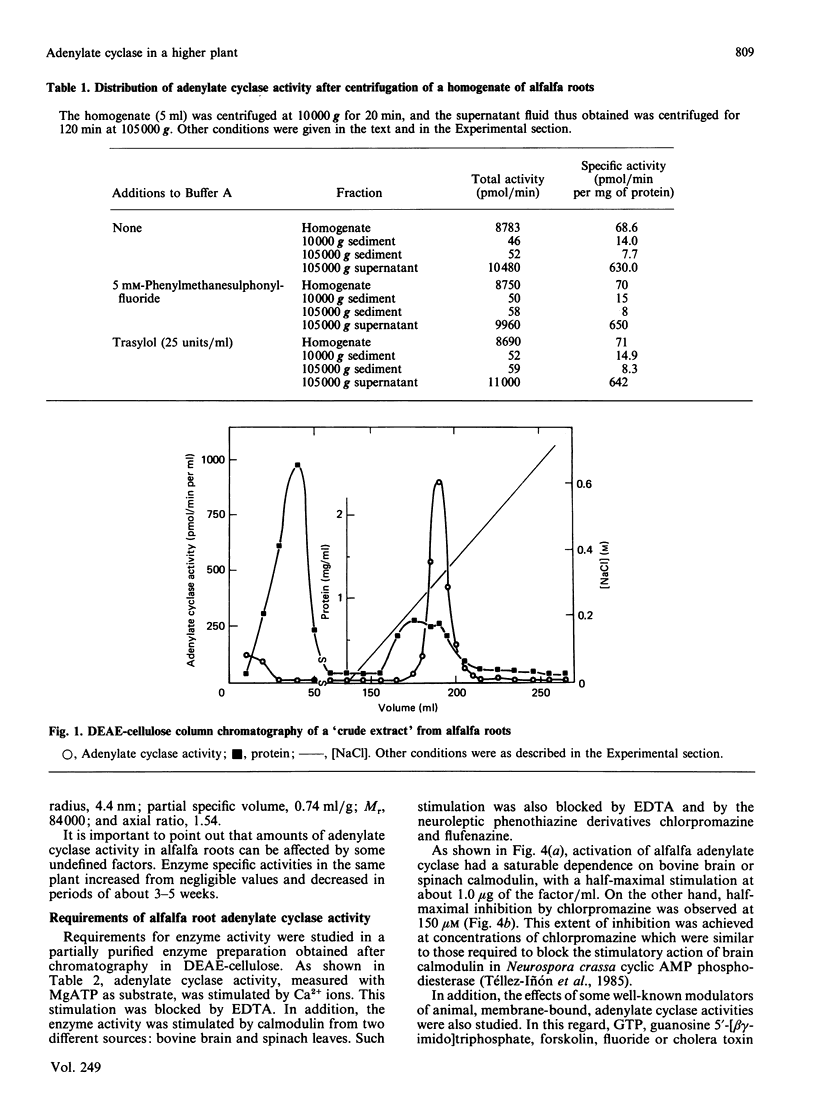
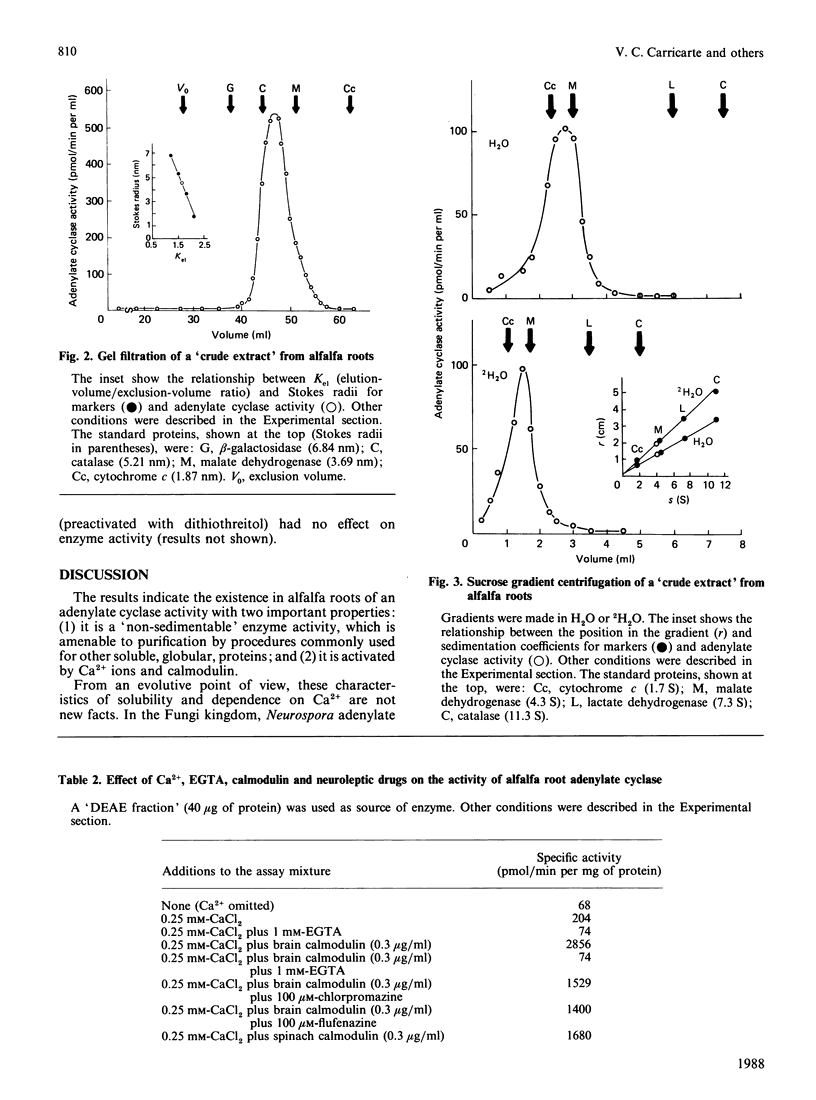
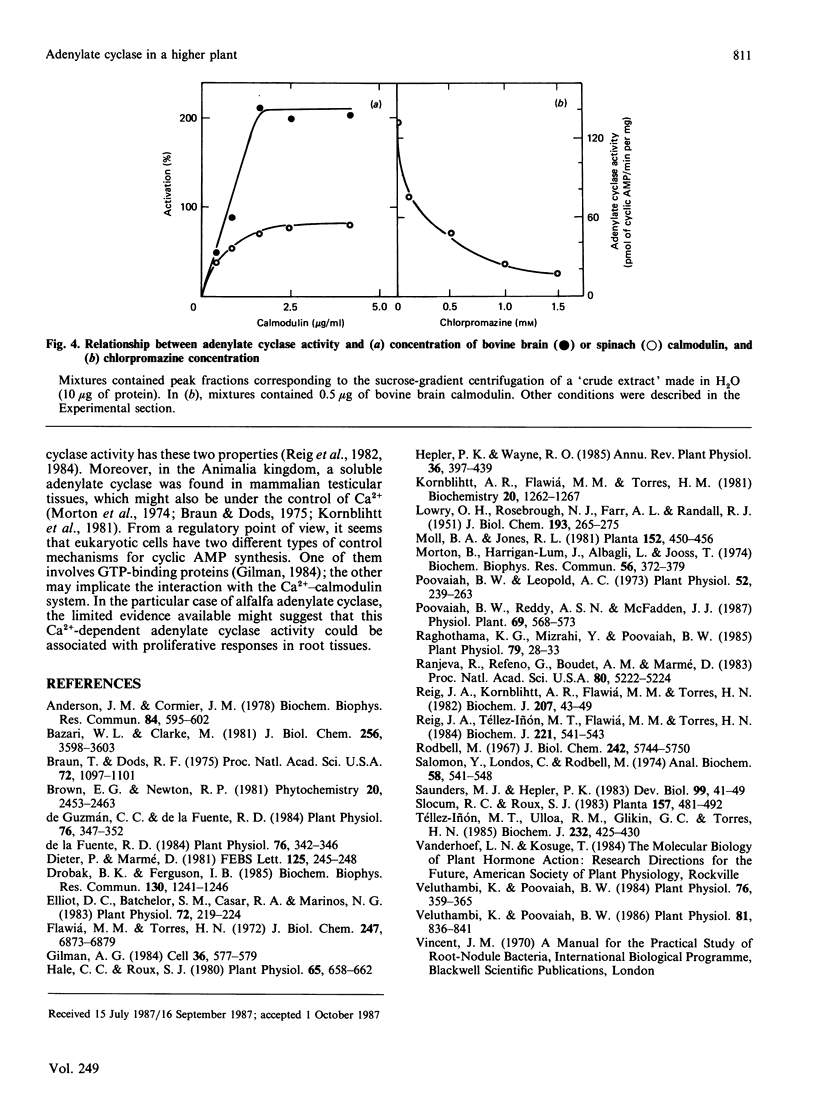
An adenylate cyclase activity in Medicago sativa L. (alfalfa) roots was partially characterized. The enzyme activity remains in the supernatant fluid after centrifugation at 105,000 g and shows in crude extracts an apparent Mr of about 84,000. The enzyme is active with Mg2+ and Ca2+ as bivalent cations, and is inhibited by EGTA and by chlorpromazine. Calmodulin from bovine brain or spinach leaves activates this adenylate cyclase.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson J. M., Cormier M. J. Calcium-dependent regulation of NAD kinase. Biochem Biophys Res Commun. 1978 Oct 16;84(3):595–602. doi: 10.1016/0006-291x(78)90747-7. [DOI] [PubMed] [Google Scholar]
- Bazari W. L., Clarke M. Characterization of a novel calmodulin from Dictyostelium discoideum. J Biol Chem. 1981 Apr 10;256(7):3598–3603. [PubMed] [Google Scholar]
- Braun T., Dods R. F. Development of a Mn-2+-sensitive, "soluble" adenylate cyclase in rat testis. Proc Natl Acad Sci U S A. 1975 Mar;72(3):1097–1101. doi: 10.1073/pnas.72.3.1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dela Fuente R. K. Role of calcium in the polar secretion of indoleacetic Acid. Plant Physiol. 1984 Oct;76(2):342–346. doi: 10.1104/pp.76.2.342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drøbak B. K., Ferguson I. B. Release of Ca2+ from plant hypocotyl microsomes by inositol-1,4,5-trisphosphate. Biochem Biophys Res Commun. 1985 Aug 15;130(3):1241–1246. doi: 10.1016/0006-291x(85)91747-4. [DOI] [PubMed] [Google Scholar]
- Elliott D. C., Batchelor S. M., Cassar R. A., Marinos N. G. Calmodulin-binding drugs affect responses to cytokinin, auxin, and gibberellic Acid. Plant Physiol. 1983 May;72(1):219–224. doi: 10.1104/pp.72.1.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flawiá M. M., Torres H. N. Adenylate cyclase activity in Neurospora crassa. I. General properties. J Biol Chem. 1972 Nov 10;247(21):6873–6879. [PubMed] [Google Scholar]
- Gilman A. G. G proteins and dual control of adenylate cyclase. Cell. 1984 Mar;36(3):577–579. doi: 10.1016/0092-8674(84)90336-2. [DOI] [PubMed] [Google Scholar]
- Hale C. C., Roux S. J. Photoreversible calcium fluxes induced by phytochrome in oat coleoptile cells. Plant Physiol. 1980 Apr;65(4):658–662. doi: 10.1104/pp.65.4.658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kornblihtt A. R., Flawia M. M., Torres H. N. Manganese ion dependent adenylate cyclase activity in rat testes: purification and properties. Biochemistry. 1981 Mar 3;20(5):1262–1267. doi: 10.1021/bi00508a033. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Morton B., Harrigan-Lum J., Albagli L., Jooss T. The activation of motility in quiescent hamster sperm from the epididymis by calcium and cyclic nucleotides. Biochem Biophys Res Commun. 1974 Jan 23;56(2):372–379. doi: 10.1016/0006-291x(74)90852-3. [DOI] [PubMed] [Google Scholar]
- Poovaiah B. W., Leopold A. C. Deferral of leaf senescence with calcium. Plant Physiol. 1973 Sep;52(3):236–239. doi: 10.1104/pp.52.3.236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poovaiah B. W., Reddy A. S., McFadden J. J. Calcium messenger system: role of protein phosphorylation and inositol bisphospholipids. Physiol Plant. 1987;69:569–573. doi: 10.1111/j.1399-3054.1987.tb09241.x. [DOI] [PubMed] [Google Scholar]
- Raghothama K. G., Mizrahi Y., Poovaiah B. W. Effect of calmodulin antagonists on auxin-induced elongation. Plant Physiol. 1985 Sep;79(1):28–33. doi: 10.1104/pp.79.1.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranjeva R., Refeno G., Boudet A. M., Marmé D. Activation of plant quinate:NAD 3-oxidoreductase by Ca and calmodulin. Proc Natl Acad Sci U S A. 1983 Sep;80(17):5222–5224. doi: 10.1073/pnas.80.17.5222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reig J. A., Kornblihtt A. R., Flawiá M. M., Torres H. N. Soluble adenylate cyclase activity in Neurospora crassa. Biochem J. 1982 Oct 1;207(1):43–49. doi: 10.1042/bj2070043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reig J. A., Téllez-Iñn M. T., Flawiá M. M., Torres H. N. Activation of Neurospora crassa soluble adenylate cyclase by calmodulin. Biochem J. 1984 Jul 15;221(2):541–543. doi: 10.1042/bj2210541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodbell M. Metabolism of isolated fat cells. V. Preparation of "ghosts" and their properties; adenyl cyclase and other enzymes. J Biol Chem. 1967 Dec 25;242(24):5744–5750. [PubMed] [Google Scholar]
- Salomon Y., Londos C., Rodbell M. A highly sensitive adenylate cyclase assay. Anal Biochem. 1974 Apr;58(2):541–548. doi: 10.1016/0003-2697(74)90222-x. [DOI] [PubMed] [Google Scholar]
- Saunders M. J., Hepler P. K. Calcium antagonists and calmodulin inhibitors block cytokinin-induced bud formation in Funaria. Dev Biol. 1983 Sep;99(1):41–49. doi: 10.1016/0012-1606(83)90252-x. [DOI] [PubMed] [Google Scholar]
- Téllez-Iñn M. T., Ulloa R. M., Glikin G. C., Torres H. N. Characterization of Neurospora crassa cyclic AMP phosphodiesterase activated by calmodulin. Biochem J. 1985 Dec 1;232(2):425–430. doi: 10.1042/bj2320425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veluthambi K., Poovaiah B. W. Calcium- and calmodulin-regulated phosphorylation of soluble and membrane proteins from corn coleoptiles. Plant Physiol. 1984 Oct;76(2):359–365. doi: 10.1104/pp.76.2.359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veluthambi K., Poovaiah B. W. In vitro and in vivo protein phosphorylation in Avena sativa L. coleoptiles: effects of Ca2+, calmodulin antagonists, and auxin. Plant Physiol. 1986 Jul;81(3):836–841. doi: 10.1104/pp.81.3.836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Guzman C. C., Dela Fuente R. K. Polar calcium flux in sunflower hypocotyl segments : I. The effect of auxin. Plant Physiol. 1984 Oct;76(2):347–352. doi: 10.1104/pp.76.2.347. [DOI] [PMC free article] [PubMed] [Google Scholar]


