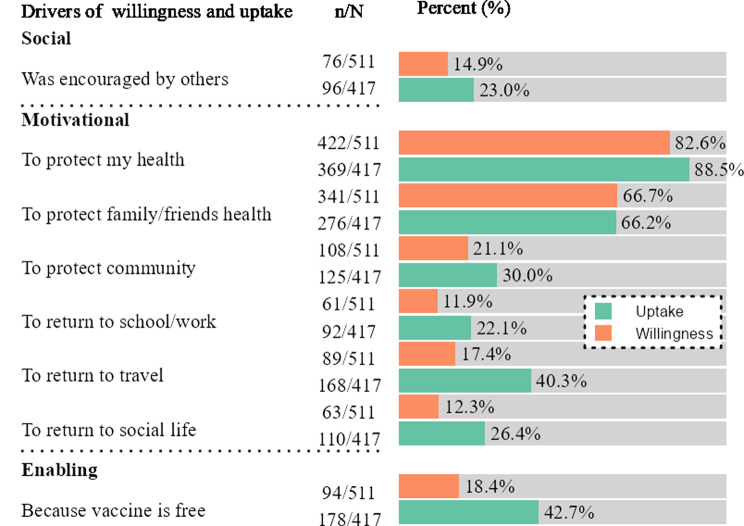Abstract
Background
The WHO set the global immunisation threshold for COVID-19 at 70% to achieve worldwide protection against the disease. To date, global COVID-19 vaccine coverage is still below this threshold, in particular in several sub-Saharan African (SSA) countries, such as Madagascar. While factors influencing COVID-19 vaccine hesitancy have been widely explored in the past few years, research on drivers of COVID-19 vaccine uptake remains scarce. This study aimed at investigating drivers associated with COVID-19 vaccine uptake in the Boeny region of Madagascar.
Methods
The study used a cross-sectional survey design to collect data on drivers of vaccine uptake from a sample of adults recruited from 12 healthcare facilities between November 2022 and February 2023. Relative and absolute frequencies were used to summarize participants’ characteristics. Prevalence ratios were estimated by Poisson regression to identify and compare sociodemographic and motivational drivers of vaccine uptake among those who were willing to get vaccinated against COVID-19 with those who had already been vaccinated.
Results
A total of 928 participants aged between 18 and 76 years were included in the study. Among those recruited, 44.9% (n = 417) had already been vaccinated and 55.1% (n = 511) were willing to receive their first dose of COVID-19 vaccine on the day of the interview. The proportions of those respondents who live in urban areas (56.5% vs. 43.8%) and who have high school or university education (46.6% vs. 35.8%) were higher for the uptake group, whereas the proportion of employed respondents (66.3% vs. 56.5%) was higher among those willing to get vaccinated. Vaccine being free of charge (aPR = 1.77 [CI 95%: 1.45–2.17]) and being able to travel again (aPR = 1.61 [CI 95%: 1.30–1.98]) were the drivers most strongly associated with higher vaccine uptake after adjustment for sociodemographic factors.
Conclusions
This study shows that actual COVID-19 vaccine uptake is influenced by a different set of factors than willingness to get vaccinated. Taking this difference in drivers into account can inform more tailored vaccination strategies to increase worldwide coverage.
Keywords: Public health, COVID-19, COVID-19 vaccination, Vaccination uptake, Vaccination willingness, Vaccination strategies
Background
Vaccination is a critical public health intervention for reducing disease burden and mortality. Despite its benefits, in 2019, the World Health Organization (WHO) declared vaccine hesitancy to be one of the top ten threats to global public health [1]. The COVID-19 pandemic has intensified the focus on vaccine hesitancy [2–7] with studies indicating variations in hesitancy due to factors like new information, or policies, or newly reported vaccine risks [2–5, 8, 9]. Vaccine hesitancy is defined as a “delay in acceptance or refusal of vaccination despite availability of vaccination services” [10], while vaccine willingness refers to the intent or motivation to be vaccinated [4]. Both concepts reflect the intentions that precede actual vaccines uptake or refusal [11].
Globally, COVID-19 vaccination uptake remains low, especially in many SSA countries [12].
Madagascar has the lowest COVID-19 vaccination rate worldwide. With only 9% of its population being vaccinated against COVID-19 with at least one dose by November 26, 2023, Madagascar falls considerably short in terms of the WHO’s target of 70% coverage [12, 13]. Low vaccination coverage in the country is primary due to infrastructural (shortage in healthcare personnel with adequate training on vaccine administration and pharmacological vigilance, shortage in cold chain equipment, information technology for and equipment for registry and control vaccine distribution), logistical (limited population access to healthcare facilities), and supply challenges [14]. In addition, the country joined the COVAX initiative comparatively late [15, 16] and had a neutral political stance towards COVID-19 vaccination. While vaccination was encouraged for at-risk groups, such as healthcare workers or older age groups (55 years old or more), it was not introduced as a mandatory requirement to resume social activities or travel [17]. Madagascar’s COVID-19 vaccination rollout begun in May 2021. Vaccination was implemented according to the national COVID-19 immunization plan [18], initially in the public primary care facilities where vaccines were available for the adult population free of charge. No further updates on vaccine recommendations for younger age groups were issued by the authorities. Various WHO-approved vaccine brands were used, namely Pfizer, Janssen, Covishield / AstraZeneca, Sinopharm, of which the Janssen vaccine was the most common one.
Several international efforts have aimed to boost coverage in the country, including the campaign that provided data for this study [19], the ‘COVID-19 vaccination campaign in the Boeny region of Madagascar: paving the road for worldwide vaccination coverage goal (CoBoGo)’. CoBoGo is a partnership between the Malagasy Ministry of Health, Malagasy academic institutions, and the Bernhard Nocht Institute for Tropical Medicine, launched under the financing umbrella of the Deutsche Gesellschaft für Internationale Zusammenarbeit GmbH aiming to deploy 25,000 doses of COVID-19 vaccines within six months, addressing infrastructural and logistical challenges in Boeny region.
An initial United Nations International Children’s Emergency Fund (UNICEF) survey in July 2021 indicated that about 53% of adults in Madagascar viewed COVID-19 vaccination as important and 29% intended to get vaccinated [18]. By September 2022, however, COVID-19 vaccine coverage was only 5.4%, revealing a significant behavioural gap between willingness and actual vaccine uptake that must be addressed to achieve coverage targets.
The WHO Technical Advisory Group on Behavioural Insights and Sciences for Health highlighted three major drivers of vaccine uptake: (1) an enabling environment, (2) social influences, and (3) motivations [20]. This framework has guided research to develop effective strategies to improve vaccination willingness and uptake. Conventional awareness campaigns seem to have had only a limited effect on vaccine uptake [11]. Extant research has shown that effective strategies to increase willingness to get vaccinated, include reducing barriers for sourcing and administration, fostering social and individual motivations, and building confidence in health workers [5, 8, 11, 20, 21]. However, far fewer studies have used actual vaccine uptake as their outcome comparing it to a willingness to get vaccinated [22], which is crucial to address the identified behavioural gap. As a part of the CoBoGo vaccination campaign, this study investigated the drivers of COVID-19 vaccine uptake in the Boeny region of Madagascar, employing the WHO framework previously described. We compared drivers among those willing to receive a vaccine with those already vaccinated, in order to strengthen the evidence base for more tailored and effective vaccination both, in Madagascar and similar contexts.
Methods
Study design and settings
This cross-sectional survey targeted adults (≥ 18 years old) in the Boeny region of the North-West Madagascar, conducted between November 2022 and February 2023, within a COVID-19 vaccination campaign implemented collaboratively by local authorities and academic partners.
Study participants
The following eligibility criteria were applied to enroll study participants: (a) residency in the Boeny region; (b) age ≥ 18 years old; (c) having received at least one dose of a COVID-19 vaccine or willingness to get vaccinated on the interview day; and (d) ability to provide informed consent and participate in French or Malagasy.
Sampling procedure and sample size determination
Study participants were recruited using a convenience sampling strategy across the 12 healthcare facilities that were included in the CoBoGo COVID-19 vaccination campaign in five Boeny municipalities, based on previously established collaborations between local authorities and academic partners.
At each site, survey staff conveniently approached and screened individuals visiting the healthcare facility for eligibility and recruited up to ten voluntary participants per day.
A target sample size of 948 individuals was determined using OpenEpi software to achieve 80% power to detect a 10% difference between groups, assuming 20% unexposed with the outcome, a significance level of 5%, and a design effect of 1.5 [23].
Data collection
Data collection was conducted by twenty-four interviewers who completed a one-day training on all survey procedures and interviewing techniques, to ensure consistency and minimize interviewer and social desirability bias.
Face-to-face interviews were conducted in French or Malagasy, using a succinct paper-based questionnaire designed to minimize interference with the vaccination procedures. The questionnaire included sections on sociodemographic information, COVID-19 vaccination status, and willingness to get vaccinated, reasons for COVID-19 uptake, and sources of information on the vaccination campaign and took no longer than ten minutes to complete. The questionnaire was developed based on existing validated instruments used in previous studies [3] and was pre-tested to ensure linguistic and cultural appropriateness.
Outcome variable
Self-reported vaccination status was assessed with the questions “Have you ever received a COVID-19 vaccine?”, and, if negative “Would you like to be vaccinated against COVID-19 today?”. Responses were combined into a dichotomous outcome “already vaccinated” vs. “willing to get vaccinated”.
Independent variables
Independent variables included sociodemographic factors, such as age, residence type (rural/urban), education level (never attended school or incomplete primary school, primary or secondary school, high school or university education), employment status, and drivers for vaccine willingness and vaccine uptake. Drivers of vaccination were assessed on the basis of the three sub-categories (enabling environment, social influence and motivation) with multiple closed and open questions. Interviewers were instructed not to prompt the responses. Multiple answers were possible, for the purpose of the analysis, each category was considered as a dichotomous variable.
Data management and analysis
Fieldwork supervisors performed daily data quality checks to identify and correct inconsistencies and data was entered into a Kobo toolbox database [24].
Analyses were conducted using the R version 4.3.1 [25] (base, car, ggplot2, sandwich, dplyr, Table 1). Relative and absolute frequencies summarized participants’ characteristics. The frequency of vaccination drivers and information sources was estimated for those vaccinated and those willing to get vaccinated. Crude and adjusted prevalence ratios were estimated using Poisson regression with robust standard errors, as they provide a more direct and interpretable measure of association in cross-sectional studies compared to odds ratios, especially when the outcome is not rare [26]. Informed by WHO’s considerations on COVID-19 vaccination acceptance and uptake [20], we included in the regression model three categories of drivers of vaccine uptake: (a) enabling environment (vaccine being free of charge, urban residency, occupation), (b) social influences (being encouraged by others to get vaccinated), (c) motivational factors (protection of own health, protection of family and friends’ health, protection of the community, return to travel, to social life, to work, education and school activities), and (d) socio-demographic factors (age group, education). Observations with missing values were excluded from the analysis. The significance level was set at 5%.
Table 1.
Characteristics of study participants, Boeny, Madagascar, November 2022-February 2023
| Total sample | Willingness | Uptake | |
|---|---|---|---|
| n = 928 | n = 511 | n = 417 | |
| Age groups | |||
| 18–29 | 405 (43.6%) | 225 (44.0%) | 180 (43.2%) |
| 30–39 | 227 (24.5%) | 129 (25.2%) | 98 (23.5%) |
| 40+ | 296 (31.9%) | 157 (30.7%) | 139 (33.3%) |
| Residence type | |||
| Rural area | 466 (50.2%) | 287 (56.2%) | 179 (42.9%) |
| Urban area | 462 (49.8%) | 224 (43.8%) | 238 (57.1%) |
| Occupation | |||
| Employed | 569 (61.9%) | 335 (66.3%) | 234 (56.5%) |
| Not employed | 350 (38.1%) | 170 (33.7%) | 180 (43.5%) |
| Education level | |||
| Incomplete/No school education | 249 (26.8%) | 134 (26.2%) | 115 (27.6%) |
| Primary/middle school | 301 (32.4%) | 194 (38.0%) | 107 (25.7%) |
| High school/University | 377 (40.6%) | 183 (35.8%) | 194 (46.6%) |
Ethical considerations
This study complied with legal and ethical requirements, receiving approval from the Ethics Committee Hamburg State Medical Chamber (protocol number: 2021-10550-BO-ff) and the National Ethics Committee of Madagascar (CERBM: IORGO000851 N°81 MSANP/SG/AMM/CERBM). Survey participation was voluntary, with no monetary incentives. Written informed consent was obtained from all participants, and an impartial witness was involved for illiterate participants. Participants willing to get vaccinated were accompanied to the vaccination service immediately after the interview to get a COVID-19 vaccine. Participants were assured of data privacy and encouraged to answer questions honestly. To ensure confidentiality, all collected data were pseudonomysed and stored securely on a password-protected Kobo toolbox database accessible only to authorized research personnel. Physical questionnaires were kept in locked cabinets to prevent unauthorized access. The study posed minimal risk to participants, primarily involving the time taken for interviews. Participants were informed that their participation would contribute to improving public health strategies for vaccine uptake in the region.
Results
Participants characteristics
Overall 928 individuals were included in this study. The majority of participants were aged 18–29 years (43.6%), with a nearly equal split between rural and urban residents (50.2% vs. 49.8%, respectively; Table 1).
61.9% (n = 569) of participants were employed, and 40.6% (n = 377) had a secondary school or university education.
Among recruited, 44.9% (n = 417) were already vaccinated, while 55.1% (n = 511) were willing to receive their first dose of the COVID-19 vaccine. Urban residency (56.5%, n = 238 vs. 43.8%, n = 224) and high school or university education (46.6%, n = 194 vs. 35.8%, n = 183) were more common in the vaccinated group, whereas employment was more common in the willing group (66.3%, n = 335 vs. 56.5%, n = 234).
Sources of information about COVID-19 vaccination
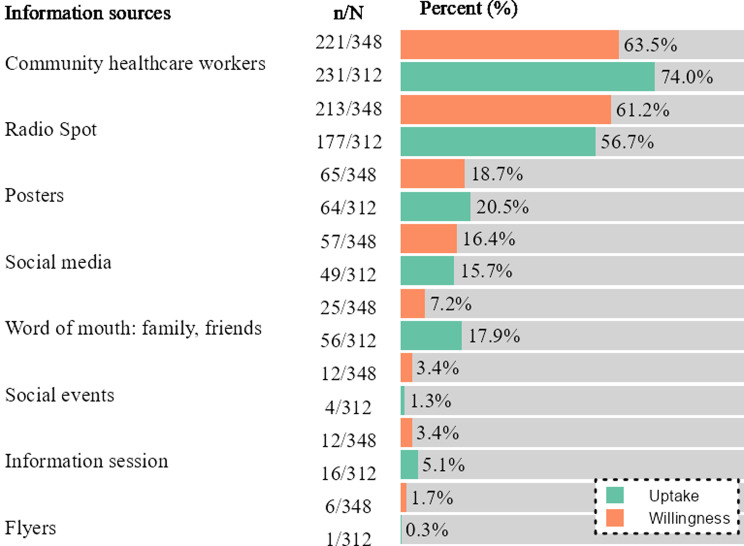
Survey participants were asked for their main sources of information about COVID-19 vaccination, specifically concerning the ongoing CoBoGo campaign. Most were aware of the CoBoGo campaign (74.8%, n = 312 vaccinated and 68.1%, n = 348 willing). Participants aware of the CoBoGo campaign reported community healthcare workers (CHW) (74.0%, n = 231 vaccinated and 63.5%, n = 221 willing), followed by radio (56.7%, n = 177 vaccinated and 61.2%, n = 213 willing), and posters (20.5%, n = 64 vaccinated and 18.7%, n = 65 willing), as the top information sources (Fig. 1). Family and friends (17.9%, n = 56) and social media (e.g. Facebook) (16.4%, n = 57) were also notable sources for vaccinated and willing participants, respectively.
Fig. 1.
Information sources on vaccination campaign in Boeny, Madagascar, November 2022-February 2023 Legend Information sources were assessed among participants aware of the CoBoGo campaign (vaccinated n = 312 and willing n = 348)
Drivers of vaccination willingness and vaccine uptake
The primary drivers for vaccination among the vaccinated were “To protect my health” (88.5%, n = 369), “To protect my family/friends’ health” (66.2%, n = 276), and “Because the vaccine is free” (42.7%, n = 178) and “To return to travel” (40.3%, n = 168) (Fig. 2).
Fig. 2.
Drivers of COVID-19 vaccination uptake and willingness reported by those already vaccinated and those willing to get vaccinated against COVID-19 in Boeny, Madagascar, November 2022-February 2023
For those willing to get vaccinated the main drivers were the protection of their own personal health (82.6%, n = 422), followed by protecting family and friends’ health (66.7%, n = 341), and protection of the community health (21.1%, n = 108) (Fig. 2). Other factors, such as “Because the vaccine is free” (18.4%, n = 94) and “To return to travel” (17.4%, n = 89) were less frequently mentioned.
Being encouraged by others, returning to work/school, and resuming a social life were among the least frequently reported drivers of COVID-19 vaccination in both groups. Those participants who stated that they had been encouraged by others specified that they had most frequently receiving the encouragement from healthcare workers and the Ministry of Health. In contract, having received encouragement from community leaders as encouraging vaccination was only mentioned once.
Factors associated with vaccine uptake
Table 2 reports the adjusted prevalence ratios (aPR) with 95% confidence intervals (CI 95%) of COVID-19 vaccine uptake as compared with willingness. Significant positive associations with vaccine uptake included free vaccination availability (aPR = 1.77, CI 95%:1.45–2.17), and the prospect of resuming travelling (aPR = 1.61, CI 95%:1.30–1.98). Urban residency was associated with a 31% higher vaccine uptake (aPR = 1.31, 95% CI: 1.07–1.60), suggesting that accessibility of healthcare facilities in urban areas may play a critical role in vaccine uptake.
Table 2.
Crude (PR) and adjusted prevalence ratios (aPR) COVID-19 vaccine uptake vs. willingness to get vaccinated in Boeny, Madagascar, November 2022-February 2023
| N | n | p | PR | aPR | |
|---|---|---|---|---|---|
| Age group | |||||
| 18-29 (ref) | 405 | 180 | 44.4 | 1 | 1 |
| 30-39 | 227 | 98 | 43.2 | 0.97 (0.76; 1.24) | 1.07 (0.88; 1.30) |
| 40+ | 296 | 139 | 47.0 | 1.06 (0.85; 1.32) | 1.17 (0.97; 1.40) |
| Urbanization | |||||
| Rural (ref) | 466 | 179 | 38.4 | 1 | 1 |
| Urban | 462 | 238 | 51.5 | 1.34 (1.16; 1.55) | 1.31 (1.07; 1.60) |
| Occupation | |||||
| Employed (ref) | 569 | 234 | 41.1 | 1 | 1 |
| Not employed | 350 | 234 | 51.4 | 1.25 (1.08; 1.44) | 1.19 (0.99; 1.41) |
| Education | |||||
| Incomplete/No formal education | 249 | 115 | 46.2 | 1 | 1 |
| Primary/Middle | 301 | 107 | 35.5 | 0.77 (0.63; 0.94) | 0.87 (0.71; 1.06) |
| High school/University | 377 | 194 | 51.5 | 1.11 (0.94; 1.32) | 1.11 (0.91; 1.35) |
| To protect my health | |||||
| No (ref) | 137 | 48 | 35.0 | 1 | 1 |
| Yes | 791 | 369 | 46.6 | 1.33 (1.06; 1.66) | 1.22 (0.95; 1.56) |
| To protect family/friends´ health | |||||
| No (ref) | 311 | 141 | 45.3 | 1 | 1 |
| Yes | 617 | 276 | 44.7 | 0.99 (0.85; 1.15) | 0.81 (0.67; 0.98) |
| To protect community | |||||
| No (ref) | 695 | 292 | 42.0 | 1 | 1 |
| Yes | 233 | 125 | 53.6 | 1.28 (1.09; 1.49) | 0.78 (0.60; 1.03) |
| To return to school/work | |||||
| No (ref) | 775 | 325 | 41.9 | 1 | 1 |
| Yes | 153 | 92 | 60.1 | 1.43 (1.21; 1.7) | 1.10 (0.88; 1.36) |
| To return to travel | |||||
| No (ref) | 671 | 249 | 37.1 | 1 | 1 |
| Yes | 257 | 168 | 65.4 | 1.76 (1.52; 2.04) | 1.61 (1.30; 1.98) |
| To return to social life | |||||
| No (ref) | 755 | 307 | 40.7 | 1 | 1 |
| Yes | 173 | 110 | 63.6 | 1.56 (1.33; 1.84) | 1.05 (0.74; 1.46) |
| Was encouraged by others | |||||
| No (ref) | 756 | 321 | 42.5 | 1 | 1 |
| Yes | 172 | 96 | 55.8 | 1.31 (1.11; 1.56) | 0.65 (0.48; 0.88) |
| Because the vaccine is free | |||||
| No (ref) | 656 | 239 | 36.4 | 1 | 1 |
| Yes | 272 | 178 | 65.4 | 1.80 (1.56; 2.07) | 1.77 (1.45; 2.17) |
Note N – total number of observations, n -number of vaccinated, p – proportion of vaccine uptake,
PR – crude prevalence ratio, aPR - adjusted prevalence ratio
Conversely, the encouragement by others (aPR = 0.65, CI 95%:0.48–0.88) and protecting family and friends’ health (aPR = 0.81, CI 95%:0.67–0.98) showed significant associations with reduced uptake as compared with willingness.
Discussion
This study aimed to identify drivers of COVID-19 vaccine uptake as compared with willingness within the context of the CoBoGo vaccination campaign in Madagascar. Our findings show that key drivers of high vaccine uptake includ the vaccine being free of charge (aPR = 1.77 [CI 95%: 1.45–2.17]), and the prospect of returning to travel (aPR = 1.61 [CI 95%: 1.30–1.98]), followed by urban residency (aPR = 1.31, 95% CI: 1.07–1.60).
Free of charge vaccination has been shown to reduce inequalities and to facilitate uptake in high-income countries among vulnerable populations with suboptimal vaccination coverage [27, 28]. It is also crucial in low-income settings, where over 80% of the population lives in extreme poverty [29]. In fact, having access to free vaccines has been recognized by the populations as a desirable feature of vaccination campaigns in 34 African countries [30]. Thus, ensuring an enabling environment in Madagascar and other limited-resource settings within which vaccines are offered free of charge can enhance and sustain vaccine uptake in adult populations.
The prospect of taking up travel again was a significant motivational driver of vaccine uptake, reported by 40.5% of vaccinated participants. While domestic travel did not require vaccination [17], international travel did. This may have been of particular relevance for those with higher levels of education within the sample, who tend to have more interest and capacity for international travel. Other studies corroborate that resuming international travel plays a critical role in influencing vaccine acceptance and uptake in both low- and high- income countries [4, 6].
Encouragement by others (aPR = 0.65 [CI 95%: 0.48–0.88]) was a less relevant driver for those already vaccinated, suggesting that the decision to get vaccinated may be more autonomous for this group. While some studies have shown that encouragement from trusted individuals, such as relatives, friends, and healthcare workers, can influence vaccine willingness for COVID-19 and other diseases [31, 32], the final decision to get vaccinated may be more autonomous, and internal [33]. This complexity of the decision-making process suggests a need for further longitudinal studies to better understand these dynamics and inform communication strategies that leverage social encouragement effectively to increase vaccine uptake [34].
In addition, this study shows that personal motivations, particularly self-protection (88.5% uptake, 82.6% willingness), were primary drivers for both groups. Family and friends’ health was also important, while community health was less frequently reported (21.2-30.0%). This finding contrasts with the widely held assumption that communities in low- and middle-income (LMICs), give greater importance to collective well-being [35] than the more individualistic high-income societies [36, 37]. These insights are crucial for designing communication strategies around the benefits of vaccination.
Consistent with other studies in LMICs and SSA [22], urban residency was associated with higher COVID-19 vaccine uptake due to better access to services [38, 39]. This is particularly concerning for Madagascar where most of the population lives in remote areas with limited access to healthcare [40]. Our data highlight the need for vaccination strategies that put greater emphasis on reaching remote populations. Community health workers (CHWs) can play an important role in helping to create an enabling environment for vaccination, as they are trusted sources of information and are effective in reaching isolated and marginalised populations [41–43]. In countries, such as Madagascar, where the health system strongly relies on CHWs [44], implementing supportive measures for CHWs, such as training and access to health records, can optimize vaccine coverage [45, 46].
Finally, in our study setting, social media are less frequently used to gather information about COVID-19 vaccinations than, for example, more traditional media, such as radio. This element is particularly relevant when considering the use of social media for the implementation of health-related awareness and information programs for the general population. In Madagascar, the use of social media for vaccination-related awareness raising campaign might not be yet as effective as in other countries [47].
This study is among the few in SSA comparing COVID-19 vaccine willingness and uptake and the first to explore social attitudes towards vaccination in Madagascar. The findings contribute to the global knowledge about drivers of vaccine uptake and provide valuable insights for adapting public health strategies in Madagascar and other countries sharing similar challenges and cultural contexts. However, this study is not without limitations. The cross-sectional survey design limits causal conclusions, the convenience sampling strategy can introduce some degree of selection bias, potentially favoring participants with higher education and better access to healthcare. This may limit the generalizability of our findings to the wider population. On the other hand, the convenience sampling strategy allowed us to optimize fieldwork time and costs in the context of externally low COVID-19 vaccine coverage in the region. Additionally, to collect the data in healthcare facilities with as little disruption as possible for vaccination rollout, we designed a succinct survey instrument, which did not address vaccine characteristics (safety, efficacy, vaccine brand), information on participants’ sex was also not collected, potentially leading to unmeasured confounding bias. Moreover, the use of self-reported data could lead to some social desirability bias, widely acknowledged in research on vaccine willingness [3, 22, 30]. Our methodological approach to the data collection helped to minimize this type of bias through non-judgemental interviewing techniques and question formulation.
Conclusions
In summary, this study demonstrates that factors influencing COVID-19 vaccine uptake differ from those influencing willingness to get vaccinated. The most influential drivers associated with higher vaccination uptake alongside access to health services included the vaccine being free of charge and the desire to resume travel, indicating that both economic and mobility incentives played a crucial role in vaccination decisions in Malagasy population.
Understanding and addressing these drivers along the continuum of decision-making around vaccinations can help to improve vaccination strategies beyond the COVID-19 pandemic and develop tailored communication campaingns in Madagascar and other countries sharing similar challenges.
Acknowledgements
The authors are grateful to study participants, so as the drivers and technicians supporting data collection and field implementation, without whom this work would not be possible, and to all the professionals involved in the data collection.
Abbreviations
- aPR
Adjusted prevalence ratio
- CI
Confidence interval
- CERBM
National Biomedical Research Ethics Committee in Madagascar
- CHW
Community healthcare worker
- CoBoGo
COVID-19 vaccination campaign in the Boeny region of Madagascar: paving the road for Worldwide vaccination coverage goal
- COVID-19
COronaVIrus Disease 19
- LMIC
Low- and middle-income countries
- SSA
Sub-Saharan African
- UNICEF
United Nations Children’s Fund
- WHO
World Health Organization
Author contributions
DF, DIP, DKA, RAR, JM, VM contributed to the conceptualization and design of the study. Field implementation was coordinated by DF, RAR, DKA and VM and performed by AOTZ, SR, AG, VP, OT, SV, RiR. Data management was performed by COD, PR and IK. DF and IK conceptualized the data analysis plan. AOTZ, SR, AG, VP, OT, SV contributed to the data collection. Data analysis was performed by IK. Interpretation of data from the Malagasy perspective was performed by LH, RaR, RiR, RRH, TR, ZAR, RAR, DKA. DF secured the funding for the study. All the authors contributed to results interpretation, critically reviewed and approved the final version of the manuscript.
Funding
This work was mainly funded by the Deutsche Gesellschaft für Internationale Zusammenarbeit (GIZ) through the project CoBoGo (Project number: 81285812) with the partial contribution of the Global Health Protection Program (GHPP) of the German Federal Ministry of Health grant number FKZ 2523GHP004, the German Center for Infection Research (DZIF) through the projects NAMASTE (grant number: 8008803819) and Else Kröner-Fresenius Stiftung (EKFS) through the project CHIMPS (project number: 2022_EKHA.101).
Open Access funding enabled and organized by Projekt DEAL.
Data availability
Raw data used in the current study are available from the corresponding author on reasonable request and will be freely available to researchers who wish to use them for non-commercial purposes.
Declarations
Ethics approval and consent to participate
Ethical approval for the study was obtained from the Ethical Committee of Hamburg (protocol number: 2021-10550-BO-ff) and Madagascar (CERBM: IORGO000851 N°81 MSANP/SG/AMM/CERBM). Survey participation was voluntary and no monetary incentives were offered to the participants. Written informed consent was obtained from all study participants.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Ten health issues WHO will. tackle this year [Internet]. [cited 2023 Jul 24]. https://www.who.int/news-room/spotlight/ten-threats-to-global-health-in-2019
- 2.Solís Arce JS, Warren SS, Meriggi NF, Scacco A, McMurry N, Voors M, et al. COVID-19 vaccine acceptance and hesitancy in low- and middle-income countries. Nat Med. 2021;27(8):1385–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Faye SLB, Krumkamp R, Doumbia S, Tounkara M, Strauss R, Ouedraogo HG, et al. Factors influencing hesitancy towards adult and child COVID-19 vaccines in rural and urban West Africa: a cross-sectional study. BMJ Open. 2022;12(4):e059138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lazarus JV, Wyka K, White TM, Picchio CA, Gostin LO, Larson HJ, et al. A survey of COVID-19 vaccine acceptance across 23 countries in 2022. Nat Med. 2023;29(2):366–75. [DOI] [PubMed] [Google Scholar]
- 5.Kalu ME, Oyinlola O, Ibekaku MC, Adandom II, Iwuagwu AO, Ezulike CJ, et al. A mapping review on the Uptake of the COVID-19 vaccine among adults in Africa using the 5As Vaccine Taxonomy. Am J Trop Med Hyg. 2022;106(6):1688–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lazarus JV, Wyka K, White TM, Picchio CA, Rabin K, Ratzan SC, et al. Revisiting COVID-19 vaccine hesitancy around the world using data from 23 countries in 2021. Nat Commun. 2022;13:3801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Abubakari SW, Workneh F, Asante KP, Hemler EC, Madzorera I, Wang D, et al. Determinants of COVID-19 vaccine readiness and hesitancy among adults in sub-saharan Africa. PLOS Glob Public Health. 2023;3(7):e0000713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Roy DN, Biswas M, Islam E, Azam MS. Potential factors influencing COVID-19 vaccine acceptance and hesitancy: a systematic review. PLoS ONE. 2022;17(3):e0265496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Deml MJ, Githaiga JN. Determinants of COVID-19 vaccine hesitancy and uptake in sub-saharan Africa: a scoping review. BMJ Open. 2022;12(11):e066615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.MacDonald NE. Vaccine hesitancy: definition, scope and determinants. Vaccine. 2015;33(34):4161–4. [DOI] [PubMed] [Google Scholar]
- 11.Brewer NT. What Works to Increase Vaccination Uptake. Acad Pediatr. 2021;21(4, Supplement):S9–16. [DOI] [PubMed]
- 12.WHO Coronavirus (COVID-19.) Dashboard [Internet]. [cited 2023 Jul 24]. https://covid19.who.int
- 13.Achieving 70%. COVID-19 Immunization Coverage by Mid-2022 [Internet]. [cited 2023 Jul 24]. https://www.who.int/news/item/23-12-2021-achieving-70-covid-19-immunization-coverage-by-mid-2022
- 14.Decouttere C, De Boeck K, Vandaele N. Advancing sustainable development goals through immunization: a literature review. Glob Health. 2021;17(1):95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rasambainarivo F, Ramiadantsoa T, Raherinandrasana A, Randrianarisoa S, Rice BL, Evans MV, et al. Prioritizing COVID-19 vaccination efforts and dose allocation within Madagascar. BMC Public Health. 2022;22(1):724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.COVAX [Internet]. [cited 2023 Jul 24]. https://www.who.int/initiatives/act-accelerator/covax
- 17.Plateforme Vaksiny pour la gestion des vaccins à Madagascar [Internet]. Unité de Gouvernance Digitale. 2021 [cited 2023 Jul 25]. https://digital.gov.mg/2021/09/08/plateforme-vaksiny-pour-la-gestion-des-vaccins-a-madagascar/
- 18.Ministre de la Santé Publique de Madagascar. Plan National de Déploiement et de Vaccination (PNDV) contre la Covid-19 à Madagascar. 2021.
- 19.Amt A. German Federal Foreign Office. [cited 2023 Jul 25]. COVID-19: Germany’s commitment to fair distribution of vaccines. https://www.auswaertiges-amt.de/en/aussenpolitik/themen/covax/2396914
- 20.WHO technical advisory group on behavioural insights and sciences for health. Behavioural considerations for acceptance and uptake of COVID-19 vaccines [Internet]. 2020 [cited 2023 Jul 24]. https://www.who.int/publications-detail-redirect/9789240016927
- 21.Brewer NT, Chapman GB, Rothman AJ, Leask J, Kempe A. Increasing vaccination: putting Psychological Science Into Action. Psychol Sci Public Interest. 2017;18(3):149–207. [DOI] [PubMed] [Google Scholar]
- 22.Whitehead HS, Songo J, Phiri K, Kalande P, Lungu E, Phiri S, et al. Correlates of uptake of COVID-19 vaccines and motivation to vaccinate among Malawian adults. Hum Vaccines Immunother. 2023;19(2):2228168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fleiss JL. Statistical methods for Rates and proportions. 3rd ed. John Wiley & Sons, Inc; 2003.
- 24.KoboToolbox [Internet]. [cited 2023 Jul 24]. KoboToolbox. https://www.kobotoolbox.org/
- 25.R: The R Project for Statistical Computing [Internet]. [cited 2023 Jul 24]. https://www.r-project.org/
- 26.Tamhane AR, Westfall AO, Burkholder GA, Cutter GR. Prevalence odds ratio versus prevalence ratio: choice comes with consequences. Stat Med. 2016;35(30):5730–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Crawshaw AF, Farah Y, Deal A, Rustage K, Hayward SE, Carter J, et al. Defining the determinants of vaccine uptake and undervaccination in migrant populations in Europe to improve routine and COVID-19 vaccine uptake: a systematic review. Lancet Infect Dis. 2022;22(9):e254–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Eiden AL, Barratt J, Nyaku MK. A review of factors influencing vaccination policies and programs for older adults globally. Hum Vaccines Immunother. 2023;19(1):2157164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.World Bank Open. Data [Internet]. [cited 2023 Jul 25]. World Bank Open Data. https://data.worldbank.org
- 30.Anjorin AA, Odetokun IA, Abioye AI, Elnadi H, Umoren MV, Damaris BF, et al. Will africans take COVID-19 vaccination? PLoS ONE. 2021;16(12):e0260575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kalunga L, Bulut E, Chen Z, Li Y, Ivanek R. Increasing vaccine uptake among employees within the non-health related critical infrastructure sectors: a review. Hum Vaccines Immunother. 2023;19(1):2135852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Froes F, Morais A, Hespanhol V, Nogueira R, Carlos JS, Jacinto N, et al. The Vacinómetro® initiative: an eleven-year monitorization of influenza vaccination coverage rates among risk groups in Portugal. Pulmonology. 2022;28(6):427–30. [DOI] [PubMed] [Google Scholar]
- 33.Schmitz M, Luminet O, Klein O, Morbée S, Van den Bergh O, Van Oost P, et al. Predicting vaccine uptake during COVID-19 crisis: a motivational approach. Vaccine. 2022;40(2):288–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tjilos M, Tamlyn AL, Ragan EJ, Assoumou SA, Barnett KG, Martin P, et al. Community members have more impact on their neighbors than celebrities: leveraging community partnerships to build COVID-19 vaccine confidence. BMC Public Health. 2023;23(1):350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pelham B, Hardin C, Murray D, Shimizu M, Vandello J. A truly global, non-WEIRD examination of collectivism: The Global Collectivism Index (GCI). Curr Res Ecol Soc Psychol. 2022;3:100030. [Google Scholar]
- 36.Nikolaev B, Boudreaux C, Salahodjaev R. Are individualistic societies less equal? Evidence from the parasite stress theory of values. J Econ Behav Organ. 2017;138:30–49. [Google Scholar]
- 37.Gorodnichenko Y, Roland G. Individualism, innovation, and long-run growth. Proc Natl Acad Sci. 2011;108(supplement4):21316–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tessema ZT, Worku MG, Tesema GA, Alamneh TS, Teshale AB, Yeshaw Y, et al. Determinants of accessing healthcare in Sub-saharan Africa: a mixed-effect analysis of recent demographic and health surveys from 36 countries. BMJ Open. 2022;12(1):e054397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bayati M, Noroozi R, Ghanbari-Jahromi M, Jalali FS. Inequality in the distribution of Covid-19 vaccine: a systematic review. Int J Equity Health. 2022;21(1):122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Weiss DJ, Nelson A, Gibson HS, Temperley W, Peedell S, Lieber A, et al. A global map of travel time to cities to assess inequalities in accessibility in 2015. Nature. 2018;553(7688):333–6. [DOI] [PubMed] [Google Scholar]
- 41.Vaughan K, Kok MC, Witter S, Dieleman M. Costs and cost-effectiveness of community health workers: evidence from a literature review. Hum Resour Health. 2015;13(1):71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ryabov I. Cost-effectiveness of Community Health Workers in controlling diabetes epidemic on the U.S.–Mexico border. Public Health. 2014;128(7):636–42. [DOI] [PubMed] [Google Scholar]
- 43.The cost-effectiveness. of community health workers delivering free diarrhoea treatment: evidence from Uganda - PMC [Internet]. [cited 2023 Jul 26]. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8757489/ [DOI] [PMC free article] [PubMed]
- 44.Evans MV, Andréambeloson T, Randriamihaja M, Ihantamalala F, Cordier L, Cowley G, et al. Geographic barriers to care persist at the community healthcare level: evidence from rural Madagascar. PLOS Glob Public Health. 2022;2(12):e0001028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.World Health Organization. WHO guideline on health policy and system support to optimize community health worker programmes. 2018. [PubMed]
- 46.Naimoli JF, Perry HB, Townsend JW, Frymus DE, McCaffery JA. Strategic partnering to improve community health worker programming and performance: features of a community-health system integrated approach. Hum Resour Health. 2015;13(1):46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Huo J, Desai R, Hong YR, Turner K, Mainous AG, Bian J. Use of Social Media in Health Communication: findings from the Health Information National Trends Survey 2013, 2014, and 2017. Cancer Control J Moffitt Cancer Cent. 2019;26(1):1073274819841442. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Raw data used in the current study are available from the corresponding author on reasonable request and will be freely available to researchers who wish to use them for non-commercial purposes.