Abstract
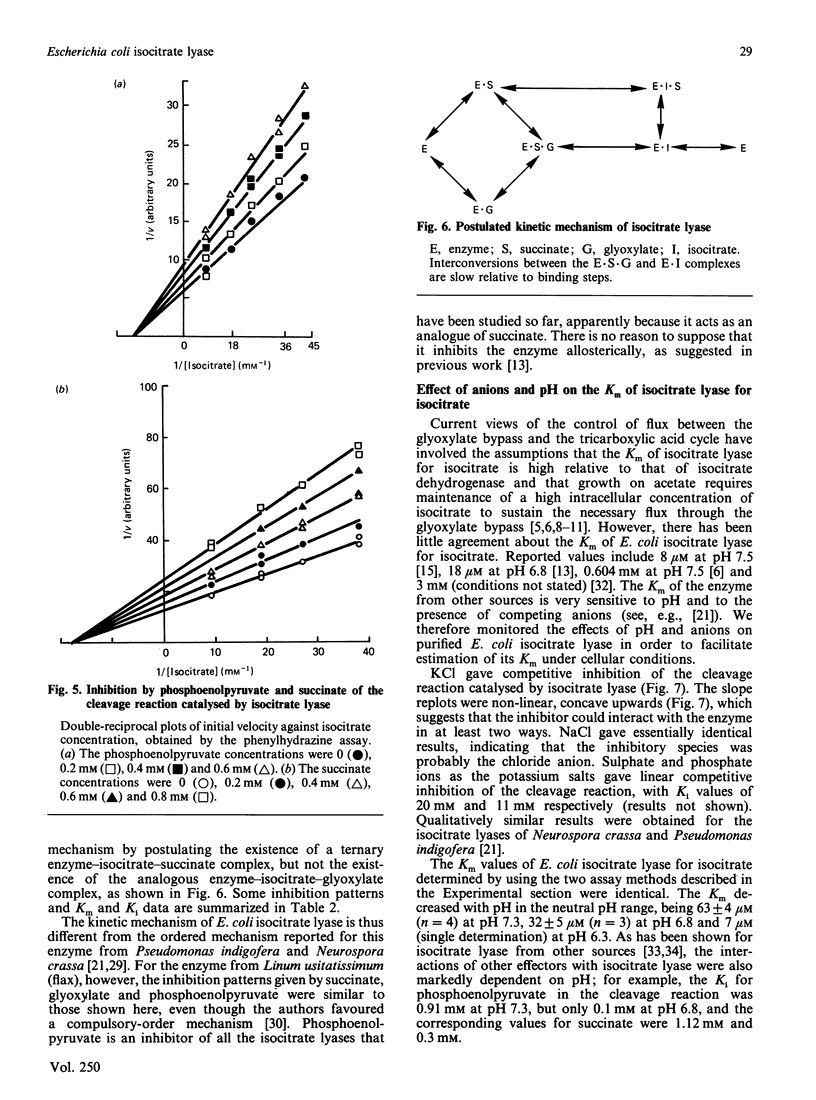
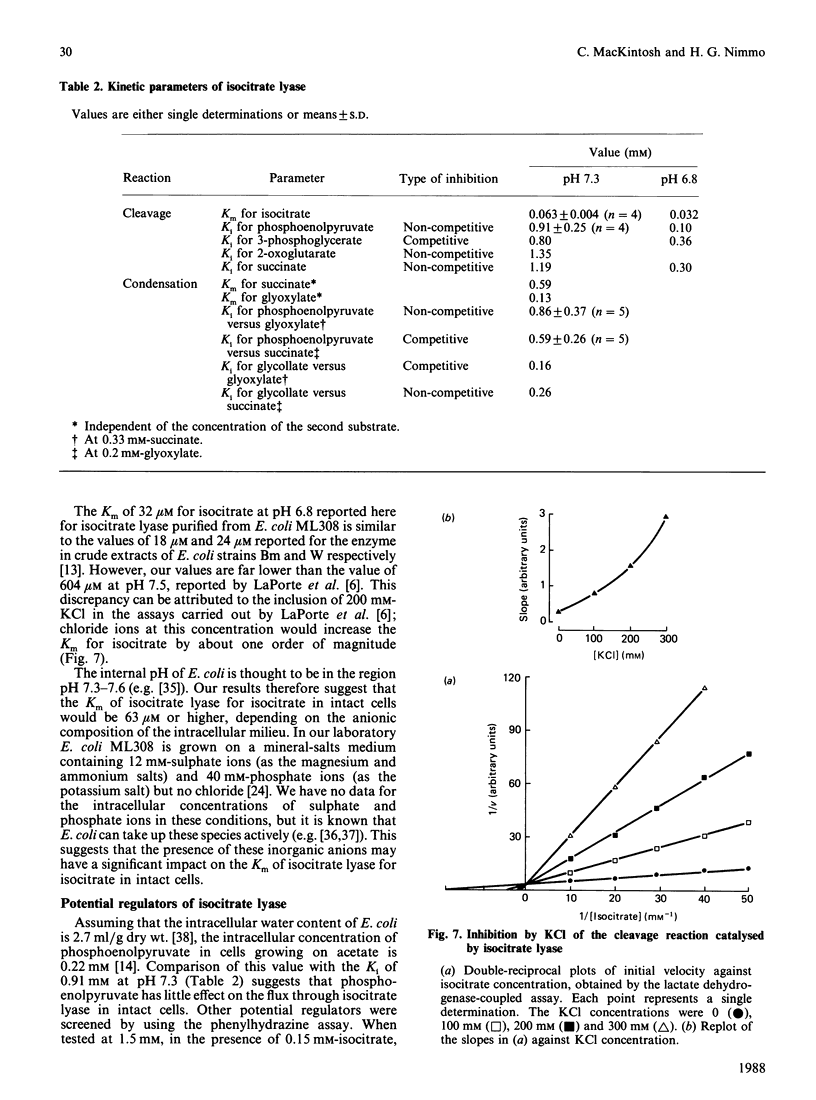
Isocitrate lyase was purified to homogeneity from Escherichia coli ML308. Its subunit Mr and native Mr were 44,670 +/- 460 and 17,000-180,000 respectively. The kinetic mechanism of the enzyme was investigated by using product and dead-end inhibitors of the cleavage and condensation reactions. The data indicated a random-order equilibrium mechanism, with formation of a ternary enzyme-isocitrate-succinate complex. In an attempt to predict the properties of isocitrate lyase in intact cells, the effects of pH, inorganic anions and potential regulatory metabolites on the enzyme were studied. The Km of the enzyme for isocitrate was 63 microM at physiological pH and in the absence of competing anions. Chloride, phosphate and sulphate ions inhibited competitively with respect to isocitrate. Phosphoenolpyruvate inhibited non-competitively with respect to isocitrate, but the Ki value suggested that this effect was unlikely to be significant in intact cells. 3-Phosphoglycerate was a competitive inhibitor. At the concentration reported to occur in intact cells, this metabolite would have a significant effect on the activity of isocitrate lyase. The available data suggest that the Km of isocitrate lyase for isocitrate is similar to the concentration of isocitrate in E. coli cells growing on acetate, about one order of magnitude higher than the Km determined in vitro in the absence of competing anions.
Full text
PDF
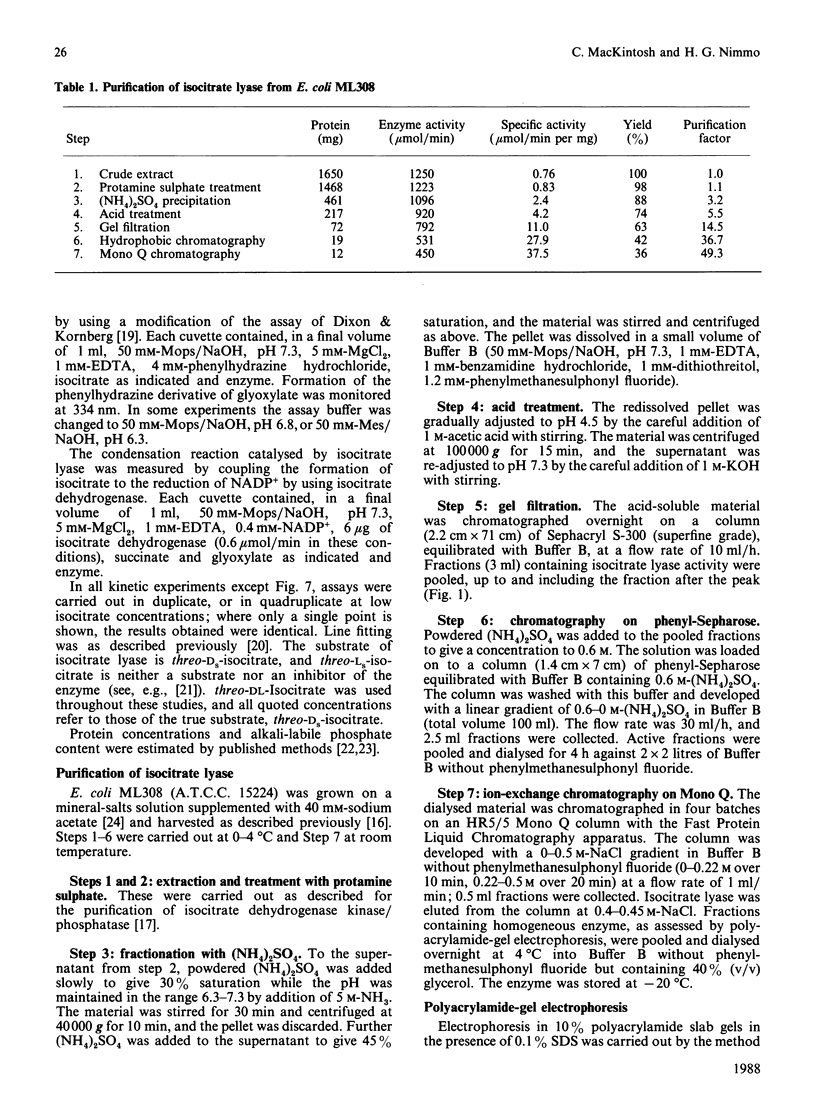
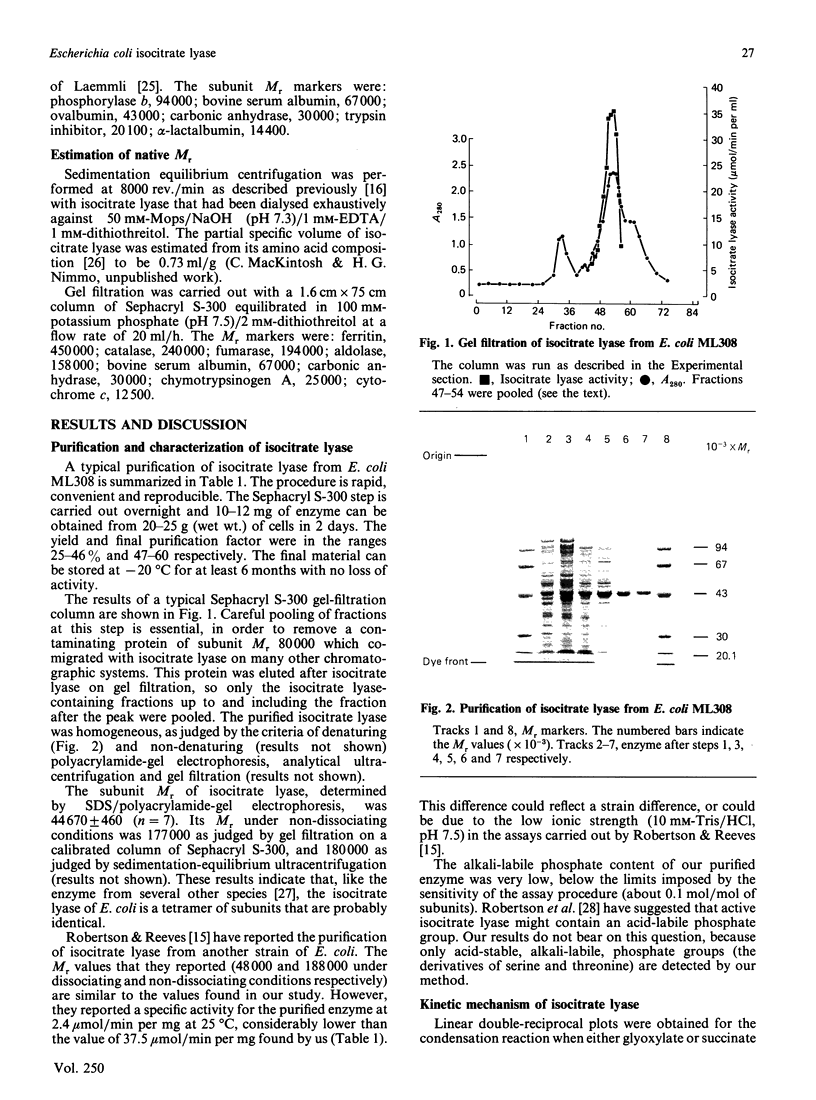
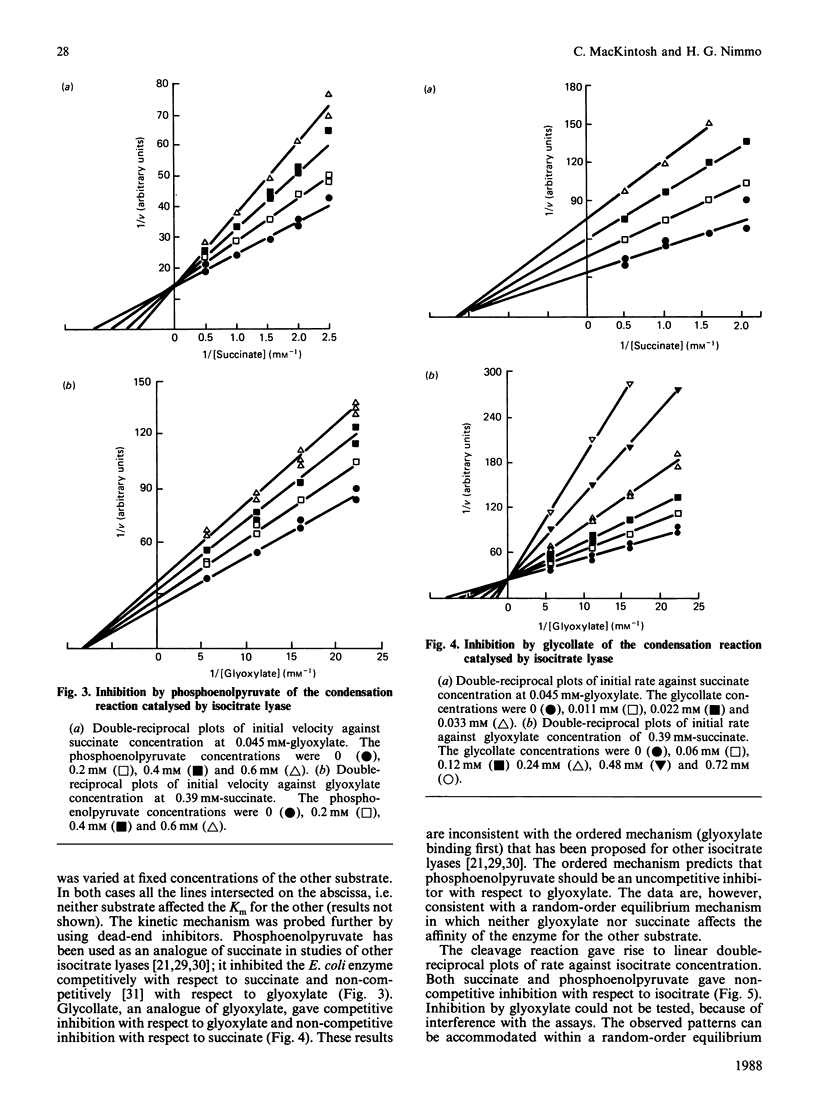

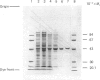
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ASHWORTH J. M., KORNBERG H. L. FINE CONTROL OF THE GLYOXYLATE CYCLE BY ALLOSTERIC INHIBITION OF ISOCITRATE LYASE. Biochim Biophys Acta. 1963 Jul 9;73:519–522. doi: 10.1016/0006-3002(63)90457-8. [DOI] [PubMed] [Google Scholar]
- Bautista J., Satrústegui J., Machado A. Evidence suggesting that the NADPH/NADP ratio modulates the splitting of the isocitrate flux between the glyoxylic and tricarboxylic acid cycles, in Escherichia coli. FEBS Lett. 1979 Sep 15;105(2):333–336. doi: 10.1016/0014-5793(79)80642-0. [DOI] [PubMed] [Google Scholar]
- Bennett P. M., Holms W. H. Reversible inactivation of the isocitrate dehydrogenase of Escherichia coli ML308 during growth on acetate. J Gen Microbiol. 1975 Mar;87(1):37–51. doi: 10.1099/00221287-87-1-37. [DOI] [PubMed] [Google Scholar]
- Borthwick A. C., Holms W. H., Nimmo H. G. Isolation of active and inactive forms of isocitrate dehydrogenase from Escherichia coli ML 308. Eur J Biochem. 1984 Jun 1;141(2):393–400. doi: 10.1111/j.1432-1033.1984.tb08204.x. [DOI] [PubMed] [Google Scholar]
- Borthwick A. C., Holms W. H., Nimmo H. G. The phosphorylation of Escherichia coli isocitrate dehydrogenase in intact cells. Biochem J. 1984 Sep 15;222(3):797–804. doi: 10.1042/bj2220797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- DREYFUSS J. CHARACTERIZATION OF A SULFATE- AND THIOSULFATE-TRANSPORTING SYSTEM IN SALMONELLA TYPHIMURIUM. J Biol Chem. 1964 Jul;239:2292–2297. [PubMed] [Google Scholar]
- Garnak M., Reeves H. C. Phosphorylation of Isocitrate dehydrogenase of Escherichia coli. Science. 1979 Mar 16;203(4385):1111–1112. doi: 10.1126/science.34215. [DOI] [PubMed] [Google Scholar]
- Gault M. H., Robertson W. G., Senciall D. R. The oxalate:phosphate ratio in urinary tract calculi by infrared revised analysis. Clin Chim Acta. 1987 Jun 30;166(1):103–105. doi: 10.1016/0009-8981(87)90203-8. [DOI] [PubMed] [Google Scholar]
- Holms W. H., Bennett P. M. Regulation of isocitrate dehydrogenase activity in Escherichia coli on adaptation to acetate. J Gen Microbiol. 1971 Jan;65(1):57–68. doi: 10.1099/00221287-65-1-57. [DOI] [PubMed] [Google Scholar]
- Holms W. H. The central metabolic pathways of Escherichia coli: relationship between flux and control at a branch point, efficiency of conversion to biomass, and excretion of acetate. Curr Top Cell Regul. 1986;28:69–105. doi: 10.1016/b978-0-12-152828-7.50004-4. [DOI] [PubMed] [Google Scholar]
- Johanson R. A., Hill J. M., McFadden B. A. Isocitrate lyase from Neurospora crassa. I. Purification, kinetic mechanism, and interaction with inhibitors. Biochim Biophys Acta. 1974 Oct 17;364(2):327–340. doi: 10.1016/0005-2744(74)90018-7. [DOI] [PubMed] [Google Scholar]
- Khan F. R., McFadden B. A. Isocitrate lyase from flax : terminal residues, composition, active site, and catalysis. Plant Physiol. 1982 Oct;70(4):943–948. doi: 10.1104/pp.70.4.943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaPorte D. C., Koshland D. E., Jr Phosphorylation of isocitrate dehydrogenase as a demonstration of enhanced sensitivity in covalent regulation. Nature. 1983 Sep 22;305(5932):286–290. doi: 10.1038/305286a0. [DOI] [PubMed] [Google Scholar]
- LaPorte D. C., Walsh K., Koshland D. E., Jr The branch point effect. Ultrasensitivity and subsensitivity to metabolic control. J Biol Chem. 1984 Nov 25;259(22):14068–14075. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lowry O. H., Carter J., Ward J. B., Glaser L. The effect of carbon and nitrogen sources on the level of metabolic intermediates in Escherichia coli. J Biol Chem. 1971 Nov;246(21):6511–6521. [PubMed] [Google Scholar]
- Meek D. W., Nimmo H. G. The interaction of fructose 2,6-bisphosphate with an allosteric site of rat liver fructose 1,6-bisphosphatase. FEBS Lett. 1983 Aug 22;160(1-2):105–109. doi: 10.1016/0014-5793(83)80946-6. [DOI] [PubMed] [Google Scholar]
- Nimmo G. A., Borthwick A. C., Holms W. H., Nimmo H. G. Partial purification and properties of isocitrate dehydrogenase kinase/phosphatase from Escherichia coli ML308. Eur J Biochem. 1984 Jun 1;141(2):401–408. doi: 10.1111/j.1432-1033.1984.tb08205.x. [DOI] [PubMed] [Google Scholar]
- Nimmo G. A., Nimmo H. G. The regulatory properties of isocitrate dehydrogenase kinase and isocitrate dehydrogenase phosphatase from Escherichia coli ML308 and the roles of these activities in the control of isocitrate dehydrogenase. Eur J Biochem. 1984 Jun 1;141(2):409–414. doi: 10.1111/j.1432-1033.1984.tb08206.x. [DOI] [PubMed] [Google Scholar]
- Nimmo H. G., Proud C. G., Cohen P. The purification and properties of rabbit skeletal muscle glycogen synthase. Eur J Biochem. 1976 Sep;68(1):21–30. doi: 10.1111/j.1432-1033.1976.tb10761.x. [DOI] [PubMed] [Google Scholar]
- Padan E., Zilberstein D., Schuldiner S. pH homeostasis in bacteria. Biochim Biophys Acta. 1981 Dec;650(2-3):151–166. doi: 10.1016/0304-4157(81)90004-6. [DOI] [PubMed] [Google Scholar]
- Rogers J. E., McFadden B. A. Isocitrate lyase from Neurospora crassa: pH dependence of catalysis and interaction with substrates and inhibitors. Arch Biochem Biophys. 1977 Apr 30;180(2):348–353. doi: 10.1016/0003-9861(77)90048-0. [DOI] [PubMed] [Google Scholar]
- Rogers J. E., McFadden B. A. Isocitrate lyase from Pseudomonas indigofera: pH dependence of catalysis and binding of substrates and inhibitors. Arch Biochem Biophys. 1976 Jun;174(2):695–704. doi: 10.1016/0003-9861(76)90400-8. [DOI] [PubMed] [Google Scholar]
- Walsh K., Koshland D. E., Jr Branch point control by the phosphorylation state of isocitrate dehydrogenase. A quantitative examination of fluxes during a regulatory transition. J Biol Chem. 1985 Jul 15;260(14):8430–8437. [PubMed] [Google Scholar]
- Walsh K., Koshland D. E., Jr Determination of flux through the branch point of two metabolic cycles. The tricarboxylic acid cycle and the glyoxylate shunt. J Biol Chem. 1984 Aug 10;259(15):9646–9654. [PubMed] [Google Scholar]
- Williams J. O., Roche T. E., McFadden B. A. Mechanism of action of isocitrate lyase from Pseudomonas indigofera. Biochemistry. 1971 Apr 13;10(8):1384–1390. doi: 10.1021/bi00784a017. [DOI] [PubMed] [Google Scholar]
- Winkler H. H., Wilson T. H. The role of energy coupling in the transport of beta-galactosides by Escherichia coli. J Biol Chem. 1966 May 25;241(10):2200–2211. [PubMed] [Google Scholar]
- el-Mansi E. M., MacKintosh C., Duncan K., Holms W. H., Nimmo H. G. Molecular cloning and over-expression of the glyoxylate bypass operon from Escherichia coli ML308. Biochem J. 1987 Mar 15;242(3):661–665. doi: 10.1042/bj2420661. [DOI] [PMC free article] [PubMed] [Google Scholar]