Abstract
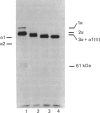
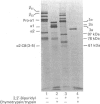
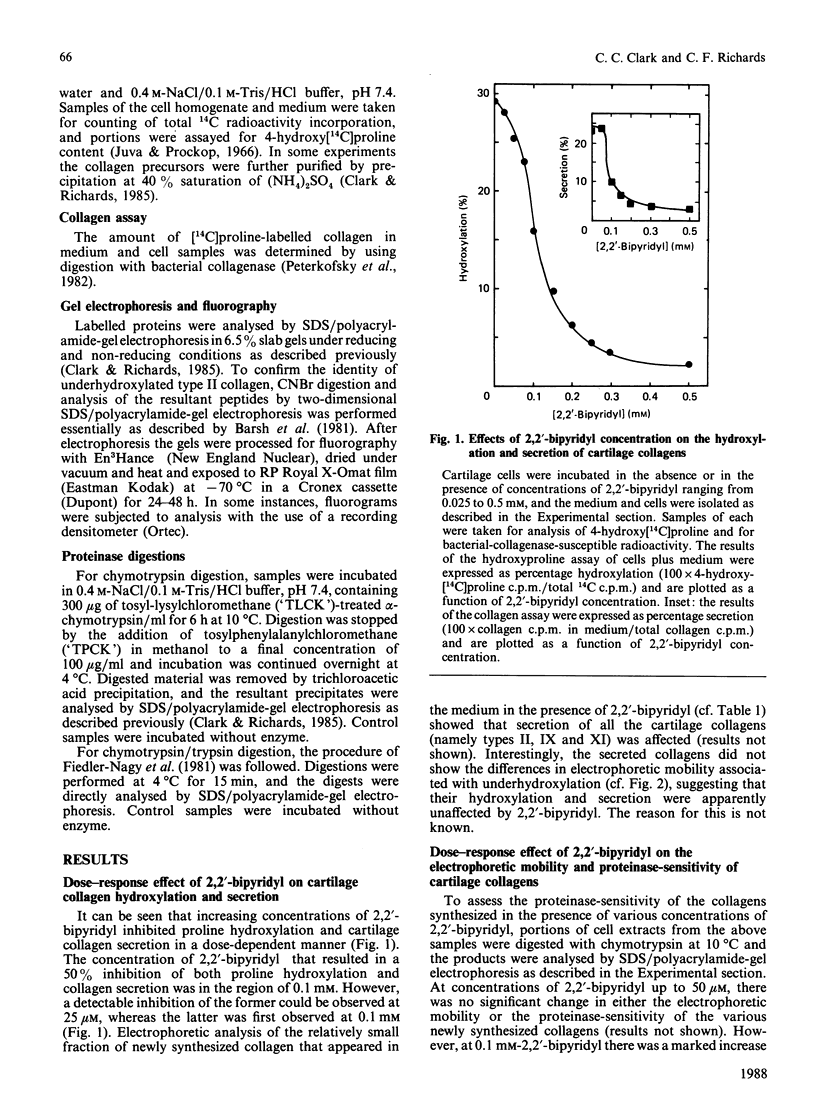
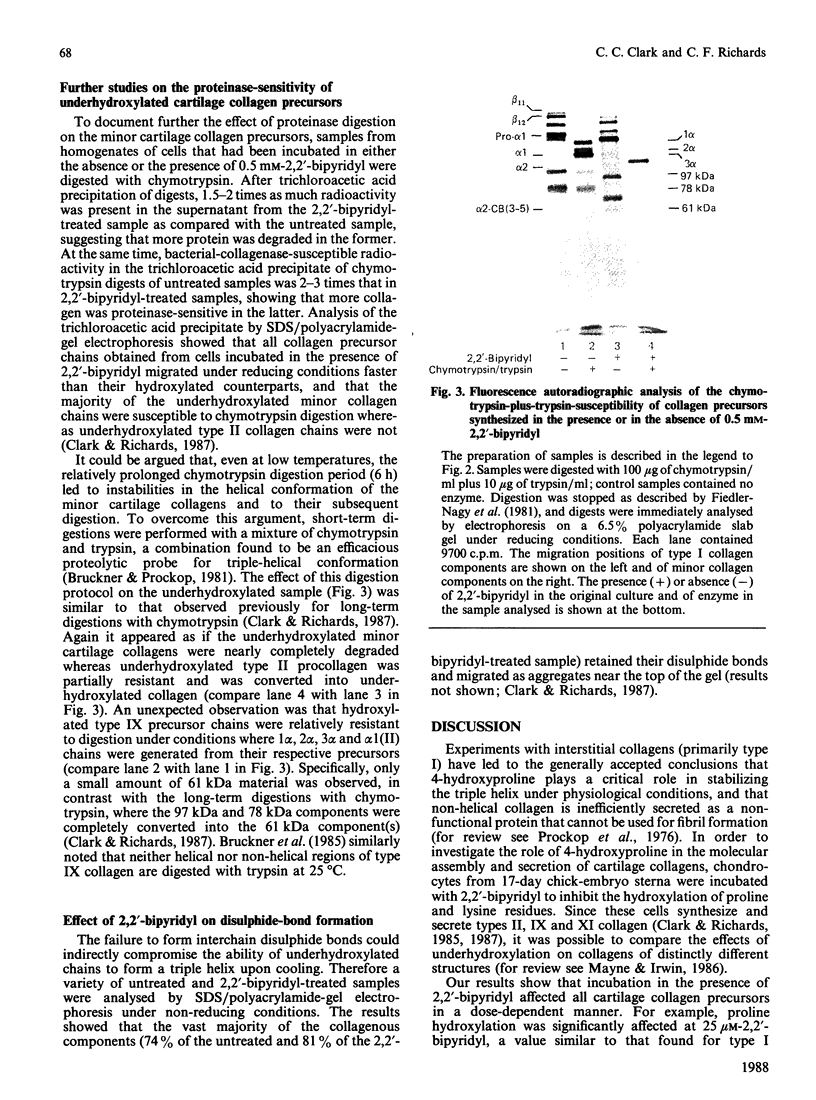
Matrix-free cells from chick-embryo sterna were incubated with various concentrations of 2,2'-bipyridyl, an iron chelator that inhibits prolyl hydroxylase and lysyl hydroxylase. At concentrations in the region of 0.1 mM, significant effects on cartilage collagen hydroxylation and secretion were observed. When the underhydroxylated collagens were subsequently digested with chymotrypsin or chymotrypsin plus trypsin at 4 degrees C for 15 min, the minor cartilage collagen precursors (namely types IX and XI) were extensively degraded; type II procollagen was only partially susceptible and was converted into underhydroxylated collagen. The results demonstrate that there were significant differences in triple-helix stability among cartilage collagens such that the underhydroxylated minor collagen precursors were unable to attain a native structure under conditions where type II procollagen was successful.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barsh G. S., Peterson K. E., Byers P. H. Peptide mapping of collagen chains using CNBr cleavage of proteins within polyacrylamide gels. Coll Relat Res. 1981 Nov;1(6):543–548. doi: 10.1016/s0174-173x(81)80035-0. [DOI] [PubMed] [Google Scholar]
- Berg R. A., Prockop D. J. The thermal transition of a non-hydroxylated form of collagen. Evidence for a role for hydroxyproline in stabilizing the triple-helix of collagen. Biochem Biophys Res Commun. 1973 May 1;52(1):115–120. doi: 10.1016/0006-291x(73)90961-3. [DOI] [PubMed] [Google Scholar]
- Bhatnagar R. S., Prockop D. J. Dissociation of the synthesis of sulphated mucopolysaccharides and the synthesis of collagen in embryonic cartilage. Biochim Biophys Acta. 1966 Dec 28;130(2):383–392. doi: 10.1016/0304-4165(66)90234-0. [DOI] [PubMed] [Google Scholar]
- Bruckner P., Vaughan L., Winterhalter K. H. Type IX collagen from sternal cartilage of chicken embryo contains covalently bound glycosaminoglycans. Proc Natl Acad Sci U S A. 1985 May;82(9):2608–2612. doi: 10.1073/pnas.82.9.2608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgeson R. E., Hebda P. A., Morris N. P., Hollister D. W. Human cartilage collagens. Comparison of cartilage collagens with human type V collagen. J Biol Chem. 1982 Jul 10;257(13):7852–7856. [PubMed] [Google Scholar]
- Capasso O., Quarto N., Descalzi-Cancedda F., Cancedda R. The low molecular weight collagen synthesized by chick tibial chondrocytes is deposited in the extracellular matrix both in culture and in vivo. EMBO J. 1984 Apr;3(4):823–827. doi: 10.1002/j.1460-2075.1984.tb01891.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark C. C., Richards C. F. Isolation and partial characterization of precursors to minor cartilage collagens. Coll Relat Res. 1985 Jun;5(3):205–223. doi: 10.1016/s0174-173x(85)80011-x. [DOI] [PubMed] [Google Scholar]
- Dehm P., Prockop D. J. Biosynthesis of cartilage procollagen. Eur J Biochem. 1973 May;35(1):159–166. doi: 10.1111/j.1432-1033.1973.tb02821.x. [DOI] [PubMed] [Google Scholar]
- Fessler L. I., Fessler J. H. Protein assembly of procollagen and effects of hydroxylation. J Biol Chem. 1974 Dec 10;249(23):7637–7646. [PubMed] [Google Scholar]
- Fiedler-Nagy C., Bruckner P., Hayashi T., Prockop D. J. Isolation of unhydroxylated type I procollagen folding of the protein in vitro. Arch Biochem Biophys. 1981 Dec;212(2):668–677. doi: 10.1016/0003-9861(81)90411-2. [DOI] [PubMed] [Google Scholar]
- GUSTAVSON K. H. The function of hydroxyproline in collanges. Nature. 1955 Jan 8;175(4445):70–74. doi: 10.1038/175070a0. [DOI] [PubMed] [Google Scholar]
- Gerard S., Puett D., Mitchell W. M. Kinetics of collagen fold formation in human type I procollagen and the effect of disulfide bonds. Biochemistry. 1981 Mar 31;20(7):1857–1865. doi: 10.1021/bi00510a022. [DOI] [PubMed] [Google Scholar]
- Harwood R., Merry A. H., Woolley D. E., Grant M. E., Jackson D. S. The disulphide-bonded nature of procollagen and the role of the extension peptides in the assembly of the molecule. Biochem J. 1977 Feb 1;161(2):405–418. doi: 10.1042/bj1610405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jimenez S. A., Harsch M., Murphy L., Rosenbloom J. Effects of temperature on conformation, hydroxylation, and secretion of chick tendon procollagen. J Biol Chem. 1974 Jul 25;249(14):4480–4486. [PubMed] [Google Scholar]
- Jimenez S., Harsch M., Rosenbloom J. Hydroxyproline stabilizes the triple helix of chick tendon collagen. Biochem Biophys Res Commun. 1973 May 1;52(1):106–114. doi: 10.1016/0006-291x(73)90960-1. [DOI] [PubMed] [Google Scholar]
- Juva K., Prockop D. J. Modified procedure for the assay of H-3-or C-14-labeled hydroxyproline. Anal Biochem. 1966 Apr;15(1):77–83. doi: 10.1016/0003-2697(66)90249-1. [DOI] [PubMed] [Google Scholar]
- Kao W. W., Prockop D. J., Berg R. A. Kinetics for the secretion of nonhelical procollagen by freshly isolated tendon cells. J Biol Chem. 1979 Apr 10;254(7):2234–2243. [PubMed] [Google Scholar]
- Oohira A., Nogami H., Kusakabe A., Kimata K., Suzuki S. Structural differences among procollagens associated with rough and smooth microsomes from chick embryo cartilage. J Biol Chem. 1979 May 10;254(9):3576–3583. [PubMed] [Google Scholar]
- Ramachandran G. N., Bansal M., Bhatnagar R. S. A hypothesis on the role of hydroxyproline in stabilizing collagen structure. Biochim Biophys Acta. 1973 Sep 21;322(1):166–171. doi: 10.1016/0005-2795(73)90187-6. [DOI] [PubMed] [Google Scholar]
- Reese C. A., Mayne R. Minor collagens of chicken hyaline cartilage. Biochemistry. 1981 Sep 15;20(19):5443–5448. doi: 10.1021/bi00522a014. [DOI] [PubMed] [Google Scholar]
- Rosenbloom J., Harsch M., Jimenez S. Hydroxyproline content determines the denaturation temperature of chick tendon collagen. Arch Biochem Biophys. 1973 Oct;158(2):478–484. doi: 10.1016/0003-9861(73)90539-0. [DOI] [PubMed] [Google Scholar]
- Uitto J., Prockop D. J. Biosynthesis of cartilage procollagen. Influence of chain association and hydroxylation of prolyl residues on the folding of the polypeptides into the triple-helical conformation. Biochemistry. 1974 Oct 22;13(22):4586–4591. doi: 10.1021/bi00719a018. [DOI] [PubMed] [Google Scholar]
- Uitto J., Prockop D. J. Rate of helix formation by intracellular procollagen and protocollagen. Evidence for a role for disulfide bonds. Biochem Biophys Res Commun. 1973 Dec 10;55(3):904–911. doi: 10.1016/0006-291x(73)91229-1. [DOI] [PubMed] [Google Scholar]
- van der Rest M., Mayne R., Ninomiya Y., Seidah N. G., Chretien M., Olsen B. R. The structure of type IX collagen. J Biol Chem. 1985 Jan 10;260(1):220–225. [PubMed] [Google Scholar]