Abstract
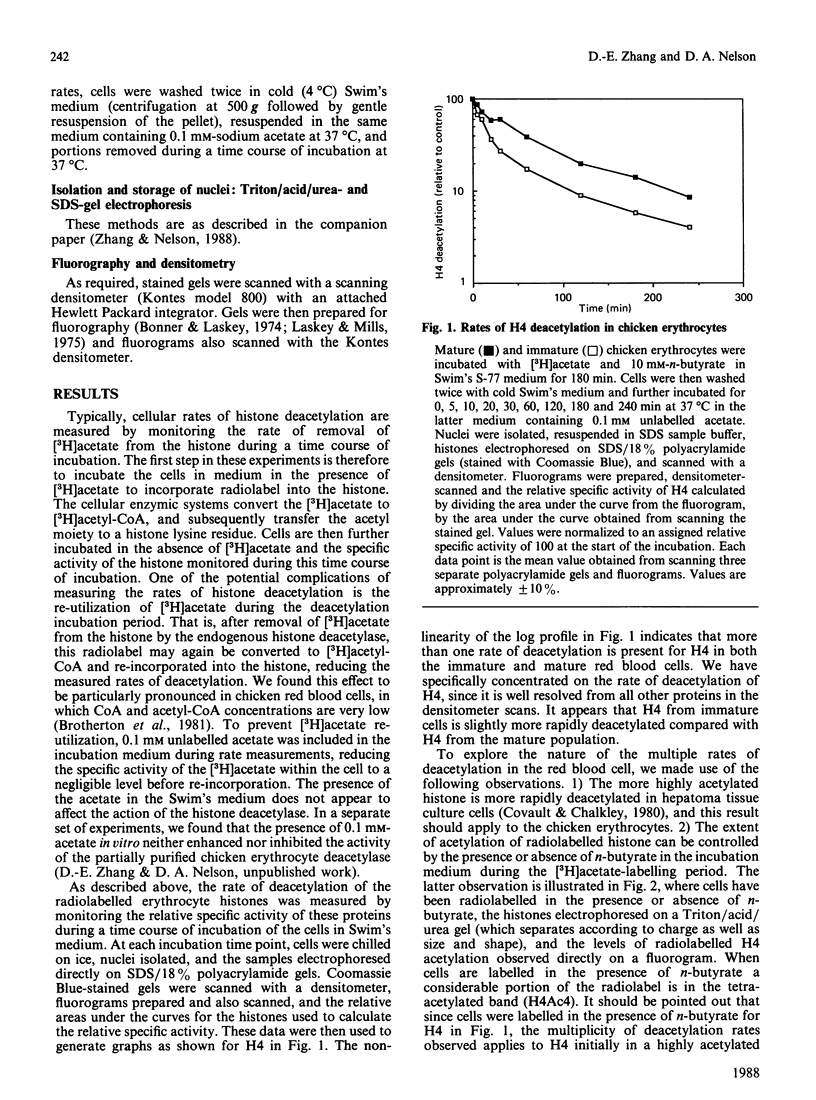
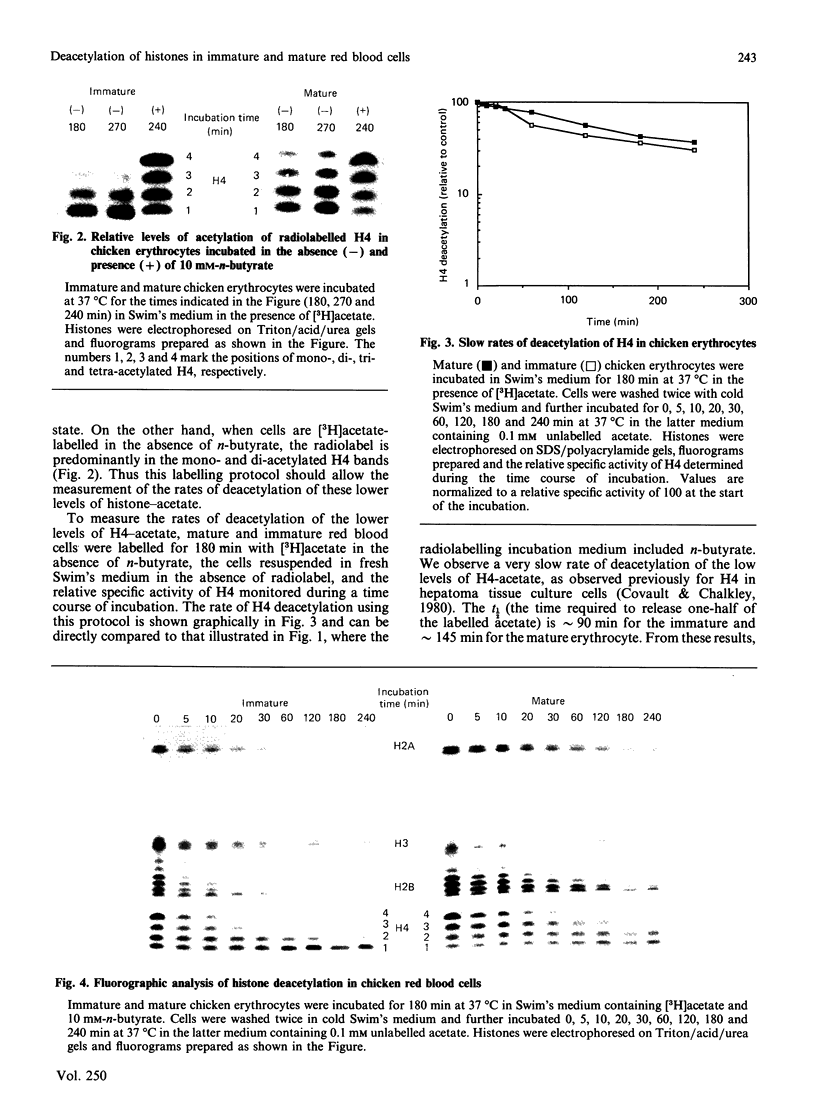
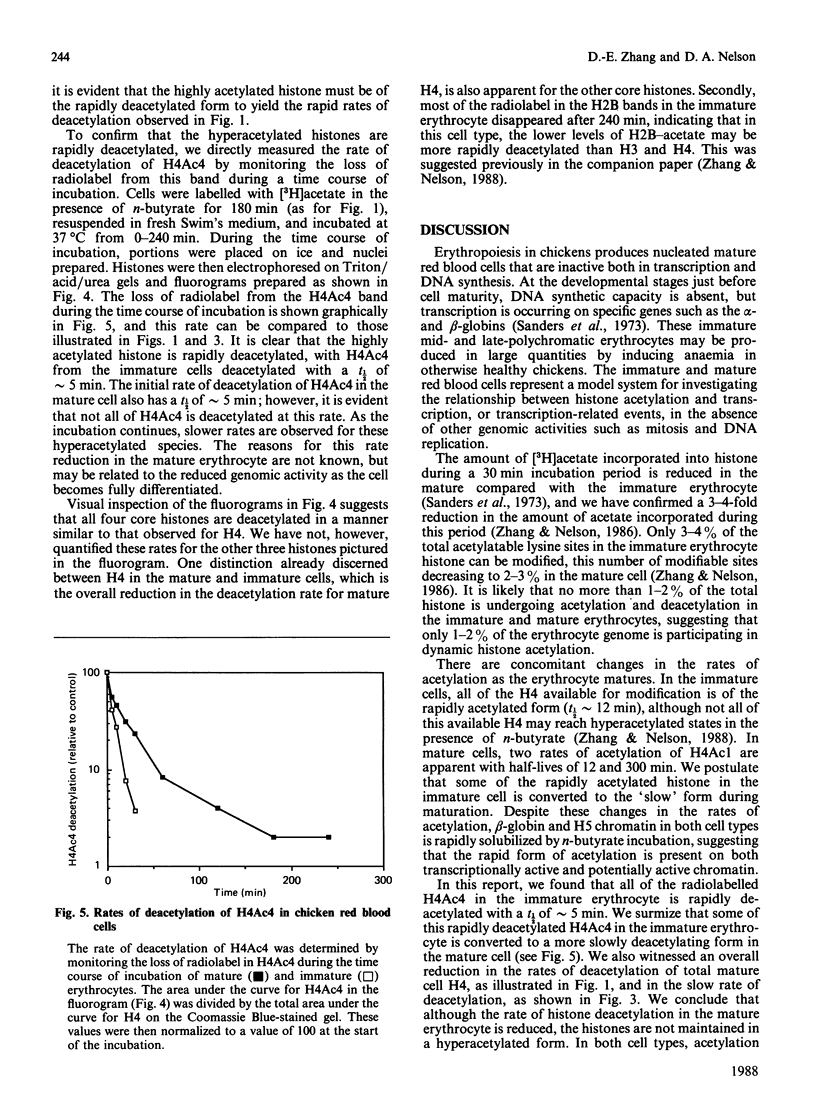
We analysed the rates of histone deacetylation in chicken mature and immature red blood cells. A multiplicity of deacetylation rates was observed for the histones and these rates may be subdivided into two major categories based on the extent of histone acetylation. In one set of experiments, cells were labelled with [3H]acetate in the presence of the deacetylase inhibitor n-butyrate, thereby accumulating radiolabel in the hyperacetylated forms of the histone. These hyperacetylated forms are deacetylated rapidly. [3H]Acetate-labelled tetra-acetylated H4 (H4Ac4) in mature cells was deacetylated with an initial half-life (t1/2) of approximately 5 min (time required for the removal of one-half of the labelled acetyl groups). In immature cells, all [3H]acetate-labelled H4Ac4 was deacetylated with a t1/2 of approximately 5 min. Erythrocytes were also labelled with [3H]acetate for extended periods in the absence of the deacetylase inhibitor. During this period, radiolabel accumulated predominantly in the mono- and di-acetylated forms of the histone. Using this protocol, the rate of deacetylation of H4Ac1 was observed to be approximately 145 min for mature cells, and approximately 90 min for immature cells, demonstrating that the less extensively acetylated histone is deacetylated slowly. These results are discussed in the context of the rates of histone acetylation in chicken red blood cells described in the companion paper [Zhang & Nelson (1988) Biochem. J. 250, 233-240].
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ALLFREY V. G., FAULKNER R., MIRSKY A. E. ACETYLATION AND METHYLATION OF HISTONES AND THEIR POSSIBLE ROLE IN THE REGULATION OF RNA SYNTHESIS. Proc Natl Acad Sci U S A. 1964 May;51:786–794. doi: 10.1073/pnas.51.5.786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Annunziato A. T., Seale R. L. Histone deacetylation is required for the maturation of newly replicated chromatin. J Biol Chem. 1983 Oct 25;258(20):12675–12684. [PubMed] [Google Scholar]
- Bonner W. M., Laskey R. A. A film detection method for tritium-labelled proteins and nucleic acids in polyacrylamide gels. Eur J Biochem. 1974 Jul 1;46(1):83–88. doi: 10.1111/j.1432-1033.1974.tb03599.x. [DOI] [PubMed] [Google Scholar]
- Brotherton T. W., Covault J., Shires A., Chalkley R. Only a small fraction of avian erythrocyte histone is involved in ongoing acetylation. Nucleic Acids Res. 1981 Oct 10;9(19):5061–5073. doi: 10.1093/nar/9.19.5061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Covault J., Chalkley R. The identification of distinct populations of acetylated histone. J Biol Chem. 1980 Oct 10;255(19):9110–9116. [PubMed] [Google Scholar]
- Doenecke D., Gallwitz D. Acetylation of histones in nucleosomes. Mol Cell Biochem. 1982 Apr 30;44(2):113–128. doi: 10.1007/BF00226895. [DOI] [PubMed] [Google Scholar]
- Imai B. S., Yau P., Baldwin J. P., Ibel K., May R. P., Bradbury E. M. Hyperacetylation of core histones does not cause unfolding of nucleosomes. Neutron scatter data accords with disc shape of the nucleosome. J Biol Chem. 1986 Jul 5;261(19):8784–8792. [PubMed] [Google Scholar]
- Laskey R. A., Mills A. D. Quantitative film detection of 3H and 14C in polyacrylamide gels by fluorography. Eur J Biochem. 1975 Aug 15;56(2):335–341. doi: 10.1111/j.1432-1033.1975.tb02238.x. [DOI] [PubMed] [Google Scholar]
- Louie A. J., Dixon G. H. Synthesis, acetylation, and phosphorylation of histone IV and its binding to DNA during spermatogenesis in trout. Proc Natl Acad Sci U S A. 1972 Jul;69(7):1975–1979. doi: 10.1073/pnas.69.7.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGhee J. D., Nickol J. M., Felsenfeld G., Rau D. C. Histone hyperacetylation has little effect on the higher order folding of chromatin. Nucleic Acids Res. 1983 Jun 25;11(12):4065–4075. doi: 10.1093/nar/11.12.4065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson D., Covault J., Chalkley R. Segregation of rapidly acetylated histones into a chromatin fraction released from intact nuclei by the action of micrococcal nuclease. Nucleic Acids Res. 1980 Apr 25;8(8):1745–1763. doi: 10.1093/nar/8.8.1745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reeves R., Candido E. P. Partial inhibition of histone deacetylase in active chromatin by HMG 14 and HMG 17. Nucleic Acids Res. 1980 May 10;8(9):1947–1963. doi: 10.1093/nar/8.9.1947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruiz-Carrillo A., Wangh L. J., Allfrey V. G. Selective synthesis and modification of nuclear proteins during maturation of avian erythroid cells. Arch Biochem Biophys. 1976 May;174(1):273–290. doi: 10.1016/0003-9861(76)90346-5. [DOI] [PubMed] [Google Scholar]
- Sanders L. A., Schechter N. M., McCarty K. S. A comparative study of histone acetylation, histone deacetylation, and ribonucleic acid synthesis in avian reticulocytes and erythrocytes. Biochemistry. 1973 Feb 27;12(5):783–791. doi: 10.1021/bi00729a001. [DOI] [PubMed] [Google Scholar]
- Simpson R. T. Structure of chromatin containing extensively acetylated H3 and H4. Cell. 1978 Apr;13(4):691–699. doi: 10.1016/0092-8674(78)90219-2. [DOI] [PubMed] [Google Scholar]
- Van Holde K. E., Sahasrabuddhe C. G., Shaw B. R. A model for particulate structure in chromatin. Nucleic Acids Res. 1974 Nov;1(11):1579–1586. doi: 10.1093/nar/1.11.1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams A. F. DNA synthesis in purified populations of avian erythroid cells. J Cell Sci. 1972 Jan;10(1):27–46. doi: 10.1242/jcs.10.1.27. [DOI] [PubMed] [Google Scholar]
- Yau P., Thorne A. W., Imai B. S., Matthews H. R., Bradbury E. M. Thermal denaturation studies of acetylated nucleosomes and oligonucleosomes. Eur J Biochem. 1982 Dec 15;129(2):281–288. doi: 10.1111/j.1432-1033.1982.tb07050.x. [DOI] [PubMed] [Google Scholar]
- Zhang D. E., Nelson D. A. Histone acetylation in chicken erythrocytes. Rates of acetylation and evidence that histones in both active and potentially active chromatin are rapidly modified. Biochem J. 1988 Feb 15;250(1):233–240. doi: 10.1042/bj2500233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang D., Nelson D. A. Histone acetylation in chicken erythrocytes. Estimation of the percentage of sites actively modified. Biochem J. 1986 Dec 15;240(3):857–862. doi: 10.1042/bj2400857. [DOI] [PMC free article] [PubMed] [Google Scholar]