Abstract
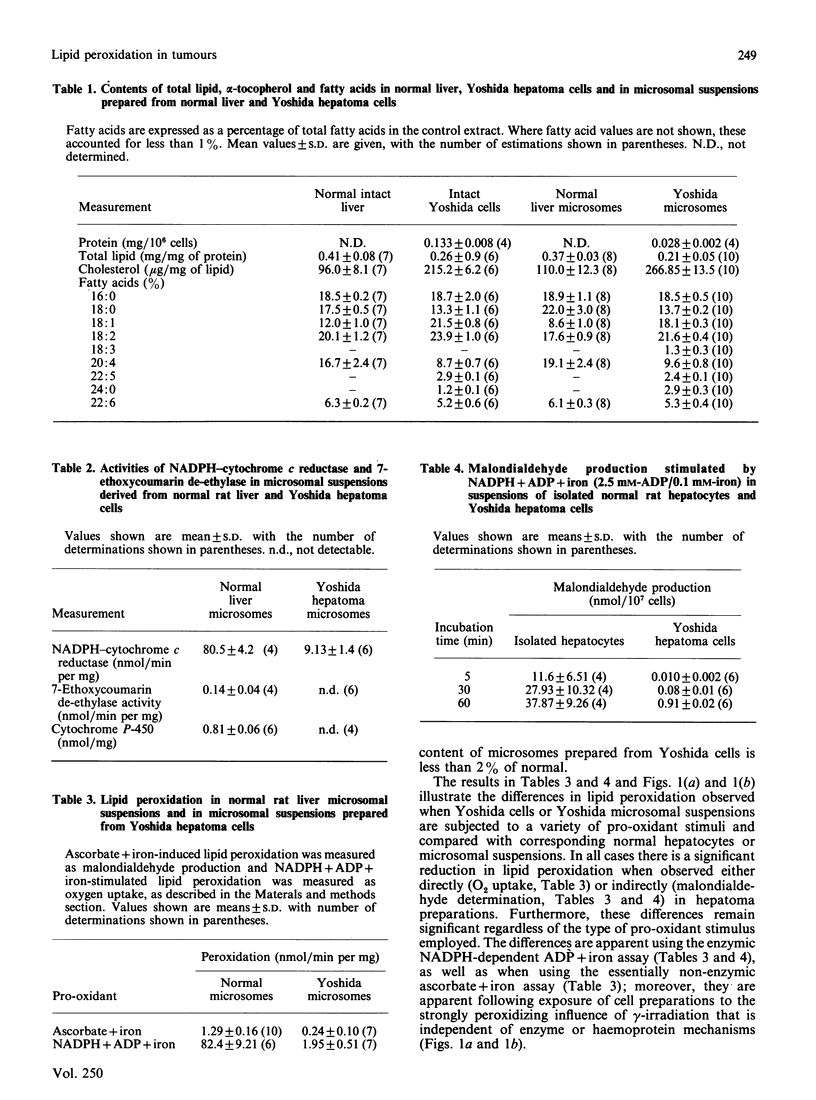
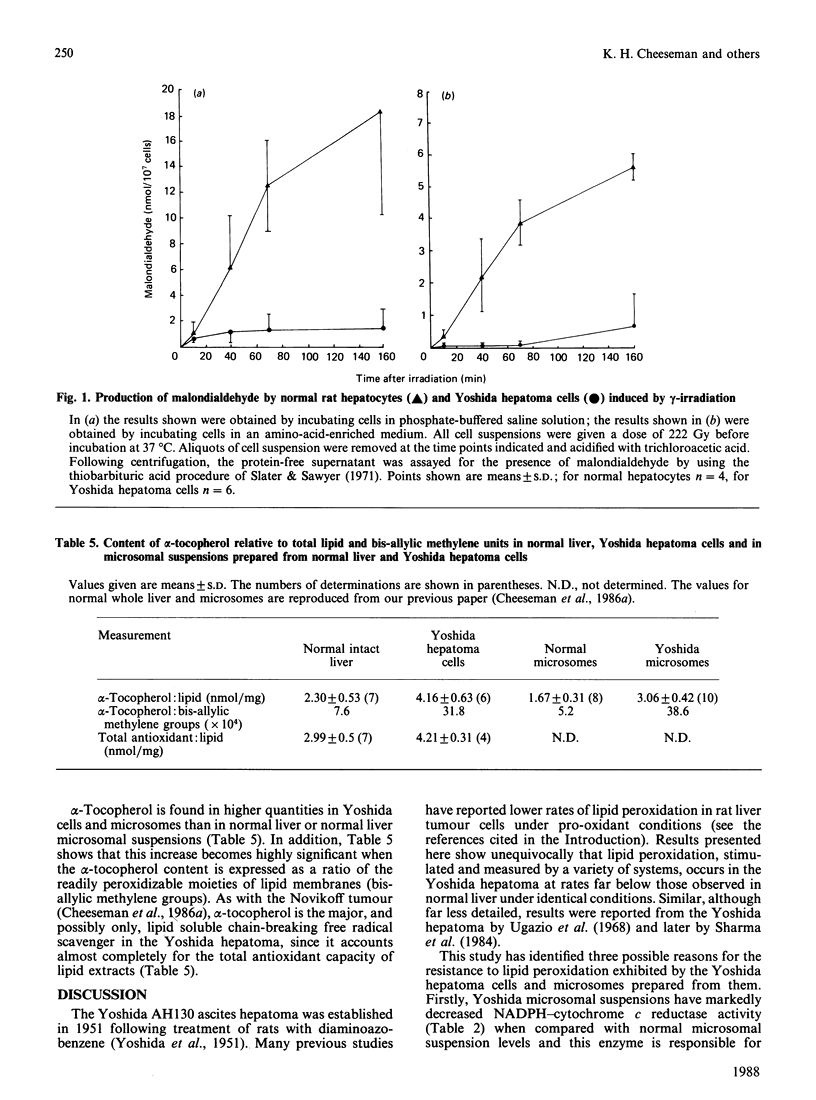
Reduced rates of lipid peroxidation have been observed in Yoshida hepatoma cells and microsomes when compared with appropriate control tissue (normal rat liver) under the same pro-oxidant conditions. The pro-oxidant conditions used were incubation with NADPH+ADP+iron or ascorbate+iron or exposure to gamma-irradiation. As previously shown with the Novikoff hepatoma, the relative concentrations of alpha-tocopherol and polyunsaturated fatty acids are important in conferring resistance to lipid peroxidation in the Yoshida hepatoma. Furthermore, NADPH-cytochrome c reductase and the NADPH-cytochrome P-450 electron transport chain, which are involved in the initiation and propagation of certain types of lipid peroxidation, are found at very much reduced levels in the Yoshida hepatoma. The relative importance of these aberrations are discussed.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bartoli G. M., Galeotti T. Growth-related lipid peroxidation in tumour microsomal membranes and mitochondria. Biochim Biophys Acta. 1979 Sep 28;574(3):537–541. doi: 10.1016/0005-2760(79)90249-2. [DOI] [PubMed] [Google Scholar]
- Benedetto C., Dianzani M. U., Ahmed M., Cheeseman K., Connelly C., Slater T. F. Activation of carbon tetrachloride, and distribution of NADPH-cytochrome c reductase, cytochrome P-450, and other microsomal enzyme activities in rat tissues. Biochim Biophys Acta. 1981 Nov 5;677(3-4):363–372. doi: 10.1016/0304-4165(81)90248-8. [DOI] [PubMed] [Google Scholar]
- Borrello S., Minotti G., Palombini G., Grattagliano A., Galeotti T. Superoxide-dependent lipid peroxidation and vitamin E content of microsomes from hepatomas with different growth rates. Arch Biochem Biophys. 1985 May 1;238(2):588–595. doi: 10.1016/0003-9861(85)90204-8. [DOI] [PubMed] [Google Scholar]
- Burlakova E. B., Molochkina E. M., Pal'mina N. P. Role of membrane lipid oxidation in control of enzymatic activity in normal and cancer cells. Adv Enzyme Regul. 1980;18:163–179. doi: 10.1016/0065-2571(80)90014-x. [DOI] [PubMed] [Google Scholar]
- Burton G. W., Joyce A., Ingold K. U. Is vitamin E the only lipid-soluble, chain-breaking antioxidant in human blood plasma and erythrocyte membranes? Arch Biochem Biophys. 1983 Feb 15;221(1):281–290. doi: 10.1016/0003-9861(83)90145-5. [DOI] [PubMed] [Google Scholar]
- Burton G. W., Webb A., Ingold K. U. A mild, rapid, and efficient method of lipid extraction for use in determining vitamin E/lipid ratios. Lipids. 1985 Jan;20(1):29–39. doi: 10.1007/BF02534359. [DOI] [PubMed] [Google Scholar]
- Cheeseman K. H., Burton G. W., Ingold K. U., Slater T. F. Lipid peroxidation and lipid antioxidants in normal and tumor cells. Toxicol Pathol. 1984;12(3):235–239. doi: 10.1177/019262338401200305. [DOI] [PubMed] [Google Scholar]
- Cheeseman K. H., Collins M., Maddix S., Milia A., Proudfoot K., Slater T. F., Burton G. W., Webb A., Ingold K. U. Lipid peroxidation in regenerating rat liver. FEBS Lett. 1986 Dec 15;209(2):191–196. doi: 10.1016/0014-5793(86)81109-7. [DOI] [PubMed] [Google Scholar]
- Cheeseman K. H., Collins M., Proudfoot K., Slater T. F., Burton G. W., Webb A. C., Ingold K. U. Studies on lipid peroxidation in normal and tumour tissues. The Novikoff rat liver tumour. Biochem J. 1986 Apr 15;235(2):507–514. doi: 10.1042/bj2350507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dianzani M. U., Canuto R. A., Rossi M. A., Poli G., Garcea R., Biocca M. E., Cecchini G., Biasi F., Ferro M., Bassi A. M. Further experiments on lipid peroxidation in transplanted and experimental hepatomas. Toxicol Pathol. 1984;12(2):189–199. doi: 10.1177/019262338401200213. [DOI] [PubMed] [Google Scholar]
- Esterbauer H., Cheeseman K. H., Dianzani M. U., Poli G., Slater T. F. Separation and characterization of the aldehydic products of lipid peroxidation stimulated by ADP-Fe2+ in rat liver microsomes. Biochem J. 1982 Oct 15;208(1):129–140. doi: 10.1042/bj2080129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esterbauer H., Lang J., Zadravec S., Slater T. F. Detection of malonaldehyde by high-performance liquid chromatography. Methods Enzymol. 1984;105:319–328. doi: 10.1016/s0076-6879(84)05041-2. [DOI] [PubMed] [Google Scholar]
- FOLCH J., LEES M., SLOANE STANLEY G. H. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem. 1957 May;226(1):497–509. [PubMed] [Google Scholar]
- Fonnesu A., Del Monte U., Olivotto M. Consumo di ossigeno e perossidazione lipidica delle frazioni citoplasmatiche extramitocondriali difegato e di epatoma-ascite di Yoshida. Sperimentale. 1967 Nov-Dec;116(6):353–372. [PubMed] [Google Scholar]
- Galeotti T., Borrello S., Palombini G., Masotti L., Ferrari M. B., Cavatorta P., Arcioni A., Stremmenos C., Zannoni C. Lipid peroxidation and fluidity of plasma membranes from rat liver and Morris hepatoma 3924A. FEBS Lett. 1984 Apr 24;169(2):169–173. doi: 10.1016/0014-5793(84)80312-9. [DOI] [PubMed] [Google Scholar]
- Gamble W., Vaughan M., Kruth H. S., Avigan J. Procedure for determination of free and total cholesterol in micro- or nanogram amounts suitable for studies with cultured cells. J Lipid Res. 1978 Nov;19(8):1068–1070. [PubMed] [Google Scholar]
- Garner A., Jamal Z., Slater T. F. Effects of 2-mercaptopropionyl glycine on radiation-induced lipid peroxidation in liposomes and in rat liver microsomal suspensions. Int J Radiat Biol Relat Stud Phys Chem Med. 1986 Aug;50(2):323–335. doi: 10.1080/09553008614550701. [DOI] [PubMed] [Google Scholar]
- Gravela E., Feo F., Canuto R. A., Garcea R., Gabriel L. Functional and structural alterations of liver ergastoplasmic membranes during DL-ethionine hepatocarcinogenesis. Cancer Res. 1975 Nov;35(11 Pt 1):3041–3047. [PubMed] [Google Scholar]
- Hammer C. T., Wills E. D. The role of lipid components of the diet in the regulation of the fatty acid composition of the rat liver endoplasmic reticulum and lipid peroxidation. Biochem J. 1978 Aug 15;174(2):585–593. doi: 10.1042/bj1740585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lash E. D. The antioxidant and prooxidant activity in ascites tumors. Arch Biochem Biophys. 1966 Aug;115(2):332–336. doi: 10.1016/0003-9861(66)90283-9. [DOI] [PubMed] [Google Scholar]
- Morisaki N., Lindsey J. A., Stitts J. M., Zhang H., Cornwell D. G. Fatty acid metabolism and cell proliferation. V. Evaluation of pathways for the generation of lipid peroxides. Lipids. 1984 Jun;19(6):381–394. doi: 10.1007/BF02537399. [DOI] [PubMed] [Google Scholar]
- OMURA T., SATO R. A new cytochrome in liver microsomes. J Biol Chem. 1962 Apr;237:1375–1376. [PubMed] [Google Scholar]
- Player T. J., Mills D. J., Horton A. A. Lipid peroxidation of the microsomal fraction and extracted microsomal lipids from DAB-induced hepatomas. Br J Cancer. 1979 Jun;39(6):773–778. doi: 10.1038/bjc.1979.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poli G., Cheeseman K., Slater T. F., Dianzani M. U. The role of lipid peroxidation in CCl4-induced damage to liver microsomal enzymes: comparative studies in vitro using microsomes and isolated liver cells. Chem Biol Interact. 1981 Oct;37(1-2):13–24. doi: 10.1016/0009-2797(81)90162-9. [DOI] [PubMed] [Google Scholar]
- Poli G., Dianzani M. U., Cheeseman K. H., Slater T. F., Lang J., Esterbauer H. Separation and characterization of the aldehydic products of lipid peroxidation stimulated by carbon tetrachloride or ADP-iron in isolated rat hepatocytes and rat liver microsomal suspensions. Biochem J. 1985 Apr 15;227(2):629–638. doi: 10.1042/bj2270629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poli G., Gravela E., Albano E., Dianzani M. U. Studies on fatty liver with isolated hepatocytes. II. The action of carbon tetrachloride on lipid peroxidation, protein, and triglyceride synthesis and secretion. Exp Mol Pathol. 1979 Feb;30(1):116–127. doi: 10.1016/0014-4800(79)90086-8. [DOI] [PubMed] [Google Scholar]
- Prough R. A., Burke M. D., Mayer R. T. Direct fluorometric methods for measuring mixed function oxidase activity. Methods Enzymol. 1978;52:372–377. doi: 10.1016/s0076-6879(78)52041-7. [DOI] [PubMed] [Google Scholar]
- Slater T. F., Cheeseman K. H., Ingold K. U. Carbon tetrachloride toxicity as a model for studying free-radical mediated liver injury. Philos Trans R Soc Lond B Biol Sci. 1985 Dec 17;311(1152):633–645. doi: 10.1098/rstb.1985.0169. [DOI] [PubMed] [Google Scholar]
- Slater T. F., Sawyer B. C. The effects of carbon tetrachloride on rat liver microsomes during the first hour of poisoning in vivo, and the modifying actions of promethazine. Biochem J. 1969 Feb;111(3):317–324. doi: 10.1042/bj1110317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slater T. F. The inhibitory effects in vitro of phenothiazines and other drugs on lipid-peroxidation systems in rat liver microsomes, and their relationship to the liver necrosis produced by carbon tetrachloride. Biochem J. 1968 Jan;106(1):155–160. doi: 10.1042/bj1060155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svingen B. A., Buege J. A., O'Neal F. O., Aust S. D. The mechanism of NADPH-dependent lipid peroxidation. The propagation of lipid peroxidation. J Biol Chem. 1979 Jul 10;254(13):5892–5899. [PubMed] [Google Scholar]
- Ugazio G., Gabriel L., Burdino E. Ricerche sugli inibitori della perossidazione lipidica presenti nelle cellule dell'epatoma ascite di Yoshida. Boll Soc Ital Biol Sper. 1968 Jan 15;44(1):30–34. [PubMed] [Google Scholar]
- Utsumi K., Yamamoto G., Inaba K. Failure of FE2+-induced lipid peroxidation and swelling in the mitochondria isolated from ascites tumor cells. Biochim Biophys Acta. 1965 Aug 24;105(2):368–371. doi: 10.1016/s0926-6593(65)80160-6. [DOI] [PubMed] [Google Scholar]
- White R. E., Coon M. J. Oxygen activation by cytochrome P-450. Annu Rev Biochem. 1980;49:315–356. doi: 10.1146/annurev.bi.49.070180.001531. [DOI] [PubMed] [Google Scholar]


