Abstract
Background:
Some pesticides have been shown to interfere with thyroid functions through changes in thyroid hormone (TH) levels. However, few human studies have explored associations between TH levels and environmental exposure to currently used pesticides, including neonicotinoids, phenylpyrazoles, phenoxy acids, and azoles. Moreover, such studies often measure biomarkers of exposure in urine or blood, and thus reveal only recent exposure. In contrast, hair has been demonstrated to be a suitable matrix for assessing chronic exposure to both persistent and nonpersistent organic pollutants.
Objectives:
We investigated 54 biomarkers of pollutant exposure in relation to tetraiodothyronine (T4), 3,3′,5-triiodothyronine (T3), 3,3′,5′-triiodothyronine (rT3), and 3,3′-diiodothyronine (T2).
Methods:
In a cross-sectional study of 196 healthy Chinese women of reproductive age (25–45 years of age), concentrations of both pollutants and THs were analyzed in the first (starting from the scalp) of the hair matrix, collected in 2016. Associations between pollutants and TH levels were explored using stability-enhanced least absolute shrinkage and selection operator (lasso) by regressing all exposures against each outcome of interest, adjusted for age, body mass index, and city.
Results:
Each TH was associated with the mixture of at least eight of the examined pesticides. We found associations of -HCH, PCP, DMP, DETP, 3Me4NP, carbofuran, , imidacloprid, 2,4-D, metolachlor, difenoconazole, and tebuconazole with THs. For example, a 2-standard deviation (SD) increase in -transformed hair DMP concentration was associated with lower hair T4 concentration [ (95% CI: , )] and higher hair T3 concentration [8.16% (95% CI: 1.73, 15.0%)] in the adjusted unpenalized regression models. We also found associations of some pesticides with T3/T4, rT3/T4, and rT3/T3 molar ratios, including PCP, DMP, 2,4-D, metolachlor, difenoconazole, and tebuconazole.
Discussion:
Our results suggest that exposure to the low levels of pesticides examined here may disrupt thyroid homeostasis in humans. Further studies are needed to confirm our results and to evaluate the long-term consequences of these subtle interferences. https://doi.org/10.1289/EHP14378
Introduction
Thyroid hormones (THs) are essential for human growth, development, metabolism, cardiovascular homeostasis, and the immune system.1 Their circulating levels are strictly regulated via a negative feedback loop along the hypothalamic–pituitary–thyroid (HPT)-axis, involving thyrotropin-releasing hormone and thyroid-stimulating hormone (TSH).2 The thyroid gland excretes tetraiodothyronine (T4) and of the circulating 3,3′,5-triiodothyronine (T3), whereas the remaining T3 is supplied through conversion of T4 by the types 1 and 2 iodothyronine deiodinases (Dio1 and Dio2) in the thyroid and in peripheral tissues, including pituitary, liver, kidney, heart, brain, and brown adipose tissue.3 Meanwhile, T4 can also be converted to inactive 3,3′,5′-triiodothyronine (rT3) through inner-ring deiodination catalyzed by Dio1 and Dio3.3 Moreover, both T3 and rT3 can be further converted to 3,3′-diiodothyronine (T2) through monodeiodination. The thyroid homeostasis can, however, be disrupted by a wide range of synthetic chemicals, including pesticides, which may have adverse consequences for human health, such as neurodevelopmental disorders and cardiovascular disease.1,4
Synthetic pesticides are a diverse group of chemicals used to kill or control pests, including disease-carrying organisms and undesirable insects, animals, and plants.5 Unlike legacy organochlorine pesticides (OCs), currently used pesticides, such as organophosphates (OPs), carbamates, pyrethroids (PYs), neonicotinoids, phenylpyrazoles, phenoxy acids, and azoles, have short biological half-lives and do not accumulate significantly in the human body. Nevertheless, owing to extensive use, the general population is considered to be exposed chronically to low-dose pesticide mixtures from different chemical families.6 Moreover, the European Food Safety Authority has reported that 101 of 287 screened pesticides can affect the thyroid or TH systems via various modes of action, including antagonism of T3 or T4 receptors; alteration in gene expression of proteins/receptors/enzymes involved in TH homeostasis; interference with transthyretin (TTR); inhibition of sodium-iodide symporter (NIS), thyroid peroxidase (TPO), and iodothyronine deiodinases; and induction of liver enzymes.7,8
Animal toxicity studies have well documented the thyroid-disrupting effects of many pesticides, as reviewed by Leemans et al.9 For example, in a study with ewes, exposure to -hexachlorocyclohexane (-HCH), pentachlorophenol (PCP), chlorpyrifos, triallate, dimethoate, or 2,4-dichlorophenoxyacetic acid (2,4-D) significantly reduced serum T4 levels, whereas carbofuran exposure resulted in a marked increase in T4 levels.10 Likewise, changes in TH levels have also been found for pesticide exposure among other animals, including rats, mice, and zebrafish.11–16 The effects of pesticide exposure on TH homeostasis have also been studied in humans, but results are less consistent than in animal studies.4,9,17 Regarding human exposure to legacy OC pesticides, positive associations were observed in Brazilian children and women between serum free T4 and total T3 levels and serum concentrations of several OC pesticides, including -HCH, -HCH, hexachlorobenzene (HCB), -endosulfan, -endosulfan, p,p′-dichlorodiphenyltrichloroethane (DDT), and methoxychlor,18,19 whereas null and inverse associations with serum free T4 levels and total T3 levels have been reported for HCB and -HCH among Canadian Inuit adults20 and pregnant Korean women.21 Similar to the findings with OC pesticides, the associations of pesticides in current use with TH levels have not been conclusive and much less data is available, with almost all such studies focusing on OPs and PYs. Urinary dialkylphosphate (DAP) metabolites of OPs have been associated with increased serum free T4, total T4, and/or free T3 levels, along with decreased serum TSH levels in pregnant Chinese women.22 However, another study among adult men reported an inverse relationship between urinary 3,5,6-trichloro-2-pyridinol (TCPy; a metabolite of chlorpyrifos and chlorpyrifos-methyl) and serum free T4 accompanied by a positive association with TSH.23 Furthermore, the US Agricultural Health Study has linked ever use of diazinon, dichlorvos, and malathion to increased hypothyroidism risk among private pesticide applicators, although not among their female spouses.24–27 Similarly, urinary metabolite measurements were used to explore PY exposure in relation to serum TH levels in pregnant women, adult men and women, and even the general population, including phenoxybenzoic acid (3-PBA), cis-3-(2,2-dicholorvinyl)-2,2-dimethyl-cyclopropane carboxylic acid (cis-), and trans-, and both null and inverse associations have been reported.28–33
However, recent studies have demonstrated that one single urine sample cannot reliably reveal the level of exposure to fast-eliminating chemicals, including currently used pesticides, and increasing the number of urine samples can only slightly improve the accuracy of exposure assessment.34 On the other hand, hair has been proposed as a potential alternative, given that it can cover a wider window of detection and thus is less sensitive to short-term variations in exposure.35 Moreover, because TH secretion shows circadian and ultradian rhythmicity, a single blood sample may not be able to provide reliable TH measurements over long time periods, whereas hair samples can. In fact, hair has been shown to be a suitable matrix for assessing endogenous hormonal homeostasis.36–40 Hair TH levels represent cumulative free circulating TH levels, given that hormones are assumed to enter the hair shaft mainly through active or passive diffusion from the bloodstream.41 However, to our knowledge, no prior human studies have so far used hair analysis to evaluate the effects of pesticide exposure on TH levels. Furthermore, prior studies have focused on only a limited number of individual pesticides, even though humans are simultaneously exposed to multiple classes of pesticides.
In the present study, we aimed to examine hair T4, T3, rT3, and T2 levels in relation to hair concentrations of mixtures of pesticides from several different chemical families among an adult female population. In addition, one polychlorinated biphenyl congener (PCB180) and two bisphenols [bisphenol A (BPA) and bisphenol S (BPS)] were also evaluated in this study owing to their pervasiveness among our study population and their endocrine disrupting properties.
Materials and Methods
Study Population and Hair Collection
The study population has been described previously.38,42–44 Briefly, the present study is part of a multiparametric clinical study conducted in 2016 to examine the impact of chronic pollution exposure on skin, scalp, and hair among a healthy Chinese female population in northern China. To this end, 102 participants per city (204 total) were recruited from the urban areas of Baoding and Dalian. The selection of these two cities was based on their following characteristics: close geographical area (distance between the two cities: ), inhabitants with similar lifestyles, similar climate, same latitude for similar ultraviolet (UV) exposure, and discriminant Air Quality Index levels according to air quality monitoring stations.42 Therefore, the exposure of study participants to UV in their daily lives can be assumed to be comparable, although they were exposed to different levels of pollution. All participants provided information on health status, medical history, and daily habits. They were nonpregnant, healthy women, aged between 25 and 45 years of age, nonsmokers, nondrinkers, had been residing in the current city for at least 15 y with indoor occupations, and had natural hair (color: black) from root to tip with the length . Pregnant women, women who used medications, or had a preexisting skin pathology that could bias the study outcome or endanger the participant’s health were excluded from the study. Here, a nonpregnant participant was defined as a woman who was not pregnant 1 y prior to sampling, and natural hair was defined as the absence of cosmetic treatment (e.g., perm, relaxing, coloring), which was validated by an expert at the time of hair collection. The study was approved by the ethics committee of the Chinese Academy of Inspection and Quarantine Cosmetics Tech Center (protocol no. 2015-033-DY-024) and conducted according to the principles expressed in the World Medical Association’s Declaration of Helsinki. Informed written consent was obtained from all participants prior to any study-related procedure.
Each woman provided a hair sample cut from the posterior vertex region (close to the scalp) of the head using curved stainless-steel scissors cleaned with sodium dodecyl sulfate (SDS), acetone, and methanol in sequence. Each hair sample contained individual hairs and was wrapped in aluminum foil with a cross indicating the root position, placed into a paper envelop, and kept at room temperature until analysis. Only the first (starting from head scalp) were used for the analysis, representing 12 months prior to hair sampling based on the average growth of per month admitted for human hair.45 Because eight participants had insufficient quantity of hair for hormone analysis, only 196 subjects were included in the present study (Table 1).
Table 1.
Characteristics of the study population ().
| Characteristic | (%) | Arithmetic | Median | Range |
|---|---|---|---|---|
| Age (y) | 196 | 34.0 | 25–45 | |
| BMI (kg/m2) | 196 | 22.1 | 16.4–34.3 | |
| Nicotine (ng/mg) | 196 | 0.41 | 0.03–44.1 | |
| City | ||||
| Baoding | 96 (49) | — | — | — |
| Dalian | 100 (51) | — | — | — |
| Education level | ||||
| High school or less | 55 (28) | — | — | — |
| University | 141 (72) | — | — | — |
Note: —, not applicable; BMI, body mass index; SD, standard deviation.
Pollutant and Hormone Analyses
Hair samples (12-cm segments) were analyzed for 153 biomarkers of pollutant exposure, four THs, and nicotine. The analyzed pollutants consisted of 4 PCB congeners (PCB101, 138, 153, and 180), 7 polybrominated diphenyl ether (PBDE) congeners (PBDE28, 33, 47, 99, 100, 153, and 154), 2 bisphenols (BPA and BPS), and 140 pesticides and their metabolites (e.g., OCs, OPs, PYs, carbamates, neonicotinoids, and azole fungicides). The THs analyzed here were T4, T3, rT3, and T2. The nicotine concentration was also analyzed in the hair samples to serve as an indicator of passive smoking. The methods and quality controls for the analysis of these chemicals have been published in detail elsewhere.38,42–44,46 In short, hair samples were successively washed with SDS followed by methanol, to remove any possible external deposit on the surface of hair. As demonstrated previously,47 this washing procedure allowed efficient removal of chemicals deposited on hair surface without removing those incorporated in the bulk matrix, presumably incorporated through biological mechanisms. For PCBs, PBDEs, bisphenols, and pesticides, after extraction overnight at 40°C by of acetonitrile/water (80:20, vol/vol) and centrifugation, the supernatants were divided into three parts for analyses by solid-phase microextraction with gas chromatography–tandem mass spectrometry (SPME-GC-MS/MS), by liquid-injection GC-MS/MS after derivatization with 2,3,4,5,6-pentafluorobenzyl bromide (PFBBr), and by ultra-performance liquid chromatography–MS/MS (UPLC-MS/MS). THs were quantified using UPLC-MS/MS after extracting samples with of methanol for 24 h at 40°C and purifying them through solid-phase extraction (SPE) cartridges. Finally, samples for nicotine analysis were digested in 1 M sodium hydroxide overnight at 40°C, neutralized with 2 M hydrochloric acid and 0.2 M acetate buffer, and further extracted by of cyclohexane/ethyl acetate (50:50, vol/vol); the aqueous layer was subsequently withdrawn, added with carbonate buffer to reach pH 10, and extracted with of ethyl acetate; and the organic layer was used for nicotine quantification by UPLC-MS/MS.
Statistical Analysis
Statistical analyses were performed using R (version 3.6.1; R Core Development Team) and restricted to biomarkers of pollutant exposure with a detection frequency (DF) of . In addition, owing to the large amount of missing data (35%) on acetamiprid caused by coelution with matrix components, we excluded this pesticide from statistical analyses. Hence, 54 pollutants were assessed, namely 1 PCB (PCB180), 2 bisphenols (BPA and BPS), and 51 pesticides, as well as their metabolites (Excel Table S1). We considered hormones and pollutants with DFs of 20%–70% dichotomous variables (not detected vs. detected), whereas those with DFs continuous variables with concentrations below the limit of detection were randomly imputed from a log-normal distribution using maximum likelihood estimation.48 Dichotomous variables included 1 hormone (T2) and 19 pollutants [PCB180, -HCH, -endosulfan, p,p′-dichlorodiphenyldichloroethylene (p,p′-DDE), carbofuran, propoxur, -cyhalothrin, 2-(4-chlorophenyl)-3-methylbutyric acid (2ClBA), thiamethoxam, pyrimethanil, flusilazole, propiconazole, tebuconazole, diflufenican, pyraclostrobin, atrazine, prometryn, terbutryn, and fenuron], whereas continuous variables included 3 hormones (T4, T3, and rT3) and 35 pollutants [BPA, BPS, -HCH, -HCH, -endosulfan, HCB, PCP, dimethyl phosphate (DMP), dimethyl thiophosphate (DMTP), diethylphosphate (DEP), diethylthiophosphate (DETP), TCPy, -nitrophenol (PNP), 3-methyl-4-nitrophenol (3Me4NP), carbendazim, cypermethrin, permethrin, 3-(2-chloro-3,3,3-trifluoro-1-propenyl)-2,2-dimethylcyclopropanecarboxylic acid (), , 3-PBA, imidacloprid, fipronil, fipronil sulfone, 2,4-dichlorophenoxyacetic acid (2,4-D), 4-chloro-2-methylphenoxyacetic acid (MCPA), mecoprop, metolachlor, difenoconazole, prochloraz, thiabendazole, trifluralin, azoxystrobin, prosulfocarb, 1-(3,4-dichlorophenyl)urea (DCPU), and 1-(3,4-dichlorophenyl)-3-methylurea (DCPMU)]. In addition to the 4 THs, we also examined 3 TH molar ratios (T3/T4, rT3/T4, and rT3/T3) in relation to pollutant exposure so as to assess potential effects of pesticide exposure on deiodinase activities (Dio1, Dio2, and Dio3).
Stability-enhanced least absolute shrinkage and selection operator (lasso)–penalized regression (package: sharp in R) was used to identify pollutant mixtures associated with hair THs and TH molar ratios (one model per outcome) because we observed multicollinearity among exposure variables using multiple linear regression models [as indicated by a variance inflation factor (VIF) of ; Excel Table S2)] This lasso modeling technique uses a penalized regression model in combination with resampling techniques to estimate variable selection probabilities. Two hyperparameters, the penalty parameter () and the threshold in selection proportion (), are jointly calibrated via maximizing a score measuring the overall stability of the lasso model.49 Lasso models were fitted on 100 subsamples of 90% of the data owing to the small sample size () of our study population and then adjusted for age (in years, continuous variable), body mass index (BMI; in kilograms per meter squared, continuous variable), and city (dichotomous variable: Baoding and Dalian) by forcing them into the model without inducing penalization. In a lasso model, an exposure variable was considered stably selected if its selection proportion was and the stably selected exposure variables were considered jointly associated with the outcome of interest. The beta coefficient for this exposure variable was obtained by taking the mean nonzero beta across 100 iterations. Lasso models were repeated 1,000 times for each outcome, each time with different seeds, to achieve more stable selection than a single repetition. Average values across the 1,000 repetitions were reported for , , selection proportion, and beta coefficient. To obtain unbiased effect estimates for the subset of pollutants stably selected in the lasso models, multipollutant ordinary least squares (OLS) regression (continuous outcomes) or logistic regression (binary outcomes) models were fitted. In the secondary analyses, all models were rerun by including education (dichotomous variable: university and high school or less) and hair nicotine concentration (in nanograms per milligram, continuous variable) as covariates in addition to age, BMI, and city, which may have resulted in over- or unnecessary adjustment given that all study participants were nonsmokers.
In all models, concentrations of pollutants and THs that were considered continuous variables, as well as nicotine and TH molar ratios, were all -transformed to reach approximate normal distributions. All predictor variables were mean-centered and the continuous ones were further standardized by two times their respective SD.50 To facilitate the interpretation of the results, regression coefficients were expressed as percentage differences in continuous outcomes (T4, T3, rT3, and TH molar ratios) and as odds ratios (ORs) for binary outcomes (T2) associated with detect vs. nondetect (binary pollutant) or with a 2-SD increase in -transformed pollutant concentration (continuous variable; Excel Table S3).
Results
Population Characteristics
Table 1 shows the characteristics of our study population. The average age () of women was , and the average BMI was . Approximately 72% of the women had a college level of education, whereas those remaining had lower levels of education. Nicotine was detected in 100% of hair samples, with concentrations ranging from 0.03 to , even though all participants were nonsmokers.
Associations between Pollutants and THs
In lasso models for T4, T3, rT3, and T2, the calibrated thresholds () were (Excel Table S4). Correspondingly, there were 13, 14, 8, and 11 stably selected pollutants, respectively (Excel Table S5). Regarding each pollutant, although 21 were not stably selected in any of the four lasso models for THs, the remaining 33 were stably selected in one to three models (Excel Table S5).
Regarding hair T4, in the lasso model its concentration was positively related to -endosulfan, tebuconazole, -HCH, PCP, 3Me4NP, , and difenoconazole (), whereas the concentration was inversely related to flusilazole, prometryn, DMP, DETP, metolachlor, and prosulfocarb ( to ; Figure 1A; Excel Table S6). Spearman correlation coefficients between these 13 pollutants ranged from to 0.45 (Figure S1, Excel Table S7). In the corresponding multipollutant OLS model, we found significant associations of T4 with 5 of these 13 stably selected pollutants (i.e., tebuconazole, -HCH, DMP, , and metolachlor); borderline significant associations with -endosulfan, prometryn, PCP, DETP, and prosulfocarb; and no associations with the remaining 3 pollutants, with VIF values of (Figure 1B; Excel Table S8). Specifically, a) women with detectable levels of tebuconazole had 25.7% [95% confidence interval (CI): 7.09, 47.5%]–higher hair T4 levels than their counterparts; b) for every 2-SD increase in -transformed hair -HCH and concentrations (equivalent to 7.36 and increase in their untransformed concentrations, respectively), hair T4 levels were 18.4% (95% CI: 0.05, 40.1%) and 24.9% (95% CI: 6.17, 47.0%) higher, respectively; and c) for every 2-SD increase in -transformed hair DMP and metolachlor concentrations (equivalent to 9.21 and increase in their untransformed concentrations, respectively), hair T4 levels were (95% CI: , ) and (95% CI: , ) lower, respectively.
Figure 1.
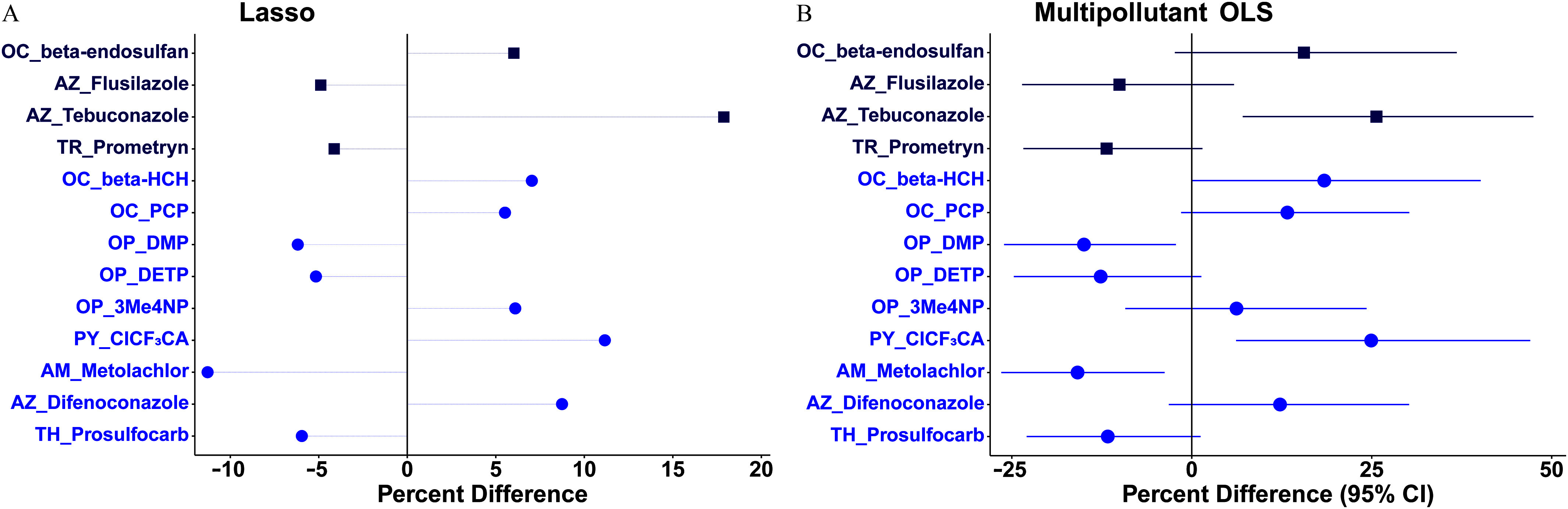
Percentage differences in hair tetraiodothyronine (T4) concentration per 2-SD increase in -transformed pollutant (continuous variable; blue circle symbol) concentration or detect vs. nondetect (binary variable; dark blue square) in (A) the multipollutant lasso model or (B) the subsequent multipollutant OLS model on the pollutants stably selected by the lasso model among 196 healthy Chinese women of reproductive age. Models were adjusted for age, body mass index, and city. Symbols in (B) represent average percentage differences and lines represent 95% CIs. Numerical values are reported in Excel Tables S6 and S8. Note: 3Me4NP, 3-methyl-4-nitrophenol; AM, amide herbicide; AZ, azole fungicide; CI, confidence interval; , 3-(2-chloro-3,3,3-trifluoro-1-propenyl)-2,2-dimethylcyclopropanecarboxylic acid; DETP, diethylthiophosphate; DMP, dimethyl thiophosphate; HCH, hexachlorocyclohexane; Lasso, least absolute shrinkage and selection operator; OC, organochlorine; OLS, ordinary least squares; OP, organophosphate; PCP, pentachlorophenol; PY, pyrethroid; SD, standard deviation; TH, thiocarbamate herbicide; TR, triazine/triazone herbicide.
In the lasso model for hair T3 (Figure 2A; Excel Table S6), positive associations were observed with pyraclostrobin, -HCH, -HCH, PCP, DMP, 3Me4NP, imidacloprid, 2,4-D, and MCPA (–6.89%), whereas inverse associations were observed with -HCH, propoxur, atrazine, , and DCPU ( to ). Spearman correlation coefficients between these 14 stably selected pollutants were in the range of to 0.42 (Figure S1, Excel Table S7). Simultaneously inputting them into an unpenalized regression model (Figure 1B; Excel Table S8), hair T3 concentration was significantly positively associated with -HCH [ (95% CI: 2.34, 19.0%)], -HCH [ (95% CI: 0.37, 16.2%)], PCP [ (95% CI: 0.06, 13.5%)], DMP [ (95% CI: 1.73, 15.0%)], 3Me4NP [ (95% CI: 0.34, 17.4%)], and MCPA [ (95% CI: 2.22, 19.5%)], whereas hair T3 concentration was significantly inversely associated with [ (95% CI: , )]. All VIF values were .
Figure 2.
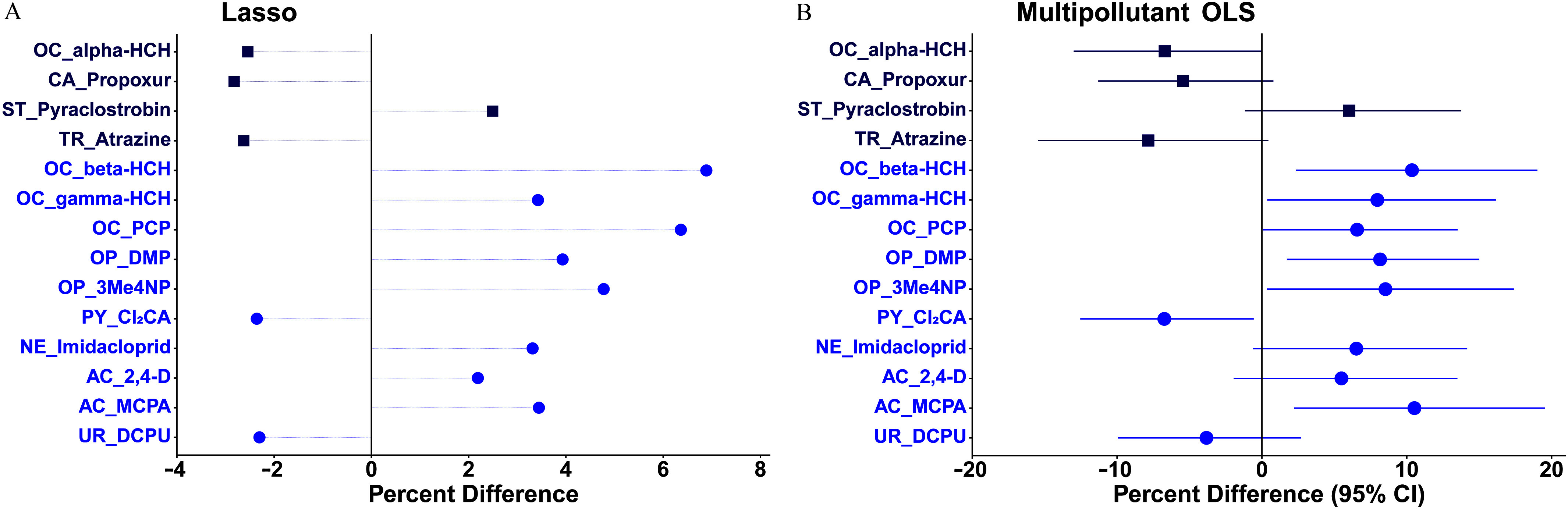
Percentage differences in hair 3,3′,5-triiodothyronine (T3) concentration per 2-SD increase in -transformed pollutant (continuous variable; blue circle symbol) concentration or detect vs. nondetect (binary variable; dark blue square) in (A) the multipollutant lasso model (B) the subsequent multipollutant OLS model on the pollutants stably selected by the lasso model among 196 healthy Chinese women of reproductive age. Models were adjusted for age, body mass index, and city. Symbols in (B) represent average percentage differences and lines represent 95% CIs. Numerical values are reported in Excel Tables S6 and S8. Note: 2,4-D, 2,4-dichlorophenoxyacetic acid; 3Me4NP, 3-methyl-4-nitrophenol; AC, acid herbicide; CA, carbamate; CI, confidence interval; , trans-3-(2,2dichlorovinyl)-2,2-dimethylcyclopropane-carboxylic acid; DCPU, 1-(3,4-dichlorophenyl)urea; DMP, dimethyl phosphate; HCH, hexachlorocyclohexane; Lasso, least absolute shrinkage and selection operator; MCPA, 4-chloro-2-methylphenoxyacetic acid; NE, neonicotinoid; OC, organochlorine; OLS, ordinary least squares; OP, organophosphate; PCP, pentachlorophenol; PY, pyrethroid; SD, standard deviation; ST, strobilurin fungicide; TR, triazine/triazone herbicide; UR, urea herbicide.
Regarding hair rT3 concentration, in the lasso model it was positively related to diflufenican, -HCH, DMTP, carbendazim, and imidacloprid (), but it was inversely related to -HCH, p,p′-DDE, and metolachlor prochloraz ( to ; Figure 3A; Excel Table S6). In the corresponding multipollutant OLS model, there was a significant positive association of hair rT3 with -HCH [ (95% CI: 11.0, 47.6%)] and an inverse association with -HCH [ (95% CI: , )], with VIF values of (Figure 3B; Excel Table S8).
Figure 3.
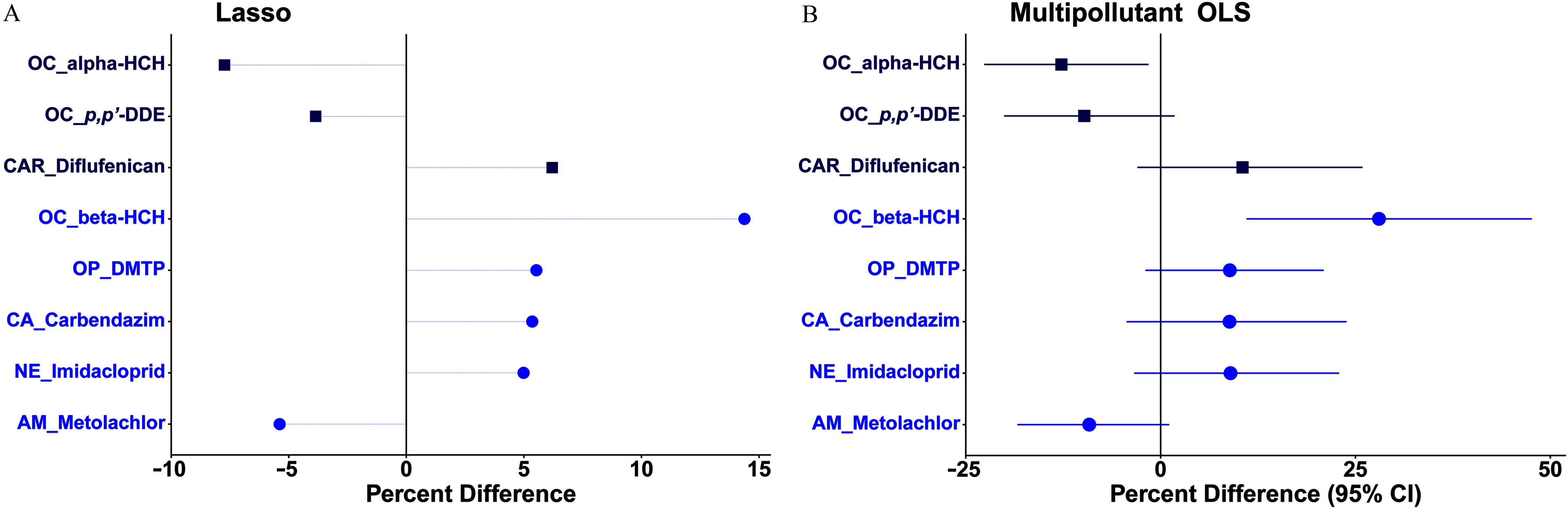
Percentage differences in hair 3,3′,5′-triiodothyronine (rT3) concentration per 2-SD increase in -transformed pollutant (continuous variable; blue circle symbol) concentration or detect vs. nondetect (binary variable; dark blue square) in (A) the multipollutant lasso model or (B) the subsequent multipollutant OLS model on the pollutants stably selected by the lasso model among 196 healthy Chinese women of reproductive age. Models were adjusted for age, body mass index, and city. Symbols in (B) represent average percentage differences and lines represent 95% CIs. Numerical values are reported in Excel Tables S6 and S8. Note: AM, amide herbicide; CA, carbamate; CAR, carboxamide herbicide; CI, confidence interval; DDE, dichlorodiphenyl dichloroethane; DMTP, dimethyl thiophosphate; HCH, hexachlorocyclohexane; Lasso, least absolute shrinkage and selection operator; NE, neonicotinoid; OC, organochlorine; OLS, ordinary least squares; OP, organophosphate; SD, standard deviation.
In the lasso model for T2 (Figure 4A; Excel Table S6), the odds of detecting T2 in hair samples was positively associated with carbofuran, terbutryn, HCB, , and metolachlor () while inversely associated with 2ClBA, thiamethoxam, flusilazole, fipronil, difenoconazole, and DCPU (). Inputting these 11 stably selected pollutants into the multipollutant unpenalized model, there were significant positive associations of T2 with carbofuran, HCB, and () and significant inverse associations with flusilazole and DCPU (), with VIF values of (Figure 4B; Excel Table S8).
Figure 4.
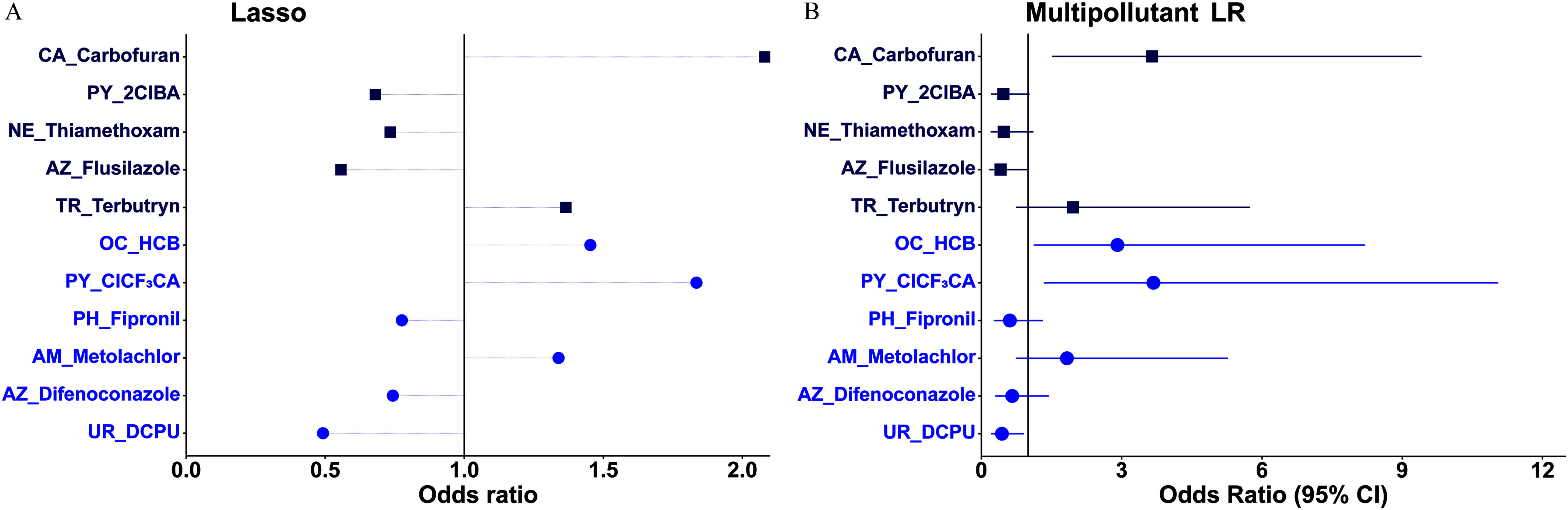
Odds ratios (ORs) of detecting 3,3′-diiodothyronine (T2) in hair with a 2-SD increase in -transformed pollutant (continuous variable; blue circle symbol) concentration or detect vs. nondetect (binary variable; dark blue square) in (A) the multipollutant lasso model or in (B) the subsequent multipollutant LR model on the pollutants stably selected by the lasso model among 196 healthy Chinese women of reproductive age. Models were adjusted for age, body mass index, and city. Symbols in (B) represent average percentage differences and lines represent 95% CIs. Numerical values are reported in Excel Tables S6 and S8. Note: 2ClBA, 2-(4-chlorophenyl)-3-methylbutyric acid; AM, amide herbicide; AZ, azole fungicide; CA, carbamate; CI, confidence interval; , 3-(2-chloro-3,3,3-trifluoro-1-propenyl)-2,2-dimethylcyclopropanecarboxylic acid; DCPU, 1-(3,4-dichlorophenyl)urea; HCB, hexachlorobenzene; Lasso, least absolute shrinkage and selection operator; LR, logistic regression; NE, neonicotinoid; OC, organochlorine; PH, phenylpyrazole insecticide; PY, pyrethroid; SD, standard deviation; TR, triazine/triazone herbicide; UR, urea herbicide.
Associations between Pollutants and TH Molar Ratios
In lasso models, 11, 10, and 11 pollutants were stably selected for T3/T4, rT3/T4, and rT3/T3 ratios, respectively (; Excel Tables S4 and S5). Specifically, hair T3/T4 ratio was positively related to flusilazole, prometryn, DMP, DETP, metolachlor, prosulfocarb, and DCPMU (–11.4%) while inversely related to tebuconazole, atrazine, , and difenoconazole ( to ) (Figure 5A; Excel Table S6), with the associations with flusilazole, tebuconazole, DMP, DETP, , metolachlor, and prosulfocarb being significant or borderline significant in the corresponding multipollutant OLS model (Figure 5B; Excel Table S8). As to rT3/T4 ratio, positive relationships were found with flusilazole, DMP, DETP, prosulfocarb, and DCPMU (–10.4%), whereas inverse relationships were found with -endosulfan, p,p′-DDE, tebuconazole, pyraclostrobin, and PCP ( to ; Figure S2A, Excel Table S6). Four of these stably selected pollutants were significantly related to the rT3/T4 ratio in the corresponding multipollutant OLS model, namely tebuconazole, PCP, DMP, and prosulfocarb (Figure S2B, Excel Table S8). With respect to the 11 pollutants stably selected for rT3/T3 ratio, the effect estimates were positive for BPA, -HCH, DMTP, and carbendazim (), but negative for -HCH, p,p′-DDE, pyraclostrobin, PCP, 2,4-D, MCPA, and metolachlor ( to ; Figure S3A, Excel Table S6), of which eight were significantly or marginally significantly associated with rT3/T3 ratio in the corresponding multipollutant OLS model (Figure S3B, Excel Table S8).
Figure 5.
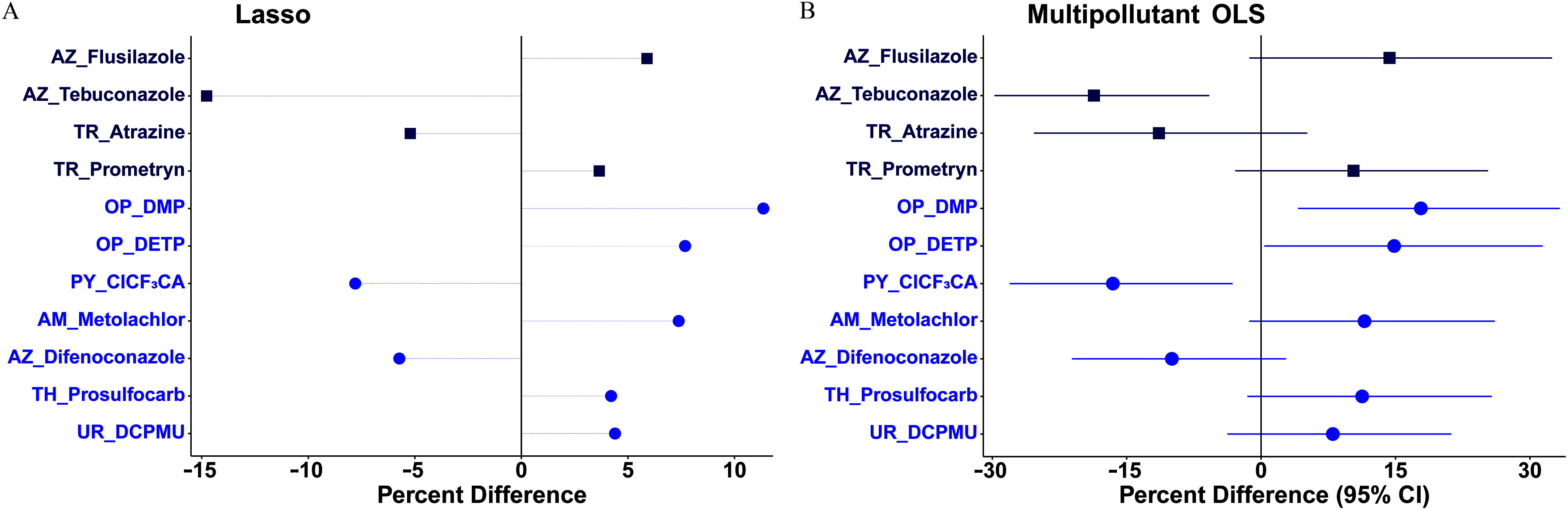
Percentage differences in hair 3,3′,5-triiodothyronine/tetraiodothyronine (T3/T4) molar ratio per 2-SD increase in -transformed pollutant (continuous variable; blue circle symbol) concentration or detect vs. nondetect (binary variable; dark blue square) in (A) the multipollutant lasso model or in (B) the subsequent multipollutant OLS model on the pollutants stably selected by the lasso model among 196 healthy Chinese women of reproductive age. Models were adjusted for age, body mass index, and city. Symbols in (B) represent average percentage differences and lines represent 95% CIs. Numerical values are reported in Excel Tables S6 and S8. Note: AM, amide herbicide; AZ, azole fungicide; CI, confidence interval; , 3-(2-chloro-3,3,3-trifluoro-1-propenyl)-2,2-dimethylcyclopropanecarboxylic acid; DCPMU, 1-(3,4-dichlorophenyl)-3-methylurea; DETP, diethyl-thiophosphate; DMP, dimethyl phosphate; Lasso, least absolute shrinkage and selection operator; OLS, ordinary least squares; OP, organophosphate; PY, pyrethroid; SD, standard deviation; TH, thiocarbamate herbicide; TR, triazine/triazone herbicide; UR, urea herbicide.
Secondary Analyses
When the lasso models were further adjusted for education and nicotine, a) the number of pollutants stably selected remained the same for T4 and increased by one for T3, T2, T3/T4 ratio, and rT3/T4 ratio; by two for rT3/T3 ratio; and by three for rT3 (Excel Table S5); and b) the magnitude of effect estimates also changed slightly (Excel Table S6). Regarding the multipollutant OLS models, with additional adjustment for education and nicotine, a) the number of observed significant associations remained the same for T4, rT3, and T3/T4 ratio; increased by two for T3 and rT3/T4 ratio; and decreased by one for T2 and rT3/T3 ratio; and b) the strength of most associations changed negligibly (Excel Table S8).
Discussion
Using hair analysis, we evaluated whether environmental exposure to pesticide mixtures were associated with TH levels among 196 healthy Chinese women of reproductive age. We found associations of -HCH with T4, T3, and rT3; metolachlor with T4, rT3, and T2; PCP, DMP, and 3Me4NP with T4 and T3; flusilazole, , and difenoconazole with T4 and T2; -HCH and imidacloprid with T3 and rT3; and DCPU with T3 and T2, among others. Moreover, we also found associations of pesticide exposure with T3/T4, rT3/T4, and rT3/T3 molar ratios, including PCP, DMP, , metolachlor, 2,4-D, and tebuconazole. However, we found no associations of PCB180, BPA, and BPS with any of the examined THs or TH molar ratios, except for the one between BPA and rT3/T3 molar ratio. Considering that hair TH levels reflect cumulative free circulating TH levels, our results suggest that chronic environmental exposure to both persistent and nonpersistent pesticides may affect TH levels and TH molar ratios among the general population. TH alterations have been associated with increased mortality risk and with compromised quality of life in the general population.2 To our knowledge, this is the first study using hair analysis to evaluate the effect of environmental exposure to pesticide mixtures on TH homeostasis in humans.
Numerous human studies have examined the associations of circulating TH levels, including free T4, total T4, free T3, and total T3 with OC exposure, and have often reported null or inverse associations.9 Here, neither T4 nor T3 levels were associated with hair HCB concentration, although free T4, free T3, and total T3 have been associated with HCB exposure in newborns from Belgium51 and in pregnant women from Spain.52 Nevertheless, a lack of association between HCB and free T4 or free T3 has been reported in three prior studies of pregnant women, newborns, and/or infants.53–55 Unlike HCB, we observed positive associations of -HCH and PCP with both T4 and T3, -endosulfan with T4, and -HCH with T3, as well as an inverse association between -HCH and T3. These positive associations differ from the findings of most previous studies relating blood OCs to TH levels in adults in which null and/or inverse relationships were reported.19,20,52,56 For example, in a cross-sectional study of 623 Canadian Inuit adults of age, where both OCs and TH parameters were measured in blood, -HCH concentration was inversely related to total T3 and thyroxine-binding globulin levels, whereas no associations were observed between -HCH and free T4 or between PCP and any of the assessed TH parameters.20 Nonetheless, significant positive relationships have been found between free T4 and -HCH in pregnant Canadian women, pregnant Spanish women, and Brazilian women19,52,57; between total T3 and -endosulfan in Brazilian women19; or between total T3 and -HCH, -HCH, and -endosulfan in Brazilian children.18 The mechanisms related to the observed positive associations of PCP with T4 and T3 might involve up-regulation of genes involved in TH synthesis, displacement of T4 from TH-binding proteins (transthyretin and albumin), and/or inhibition of iodothyronine sulfation.4,58–62 Indeed, we found an inverse relationship between hair PCP and rT3/T3 ratios, which is in line with the transcriptional up-regulation of genes encoding Dio 1 and Dio 2 observed in larval and adult zebrafish.61,62 Given that -HCH, PCP, and -HCH were frequently detected in nonoccupationally exposed populations,63–65 our results suggest that the thyroid-disrupting effects related to chronic OC exposure are still relevant for the general population.
Few human studies have explored TH levels in relation to nonpersistent pesticides other than OPs and PYs, and those that did reported inconsistent findings.9,17 Taking into account multiple pesticides simultaneously, here we found associations of DMP and 3Me4NP with both T4 and T3; metolachlor with T4, rT3, and T2; , flusilazole, and difenoconazole with both T4 and T2; imidacloprid with both T3 and rT3; and DCPU with both T3 and T2. Partially in line with our results, in a cross-sectional study of the relationships between OP metabolites in spot urine samples and THs in blood samples collected from 325 pregnant Chinese women during hospital admission for delivery, Wang et al.22 found significant positive relationships between urinary DMP levels and serum free T4 and free T3 levels, along with positive relationships of urinary DEP and DETP levels with serum total T4 levels. In contrast, another study conducted in the Netherlands on 715 pregnant women with gestational age of found no associations between urinary levels of dialkylphosphate metabolites [i.e., DMP, DMTP, DMDTP, DEP, DETP, and diethyldithiophosphate (DEDTP)] and serum free T4, total T4, or TSH levels.66 Such differences in findings among the three studies can be attributed to differences in biological matrixes for analysis of TH levels and biomarkers of OP exposure (urine/blood vs. hair), which revealed different temporal windows of detection (recent vs. chronic exposure), study population characteristics (e.g., metabolism of chemicals and baseline hormone levels during pregnancy compared with outside of pregnancy), regression approaches (single-pollutant vs. multipollutant), covariate adjustment, and so on. The associations of metolachlor with TH levels agree partly with the findings of an occupational study that found no associations between metolachlor use and TSH, T4, or T3 among male pesticide applicators.67 However, metolachlor use has been associated with decreased hypothyroidism risk and increased hyperthyroidism risk among female spouses of private pesticide applicators,26 although null associations were often reported in the US Agricultural Health Study.24,25,27
Similar to human studies, in animal studies there is also limited data on altered TH levels resulting from exposure to these pesticides or those from the same chemical families. The inverse relations of DMP and DETP to hair T4 concentration are in accordance with animal studies of malathion exposure among male rats and chlorpyrifos exposure among ewes and pregnant mice, where significant decreases in serum T4 levels were observed.10,11,68 Similarly, the positive relation of DMP to hair T3 concentration is corroborated by an experimental study of female rats in which prenatal coupled with postnatal chlorpyrifos exposure caused significantly elevated serum T3 levels,68 although another study found no effect of chlorpyrifos-methyl exposure on serum T3 levels in pregnant rats.13 Likewise, the inverse association of metolachlor with T4 but not with T3 are in partial agreement with animal studies that found no effects of metolachlor exposure on serum T4, T3, TSH, thyroid histopathology, or T4–uridine-5′-diphospho-glucuronosyltransferase activity in male rats but, rather, an association with increased total T4 levels in female rats.69,70 However, in these two studies, high doses of metolachlor were applied (150 and , respectively). The positive association between imidacloprid and T3 levels is in line with a study of wild male finches that found a significant increase in plasma T3 levels following 30 d of exposure to body weight of imidacloprid during the preparatory phase.71 However, this study also reported significantly reduced plasma T3 levels during the breeding phase and significantly reduced plasma T4 levels during both preparatory and breeding phases. Based on these conflicting findings, the associations between TH levels and exposure to pesticides in current use need to be investigated in other epidemiological studies.
Some nonpersistent pesticides were associated only with single THs, including tebuconazole with T4, 2,4-D with T3, carbendazim with rT3, and fipronil with T2. However, to our knowledge, no human studies have so far examined TH levels in relation to these pesticides except for 2,4-D and fipronil. In contrast with our results, 2,4-D use was associated with elevated hypothyroidism risk among private pesticide applicators.25,27 Moreover, animal studies of rats and ewes found significantly reduced serum T4 levels following 2,4-D exposure, which was partly caused by the competition for binding proteins and/or direct effects on the thyroid.10,72 As to fipronil, the absence of associations with T4 and T3 has also been found in an occupational study of workers from a factory manufacturing fipronil-containing veterinary drugs.73 In addition, these results agree partly with a study of Korean newborns that found no associations of prenatal exposure to fipronil sulfone with free T4 and total T4 levels but inverse associations with free T3 and total T3 levels.74
Unlike OPs, among the seven examined PYs (-cyhalothrin, 2ClBA, permethrin, cypermethrin, , , and 3-PBA), we only found inverse associations of 2ClBA with T2 and of with T3 and positive associations of with T4 and T2. The lack of associations between PYs and hair T4 or T3 levels together with the inverse association between and T3 are in partial agreement with prior studies that found null or inverse relationships of urinary 3-PBA, cis-, and trans- with serum free T4, total T4, free T3, and/or total T3 levels in adult men, pregnant women, or general populations.28–33 In addition, the US Agricultural Health Study has associated permethrin use with elevated hypothyroidism risk among private pesticide applicators and their female spouses.26,27 In support of the inverse associations found in the present study, in vitro studies have shown that PYs, including cyfluthrin, cyhalothrin, cypermethrin, deltamethrin, fenvalerate, permethrin, tetramethrin, and 3-PBA displayed antagonistic effects on a TH receptor in a reporter gene assay, whereas tau-fluvalinate and cypermethrin also inhibited the T3-induced GH3 cell proliferation.75,76 These results are also partly corroborated by animal studies of bifenthrin, -cyhalothrin, fenvalerate, and deltamethrin exposure among rats or mice, where reduced serum TH levels were observed.11,12,15,77,78 For example, fenvalerate exposure among rats significantly inhibited hepatic D1 activity mediated by lipid peroxidation and resulted in a significant decrease in serum T4 and T3 levels.12 However, in another study, fenvalerate was found to elevate serum total T4 and T3 levels in rats.79 Nevertheless, here we also found positive relationships of with T4 and T2 in multipollutant models.
We observed discordance between associations of different TH levels with a specific pollutant and between associations of the same TH with different metabolites from the same chemical families (e.g., OP and PY). We could not clarify the exact reason for these results; however, one possible explanation could be moderate correlations generally observed between TH levels, between OP metabolites and between PY metabolites. In addition, different molecules may impact TH levels through different modes of action,4 such as interference with TH synthesis, secretion, transport, and/or metabolism, which could lead to inconsistent associations with the same TH.
Strengths of this study included the use of hair analysis to analyze both pollutants and hormones that, to our knowledge, has seldom been applied in previous epidemiological studies. Given that hair measurements in the present study corresponded to average levels over the year before sampling,45 our results reflect long-term effects of pollutant exposure on TH levels, differing from prior studies on biological fluids providing information on the short-term regarding concentrations of THs and pesticides in current use. Correspondingly, we were not able to distinguish chronic low-level exposure from short-term intensive ones; however, this was beyond the scope of the present study. Another strength was the homogeneity of our study population in terms of gender and age, which likely lowered the confounding effects of such factors on associations observed here. Our study also had some potential limitations. First, causality could not be inferred because of the cross-sectional study design. Second, because lasso tends to select only one variable from a group of highly correlated variables, there might be stably selected pesticides reflective of others. Nevertheless, Spearman correlation coefficients or were observed only between propiconazole and diflufenican (0.69), -endosulfan, and trifluralin (0.70); DETP and TCPy (0.66); PNP and 3Me4NP (0.72); and MCPA and mecoprop (0.80). Third, we did not have information on iodine, which is crucial for the TH synthesis. Nevertheless, Alvarez-Pedrerol et al.52 found no effects of iodine intake and urinary iodine concentrations on associations between OCs and TH levels in pregnant Spanish women. Iodine is therefore unlikely to have been a major confounder in our study. Last, residual confounding by diet may have been possible given that associations have been well established between numerous dietary factors and both pollutant exposure and TH levels.80,81 However, because the hair levels of the examined pesticides in our study population are comparable to those in pregnant French women, French children, and the general Luxembourgish population,63–65 the associations noted in the present study warrant further investigations in longitudinal studies.
In conclusion, to our knowledge, this represents the first study based on hair analysis exploring the effect of pesticide mixtures on TH levels among an environmentally exposed adult female population. We found associations of PCP, -HCH, DMP, and 3Me4NP with both hair T4 and T3 levels, DETP, , metolachlor, difenoconazole, and tebuconazole with T4, , imidacloprid, and 2,4-D with T3, among others. In addition, we also found several associations between pesticides and rT3 and T2. These results are not fully consistent with prior human studies based on blood and/or urine matrixes and should thus be confirmed in future epidemiological studies.
Supplementary Material
Conclusions and opinions are those of the individual authors and do not necessarily reflect the policies or views of EHP Publishing or the National Institute of Environmental Health Sciences.
References
- 1.Oliveira KJ, Chiamolera MI, Giannocco G, Pazos-Moura CC, Ortiga-Carvalho TM. 2019. Thyroid function disruptors: from nature to chemicals. J Mol Endocrinol 62(1):R1–R19, PMID: 30006341, 10.1530/JME-18-0081. [DOI] [PubMed] [Google Scholar]
- 2.Gauthier BR, Sola-García A, Cáliz-Molina MÁ, Lorenzo PI, Cobo-Vuilleumier N, Capilla-González V, et al. 2020. Thyroid hormones in diabetes, cancer, and aging. Aging Cell 19(11):e13260, PMID: 33048427, 10.1111/acel.13260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bianco AC, Salvatore D, Gereben B, Berry MJ, Larsen PR. 2002. Biochemistry, cellular and molecular biology, and physiological roles of the iodothyronine selenodeiodinases. Endocr Rev 23(1):38–89, PMID: 11844744, 10.1210/edrv.23.1.0455. [DOI] [PubMed] [Google Scholar]
- 4.Hernández AF, Bennekou SH, Hart A, Mohimont L, Wolterink G. 2020. Mechanisms underlying disruptive effects of pesticides on the thyroid function. Curr Opin Toxicol 19:34–41, 10.1016/j.cotox.2019.10.003. [DOI] [Google Scholar]
- 5.EFSA (European Food Safety Authority). https://www.efsa.europa.eu/en/glossary-taxonomy-terms/p#glossary-term-513 [accessed 13 June 2023].
- 6.Hernández AF, Gil F, Lacasaña M. 2017. Toxicological interactions of pesticide mixtures: an update. Arch Toxicol 91(10):3211–3223, PMID: 28845507, 10.1007/s00204-017-2043-5. [DOI] [PubMed] [Google Scholar]
- 7.EFSA Panel on Plant Protection Products and their Residues. 2013. Scientific Opinion on the identification of pesticides to be included in cumulative assessment groups on the basis of their toxicological profile. EFSA J 11(7):3293, 10.2903/j.efsa.2013.3293. [DOI] [Google Scholar]
- 8.EFSA, Crivellente F, Hart A, Hernandez-Jerez AF, Hougaard Bennekou S, Pedersen R, et al. 2019. Establishment of cumulative assessment groups of pesticides for their effects on the thyroid. EFSA J 17(9):e05801, PMID: 32626429, 10.2903/j.efsa.2019.5801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Leemans M, Couderq S, Demeneix B, Fini J-B. 2019. Pesticides with potential thyroid hormone-disrupting effects: a review of recent data. Front Endocrinol (Lausanne) 10:743, PMID: 31920955, 10.3389/fendo.2019.00743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rawlings NC, Cook SJ, Waldbillig D. 1998. Effects of the pesticides carbofuran, chlorpyrifos, dimethoate, lindane, triallate, trifluralin, 2,4-D, and pentachlorophenol on the metabolic endocrine and reproductive endocrine system in ewes. J Toxicol Environ Health A 54(1):21–36, PMID: 9588346, 10.1080/009841098159006. [DOI] [PubMed] [Google Scholar]
- 11.Akhtar N, Kayani SA, Ahmad MM, Shahab M. 1996. Insecticide-induced changes in secretory activity of the thyroid gland in rats. J Appl Toxicol 16(5):397–400, PMID: 8889791, . [DOI] [PubMed] [Google Scholar]
- 12.Maiti PK, Kar A. 1998. Is triiodothyronine capable of ameliorating pyrethroid-induced thyroid dysfunction and lipid peroxidation? J Appl Toxicol 18(2):125–128, PMID: 9570695, . [DOI] [PubMed] [Google Scholar]
- 13.Jeong S-H, Kim B-Y, Kang H-G, Ku H-O, Cho J-H. 2006. Effect of chlorpyrifos-methyl on steroid and thyroid hormones in rat F0- and F1-generations. Toxicology 220(2–3):189–202, PMID: 16472551, 10.1016/j.tox.2006.01.005. [DOI] [PubMed] [Google Scholar]
- 14.Yu L, Chen M, Liu Y, Gui W, Zhu G. 2013. Thyroid endocrine disruption in zebrafish larvae following exposure to hexaconazole and tebuconazole. Aquat Toxicol 138–139:35–42, PMID: 23685399, 10.1016/j.aquatox.2013.04.001. [DOI] [PubMed] [Google Scholar]
- 15.Sekeroglu V, Sekeroglu ZA, Demirhan E. 2014. Effects of commercial formulations of deltamethrin and/or thiacloprid on thyroid hormone levels in rat serum. Toxicol Ind Health 30(1):40–46, PMID: 22677783, 10.1177/0748233712448114. [DOI] [PubMed] [Google Scholar]
- 16.Liang X, Yu L, Gui W, Zhu G. 2015. Exposure to difenoconazole causes changes of thyroid hormone and gene expression levels in zebrafish larvae. Environ Toxicol Pharmacol 40(3):983–987, PMID: 26590868, 10.1016/j.etap.2015.10.005. [DOI] [PubMed] [Google Scholar]
- 17.Campos É, Freire C. 2016. Exposure to non-persistent pesticides and thyroid function: a systematic review of epidemiological evidence. Int J Hyg Environ Health 219(6):481–497, PMID: 27265299, 10.1016/j.ijheh.2016.05.006. [DOI] [PubMed] [Google Scholar]
- 18.Freire C, Koifman RJ, Sarcinelli P, Rosa AC, Clapauch R, Koifman S. 2012. Long term exposure to organochlorine pesticides and thyroid function in children from Cidade dos Meninos, Rio de Janeiro, Brazil. Environ Res 117:68–74, PMID: 22776325, 10.1016/j.envres.2012.06.009. [DOI] [PubMed] [Google Scholar]
- 19.Freire C, Koifman RJ, Sarcinelli PN, Simões Rosa AC, Clapauch R, Koifman S. 2013. Long-term exposure to organochlorine pesticides and thyroid status in adults in a heavily contaminated area in Brazil. Environ Res 127:7–15, PMID: 24183346, 10.1016/j.envres.2013.09.001. [DOI] [PubMed] [Google Scholar]
- 20.Dallaire R, Dewailly E, Pereg D, Dery S, Ayotte P. 2009. Thyroid function and plasma concentrations of polyhalogenated compounds in Inuit adults. Environ Health Perspect 117(9):1380–1386, PMID: 19750101, 10.1289/ehp.0900633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kim S, Park J, Kim H-J, Lee JJ, Choi G, Choi S, et al. 2013. Association between several persistent organic pollutants and thyroid hormone levels in serum among the pregnant women of Korea. Environ Int 59:442–448, PMID: 23928038, 10.1016/j.envint.2013.07.009. [DOI] [PubMed] [Google Scholar]
- 22.Wang Y, Chen L, Wang C, Hum Y, Gao Y, Zhou Y, et al. 2017. Association between organophosphate pesticide exposure and thyroid hormones in pregnant women. Epidemiology 28(suppl 1):S35–S40, PMID: 29028673, 10.1097/EDE.0000000000000721. [DOI] [PubMed] [Google Scholar]
- 23.Meeker JD, Barr DB, Hauser R. 2006. Thyroid hormones in relation to urinary metabolites of non-persistent insecticides in men of reproductive age. Reprod Toxicol 22(3):437–442, PMID: 16584866, 10.1016/j.reprotox.2006.02.005. [DOI] [PubMed] [Google Scholar]
- 24.Goldner WS, Sandler DP, Yu F, Hoppin JA, Kamel F, LeVan TD. 2010. Pesticide use and thyroid disease among women in the Agricultural Health Study. Am J Epidemiol 171(4):455–464, PMID: 20061368, 10.1093/aje/kwp404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Goldner WS, Sandler DP, Yu F, Shostrom V, Hoppin JA, Kamel F, et al. 2013. Hypothyroidism and pesticide use among male private pesticide applicators in the Agricultural Health Study. J Occup Environ Med 55(10):1171–1178, PMID: 24064777, 10.1097/JOM.0b013e31829b290b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shrestha S, Parks CG, Goldner WS, Kamel F, Umbach DM, Ward MH, et al. 2018. Incident thyroid disease in female spouses of private pesticide applicators. Environ Int 118:282–292, PMID: 29908479, 10.1016/j.envint.2018.05.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shrestha S, Parks CG, Goldner WS, Kamel F, Umbach DM, Ward MH, et al. 2018. Pesticide use and incident hypothyroidism in pesticide applicators in the Agricultural Health Study. Environ Health Perspect 126(9):097008, PMID: 30256155, 10.1289/EHP3194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Meeker JD, Barr DB, Hauser R. 2009. Pyrethroid insecticide metabolites are associated with serum hormone levels in adult men. Reprod Toxicol 27(2):155–160, PMID: 19429394, 10.1016/j.reprotox.2008.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhang J, Hisada A, Yoshinaga J, Shiraishi H, Shimodaira K, Okai T, et al. 2013. Exposure to pyrethroids insecticides and serum levels of thyroid-related measures in pregnant women. Environ Res 127:16–21, PMID: 24210131, 10.1016/j.envres.2013.10.001. [DOI] [PubMed] [Google Scholar]
- 30.Yoshinaga J, Imai K, Shiraishi H, Nozawa S, Yoshiike M, Mieno MN, et al. 2014. Pyrethroid insecticide exposure and reproductive hormone levels in healthy Japanese male subjects. Andrology 2(3):416–420, PMID: 24634311, 10.1111/j.2047-2927.2014.00202.x. [DOI] [PubMed] [Google Scholar]
- 31.Jain RB. 2016. Variability in the levels of 3-phenoxybenzoic acid by age, gender, and race/ethnicity for the period of 2001–2002 versus 2009–2010 and its association with thyroid function among general US population. Environ Sci Pollut Res Int 23(7):6934–6939, PMID: 26676543, 10.1007/s11356-015-5954-9. [DOI] [PubMed] [Google Scholar]
- 32.Hu Y, Zhang Z, Qin K, Zhang Y, Pan R, Wang Y, et al. 2019. Environmental pyrethroid exposure and thyroid hormones of pregnant women in Shandong, China. Chemosphere 234:815–821, PMID: 31247491, 10.1016/j.chemosphere.2019.06.098. [DOI] [PubMed] [Google Scholar]
- 33.Hwang M, Lee Y, Choi K, Park C. 2019. Urinary 3-phenoxybenzoic acid levels and the association with thyroid hormones in adults: Korean National Environmental Health Survey 2012–2014. Sci Total Environ 696:133920, PMID: 31446285, 10.1016/j.scitotenv.2019.133920. [DOI] [PubMed] [Google Scholar]
- 34.Faÿs F, Palazzi P, Hardy EM, Schaeffer C, Phillipat C, Zeimet E, et al. 2020. Is there an optimal sampling time and number of samples for assessing exposure to fast elimination endocrine disruptors with urinary biomarkers? Sci Total Environ 747:141185, PMID: 32771784, 10.1016/j.scitotenv.2020.141185. [DOI] [PubMed] [Google Scholar]
- 35.Fäys F, Hardy EM, Palazzi P, Haan S, Beausoleil C, Appenzeller BMR. 2021. Biomonitoring of fast-elimination endocrine disruptors—results from a 6-month follow up on human volunteers with repeated urine and hair collection. Sci Total Environ 778:146330, PMID: 34030378, 10.1016/j.scitotenv.2021.146330. [DOI] [PubMed] [Google Scholar]
- 36.Stalder T, Steudte-Schmiedgen S, Alexander N, Klucken T, Vater A, Wichmann S, et al. 2017. Stress-related and basic determinants of hair cortisol in humans: a meta-analysis. Psychoneuroendocrinology 77:261–274, PMID: 28135674, 10.1016/j.psyneuen.2016.12.017. [DOI] [PubMed] [Google Scholar]
- 37.Gao W, Penz M, Wekenborg M, Walther A, Kirschbaum C. 2019. Determination of thyroid hormones in human hair with online SPE LC-MS/MS: analytical protocol and application in study of burnout. Psychoneuroendocrinology 106:129–137, PMID: 30978532, 10.1016/j.psyneuen.2019.03.032. [DOI] [PubMed] [Google Scholar]
- 38.Peng F-J, Palazzi P, Mezzache S, Bourokba N, Soeur J, Appenzeller BMR. 2022. Profiling steroid and thyroid hormones with hair analysis in a cohort of women aged 25 to 45 years old. Eur J Endocrinol 186(5):K9–K15, PMID: 35192511, 10.1530/EJE-22-0081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Peng F-J, Palazzi P, Viguié C, Appenzeller BMR. 2022. Hormonal profile changes induced by pesticide mixture exposure in female rats revealed by hair analysis. Chemosphere 303(pt 2):135059, PMID: 35643162, 10.1016/j.chemosphere.2022.135059. [DOI] [PubMed] [Google Scholar]
- 40.Peng F-J, Palazzi P, Viguié C, Appenzeller BMR. 2022. Measurement of hair thyroid and steroid hormone concentrations in the rat evidence endocrine disrupting potential of a low dose mixture of polycyclic aromatic hydrocarbons. Environ Pollut 313:120179, PMID: 36116566, 10.1016/j.envpol.2022.120179. [DOI] [PubMed] [Google Scholar]
- 41.Pragst F, Balikova MA. 2006. State of the art in hair analysis for detection of drug and alcohol abuse. Clin Chim Acta 370(1–2):17–49, PMID: 16624267, 10.1016/j.cca.2006.02.019. [DOI] [PubMed] [Google Scholar]
- 42.Palazzi P, Mezzache S, Bourokba N, Hardy EM, Schritz A, Bastien P, et al. 2018. Exposure to polycyclic aromatic hydrocarbons in women living in the Chinese cities of BaoDing and Dalian revealed by hair analysis. Environ Int 121(pt 2):1341–1354, PMID: 30420128, 10.1016/j.envint.2018.10.056. [DOI] [PubMed] [Google Scholar]
- 43.Peng F-J, Hardy EM, Mezzache S, Bourokba N, Palazzi P, Stojiljkovic N, et al. 2020. Exposure to multiclass pesticides among female adult population in two Chinese cities revealed by hair analysis. Environ Int 138:105633, PMID: 32179318, 10.1016/j.envint.2020.105633. [DOI] [PubMed] [Google Scholar]
- 44.Peng F-J, Hardy EM, Béranger R, Mezzache S, Bourokba N, Bastien P, et al. 2020. Human exposure to PCBs, PBDEs and bisphenols revealed by hair analysis: a comparison between two adult female populations in China and France. Environ Pollut 267:115425, PMID: 32882460, 10.1016/j.envpol.2020.115425. [DOI] [PubMed] [Google Scholar]
- 45.Harkey MR. 1993. Anatomy and physiology of hair. Forensic Sci Int 63(1–3):9–18, PMID: 8138238, 10.1016/0379-0738(93)90255-9. [DOI] [PubMed] [Google Scholar]
- 46.Hardy EM, Duca RC, Salquebre G, Appenzeller BMR. 2015. Multi-residue analysis of organic pollutants in hair and urine for matrices comparison. Forensic Sci Int 249:6–19, PMID: 25553512, 10.1016/j.forsciint.2014.12.003. [DOI] [PubMed] [Google Scholar]
- 47.Duca R-C, Hardy E, Salquèbre G, Appenzeller BMR. 2014. Hair decontamination procedure prior to multi-class pesticide analysis. Drug Test Anal 6(suppl 1)55–66, PMID: 24817049, 10.1002/dta.1649. [DOI] [PubMed] [Google Scholar]
- 48.Lubin JH, Colt JS, Camann D, Davis S, Cerhan JR, Severson RK, et al. 2004. Epidemiologic evaluation of measurement data in the presence of detection limits. Environ Health Perspect 112(17):1691–1696, PMID: 15579415, 10.1289/ehp.7199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bodinier B. 2022. sharp: stability-enHanced Approaches using Resampling Procedures. https://github.com/barbarabodinier/sharp [accessed 10 August 2022].
- 50.Lenters V, Portengen L, Rignell-Hydbom A, Jönsson BAG, Lindh CH, Piersma AH, et al. 2016. Prenatal phthalate, perfluoroalkyl acid, and organochlorine exposures and term birth weight in three birth cohorts: multi-pollutant models based on elastic net regression. Environ Health Perspect 124(3):365–372, PMID: 26115335, 10.1289/ehp.1408933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Maervoet J, Vermeir G, Covaci A, Van Larebeke N, Koppen G, Schoeters G, et al. 2007. Association of thyroid hormone concentrations with levels of organochlorine compounds in cord blood of neonates. Environ Health Perspect 115(12):1780–1786, PMID: 18087600, 10.1289/ehp.10486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Alvarez-Pedrerol M, Guxens M, Ibarluzea J, Rebagliato M, Rodriguez A, Espada M, et al. 2009. Organochlorine compounds, iodine intake, and thyroid hormone levels during pregnancy. Environ Sci Technol 43(20):7909–7915, PMID: 19921913, 10.1021/es9007273. [DOI] [PubMed] [Google Scholar]
- 53.Dallaire R, Muckle G, Dewailly E, Jacobson SW, Jacobson JL, Sandanger TM, et al. 2009. Thyroid hormone levels of pregnant Inuit women and their infants exposed to environmental contaminants. Environ Health Perspect 117(6):1014–1020, PMID: 19590699, 10.1289/ehp.0800219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Julvez J, Debes F, Weihe P, Choi AL, Grandjean P. 2011. Thyroid dysfunction as a mediator of organochlorine neurotoxicity in preschool children. Environ Health Perspect 119(10):1429–1435, PMID: 21719373, 10.1289/ehp.1003172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kim S, Park J, Kim H-J, Lee JJ, Choi G, Choi S, et al. 2015. Association between several persistent organic pollutants and thyroid hormone levels in cord blood serum and bloodspot of the newborn infants of Korea. PLoS One 10(5):e0125213, PMID: 25965908, 10.1371/journal.pone.0125213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hagmar L, Rylander L, Dyremark E, Klasson-Wehler E, Erfurth EM. 2001. Plasma concentrations of persistent organochlorines in relation to thyrotropin and thyroid hormone levels in women. Int Arch Occup Environ Health 74(3):184–188, PMID: 11355292, 10.1007/s004200000213. [DOI] [PubMed] [Google Scholar]
- 57.Takser L, Mergler D, Baldwin M, de Grosbois S, Smargiassi A, Lafond J. 2005. Thyroid hormones in pregnancy in relation to environmental exposure to organochlorine compounds and mercury. Environ Health Perspect 113(8):1039–1045, PMID: 16079076, 10.1289/ehp.7685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.van den Berg KJ. 1990. Interaction of chlorinated phenols with thyroxine binding sites of human transthyretin, albumin and thyroid binding globulin. Chem Biol Interact 76(1):63–75, PMID: 2393944, 10.1016/0009-2797(90)90034-k. [DOI] [PubMed] [Google Scholar]
- 59.Visser TJ, Kaptein E, Glatt H, Bartsch I, Hagen M, Coughtrie MWH. 1998. Characterization of thyroid hormone sulfotransferases. Chem Biol Interact 109(1–3):279–291, PMID: 9566752, 10.1016/s0009-2797(97)00139-7. [DOI] [PubMed] [Google Scholar]
- 60.Schuur AG, Bergman Å, Brouwer A, Visser TJ. 1999. Effects of pentachlorophenol and hydroxylated polychlorinated biphenyls on thyroid hormone conjugation in a rat and a human hepatoma cell line. Toxicol In Vitro 13(3):417–425, PMID: 20654499, 10.1016/s0887-2333(99)00005-3. [DOI] [PubMed] [Google Scholar]
- 61.Guo Y, Zhou B. 2013. Thyroid endocrine system disruption by pentachlorophenol: an in vitro and in vivo assay. Aquat Toxicol 142–143:138–145, PMID: 24001430, 10.1016/j.aquatox.2013.08.005. [DOI] [PubMed] [Google Scholar]
- 62.Yu L-Q, Zhao G-F, Feng M, Wen W, Li K, Zhang P-W, et al. 2014. Chronic exposure to pentachlorophenol alters thyroid hormones and thyroid hormone pathway mRNAs in zebrafish. Environ Toxicol Chem 33(1):170–176, PMID: 24123209, 10.1002/etc.2408. [DOI] [PubMed] [Google Scholar]
- 63.Béranger R, Hardy EM, Dexet C, Guldner L, Zaros C, Nougadère A, et al. 2018. Multiple pesticide analysis in hair samples of pregnant French women: results from the ELFE national birth cohort. Environ Int 120:43–53, PMID: 30064054, 10.1016/j.envint.2018.07.023. [DOI] [PubMed] [Google Scholar]
- 64.Iglesias-González A, Hardy EM, Appenzeller BMR. 2020. Cumulative exposure to organic pollutants of French children assessed by hair analysis. Environ Int 134:105332, PMID: 31785528, 10.1016/j.envint.2019.105332. [DOI] [PubMed] [Google Scholar]
- 65.Peng F-J, Emond C, Hardy EM, Sauvageot N, Alkerwi A, Lair M-L, et al. 2021. Population-based biomonitoring of exposure to persistent and non-persistent organic pollutants in the Grand Duchy of Luxembourg: results from hair analysis. Environ Int 153:106526, PMID: 33839549, 10.1016/j.envint.2021.106526. [DOI] [PubMed] [Google Scholar]
- 66.Mulder TA, van den Dries MA, Korevaar TIM, Ferguson KK, Peeters RP, Tiemeier H. 2019. Organophosphate pesticides exposure in pregnant women and maternal and cord blood thyroid hormone concentrations. Environ Int 132:105124, PMID: 31479957, 10.1016/j.envint.2019.105124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lerro CC, Beane Freeman LE, DellaValle CT, Kibriya MG, Aschebrook-Kilfoy B, Jasmine F, et al. 2018. Occupational pesticide exposure and subclinical hypothyroidism among male pesticide applicators. Occup Environ Med 75(2):79–89, PMID: 28775130, 10.1136/oemed-2017-104431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.De Angelis S, Tassinari R, Maranghi F, Eusepi A, Di Virgilio A, Chiarotti F, et al. 2009. Developmental exposure to chlorpyrifos induces alterations in thyroid and thyroid hormone levels without other toxicity signs in CD1 mice. Toxicol Sci 108(2):311–319, PMID: 19190125, 10.1093/toxsci/kfp017. [DOI] [PubMed] [Google Scholar]
- 69.Dalton SR, Miller RT, Meyer SA. 2003. The herbicide metolachlor induces liver cytochrome P450s 2B1/2 and 3A1/2, but not thyroxine-uridine dinucleotide phosphate glucuronosyltransferase and associated thyroid gland activity. Int J Toxicol 22(4):287–295, PMID: 12933323, 10.1080/10915810305121. [DOI] [PubMed] [Google Scholar]
- 70.US EPA (US Environmental Protection Agency). 2015. EDSP: weight of evidence analysis of potential interaction with the estrogen, androgen or thyroid pathways. In: Chemical: Metolachlor. Office of Pesticide Programs. https://www.epa.gov/endocrine-disruption/endocrine-disruptor-screening-program-tier-1-screening-determinations-and [accessed 29 December 2021].
- 71.Pandey SP, Mohanty B. 2015. The neonicotinoid pesticide imidacloprid and the dithiocarbamate fungicide mancozeb disrupt the pituitary–thyroid axis of a wildlife bird. Chemosphere 122:227–234, PMID: 25496744, 10.1016/j.chemosphere.2014.11.061. [DOI] [PubMed] [Google Scholar]
- 72.Gorzinski SJ, Kociba RJ, Campbell RA, Smith FA, Nolan RJ, Eisenbrandt DL. 1987. Acute, pharmacokinetic, and subchronic toxicological studies of 2,4-dichlorophenoxyacetic acid. Fundam Appl Toxicol 9(3):423–435, PMID: 3692002, 10.1016/0272-0590(87)90025-x. [DOI] [PubMed] [Google Scholar]
- 73.Herin F, Boutet-Robinet E, Levant A, Dulaurent S, Manika M, Galatry-Bouju F, et al. 2011. Thyroid function tests in persons with occupational exposure to fipronil. Thyroid 21(7):701–706, PMID: 21615307, 10.1089/thy.2010.0449. [DOI] [PubMed] [Google Scholar]
- 74.Kim YA, Yoon YS, Kim HS, Jeon SJ, Cole E, Lee J, et al. 2019. Distribution of fipronil in humans, and adverse health outcomes of in utero fipronil sulfone exposure in newborns. Int J Hyg Environ Health 222(3):524–532, PMID: 30718154, 10.1016/j.ijheh.2019.01.009. [DOI] [PubMed] [Google Scholar]
- 75.Du G, Shen O, Sun H, Fei J, Lu C, Song L, et al. 2010. Assessing hormone receptor activities of pyrethroid insecticides and their metabolites in reporter gene assays. Toxicol Sci 116(1):58–66, PMID: 20410157, 10.1093/toxsci/kfq120. [DOI] [PubMed] [Google Scholar]
- 76.Ghisari M, Long M, Tabbo A, Bonefeld-Jørgensen EC. 2015. Effects of currently used pesticides and their mixtures on the function of thyroid hormone and aryl hydrocarbon receptor in cell culture. Toxicol Appl Pharmacol 284(3):292–303, PMID: 25684042, 10.1016/j.taap.2015.02.004. [DOI] [PubMed] [Google Scholar]
- 77.Maiti PK, Kar A, Gupta P, Chaurasia SS. 1995. Loss of membrane integrity and inhibition of type-I iodothyronine 5′-monodeiodinase activity by fenvalerate in female mouse. Biochem Biophys Res Commun 214(3):905–909, PMID: 7575562, 10.1006/bbrc.1995.2372. [DOI] [PubMed] [Google Scholar]
- 78.Giray B, Cağlayan A, Erkekoğlu P, Hincal F. 2010. Fenvalerate exposure alters thyroid hormone status in selenium- and/or iodine-deficient rats. Biol Trace Elem Res 135(1–3):233–241, PMID: 19727571, 10.1007/s12011-009-8506-7. [DOI] [PubMed] [Google Scholar]
- 79.Kaul PP, Rastogi A, Hans RK, Seth TD, Seth PK, Srimal RC. 1996. Fenvalerate-induced alterations in circulatory thyroid hormones and calcium stores in rat brain. Toxicol Lett 89(1):29–33, PMID: 8952708, 10.1016/s0378-4274(96)03778-2. [DOI] [PubMed] [Google Scholar]
- 80.Brdar D, Gunjača I, Pleić N, Torlak V, Knežević P, Punda A, et al. 2021. The effect of food groups and nutrients on thyroid hormone levels in healthy individuals. Nutrition 91–92:111394, PMID: 34303955, 10.1016/j.nut.2021.111394. [DOI] [PubMed] [Google Scholar]
- 81.Merchan-Ramirez E, Sanchez-Delgado G, Jurado-Fasoli L, Acosta FM, Muñoz-Torres M, Llamas-Elvira JM, et al. 2024. Association between lifestyle factors and thyroid function in young euthyroid adults. Food Sci Hum Wellness 13(1):265–275, 10.26599/FSHW.2022.9250022. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


