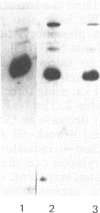
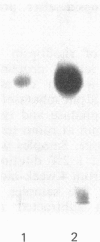
Abstract
On the basis of the amino acid sequence of bovine rhodopsin, a series of peptides from the C-terminus (Rhod-4 and Rhod-1) and external loops (Rhod-10) were synthesized. Rabbit antisera to these peptides recognize the rhodopsin molecule in whole retina from 8-week-old normal and affected rcdl (rod/cone-dysplasic) Irish setters (8- and 4-weeks-old). When the rhodopsin content was equalized by using a solid-phase radioimmunoassay, the reaction with anti-peptide antisera to the C-terminal octapeptide (residues 341-348) is severely decreased in the rcdl-dog retinas. The results of mixing experiments suggest that this is not due to proteolytic clipping of the rhodopsin C-terminus from the affected dogs. Treatment of retinas with 1.0 mM-NaF, a phosphatase inhibitor, or pretreatment with alkaline and acid phosphatases does alter the reaction of the rhodopsin with anti-rhodopsin antisera. This suggests that the decreased reaction of the affected rhodopsin with the anti-peptide antisera may partially result from differences in intrinsic rhodopsin phosphorylation. However, since the reaction of rcdl retinas cannot be restored to that of the normals, these results suggest that the rhodopsin molecule from the rcdl dogs may be structurally altered in other ways.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aguirre G. D., Rubin L. F. Rod-cone dysplasia (progressive retinal atrophy) in Irish setters. J Am Vet Med Assoc. 1975 Jan 15;166(2):157–164. [PubMed] [Google Scholar]
- Aguirre G., Farber D., Lolley R., O'Brien P., Alligood J., Fletcher R. T., Chader G. Retinal degeneration in the dog. III. Abnormal cyclic nucleotide metabolism in rod-cone dysplasia. Exp Eye Res. 1982 Dec;35(6):625–642. doi: 10.1016/s0014-4835(82)80075-4. [DOI] [PubMed] [Google Scholar]
- Aquirre G., Farber D., Lolley R., Fletcher R. T., Chader G. J. Rod-cone dysplasia in Irish setters: a defect in cyclic GMP metabolism in visual cells. Science. 1978 Sep 22;201(4361):1133–1134. doi: 10.1126/science.210508. [DOI] [PubMed] [Google Scholar]
- Aton B., Litman B. J. Activation of rod outer segment phosphodiesterase by enzymatically altered rhodopsin: a regulatory role for the carboxyl terminus of rhodopsin. Exp Eye Res. 1984 Jun;38(6):547–559. doi: 10.1016/0014-4835(84)90173-8. [DOI] [PubMed] [Google Scholar]
- Baehr W., Devlin M. J., Applebury M. L. Isolation and characterization of cGMP phosphodiesterase from bovine rod outer segments. J Biol Chem. 1979 Nov 25;254(22):11669–11677. [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Farber D. B., Lolley R. N. Cyclic guanosine monophosphate: elevation in degenerating photoreceptor cells of the C3H mouse retina. Science. 1974 Nov 1;186(4162):449–451. doi: 10.1126/science.186.4162.449. [DOI] [PubMed] [Google Scholar]
- Gorman J. J. An apparatus for simultaneous manual solid-phase synthesis of multiple peptide analogs. Anal Biochem. 1984 Feb;136(2):397–406. doi: 10.1016/0003-2697(84)90235-5. [DOI] [PubMed] [Google Scholar]
- Hargrave P. A., Fong S. L. The amino- and carboxyl-terminal sequence of bovine rhodopsin. J Supramol Struct. 1977;6(4):559–570. doi: 10.1002/jss.400060409. [DOI] [PubMed] [Google Scholar]
- Hodges R. S., Merrifield R. B. Monitoring of solid phase peptide synthesis by an automated spectrophotometric picrate method. Anal Biochem. 1975 May 12;65(1-2):241–272. doi: 10.1016/0003-2697(75)90509-6. [DOI] [PubMed] [Google Scholar]
- Kwok-Keung Fung B., Stryer L. Photolyzed rhodopsin catalyzes the exchange of GTP for bound GDP in retinal rod outer segments. Proc Natl Acad Sci U S A. 1980 May;77(5):2500–2504. doi: 10.1073/pnas.77.5.2500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kühn H., Hargrave P. A. Light-induced binding of guanosinetriphosphatase to bovine photoreceptor membranes: effect of limited proteolysis of the membranes. Biochemistry. 1981 Apr 28;20(9):2410–2417. doi: 10.1021/bi00512a007. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lee R. H., Lieberman B. S., Hurwitz R. L., Lolley R. N. Phosphodiesterase-probes show distinct defects in rd mice and Irish setter dog disorders. Invest Ophthalmol Vis Sci. 1985 Nov;26(11):1569–1579. [PubMed] [Google Scholar]
- Lolley R. N., Farber D. B. Abnormal guanosine 3', 5'-monophosphate during photoreceptor degeneration in the inherited retinal disorder of C3H/HeJ mice. Ann Ophthalmol. 1976 Apr;8(4):469–473. [PubMed] [Google Scholar]
- Lookhart G. L., Jones B. L., Cooper D. B., Hall S. B. A method for hydrolyzing and determining the amino acid compositions of picomole quantities of proteins in less than 3 hours. J Biochem Biophys Methods. 1982 Dec;7(1):15–23. doi: 10.1016/0165-022x(82)90032-x. [DOI] [PubMed] [Google Scholar]
- MERRIFIELD R. B. SOLID-PHASE PEPTIDE SYNTHESIS. 3. AN IMPROVED SYNTHESIS OF BRADYKININ. Biochemistry. 1964 Sep;3:1385–1390. doi: 10.1021/bi00897a032. [DOI] [PubMed] [Google Scholar]
- Suter M. A modified ELISA technique for anti-hapten antibodies. J Immunol Methods. 1982 Aug 27;53(1):103–108. doi: 10.1016/0022-1759(82)90244-7. [DOI] [PubMed] [Google Scholar]
- Takemoto D. J., Cunnick J., Takemoto L. J. Reduced rhodopsin phosphorylation during retinal dystrophy. Biochem Biophys Res Commun. 1986 Mar 28;135(3):1022–1028. doi: 10.1016/0006-291x(86)91030-2. [DOI] [PubMed] [Google Scholar]
- Takemoto D. J., Morrison D., Davis L. C., Takemoto L. J. C-terminal peptides of rhodopsin. Determination of the optimum sequence for recognition of retinal transducin. Biochem J. 1986 Apr 1;235(1):309–312. doi: 10.1042/bj2350309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takemoto D. J., Spooner B., Takemoto L. J. Antisera to synthetic peptides of bovine rhodopsin: use as site-specific probes of disc membrane changes in retinal dystrophic dogs. Biochem Biophys Res Commun. 1985 Oct 15;132(1):438–444. doi: 10.1016/0006-291x(85)91041-1. [DOI] [PubMed] [Google Scholar]
- Takemoto D. J., Takemoto L. J., Hansen J., Morrison D. Regulation of retinal transducin by C-terminal peptides of rhodopsin. Biochem J. 1985 Dec 15;232(3):669–672. doi: 10.1042/bj2320669. [DOI] [PMC free article] [PubMed] [Google Scholar]