Abstract
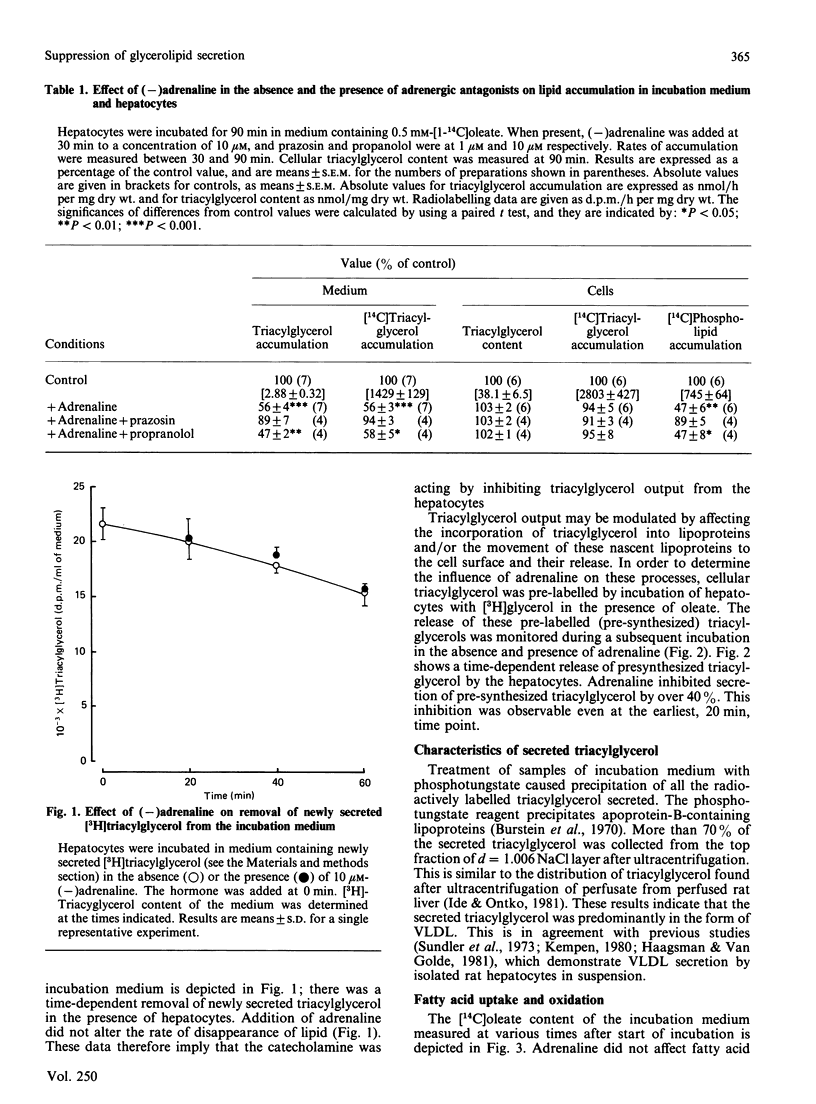
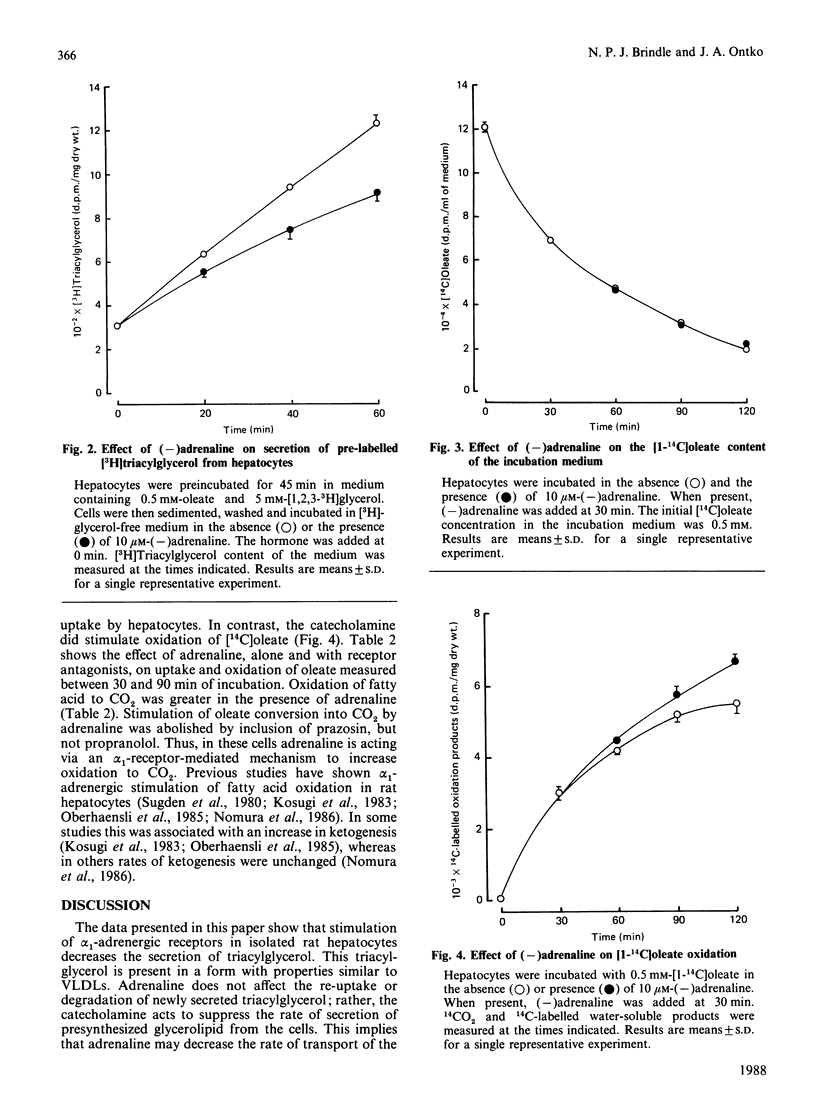
The effect of adrenaline on triacylglycerol synthesis and secretion was examined in isolated rat hepatocytes. Cells were incubated with 0.5 mM-[1-14C]oleate, and the accumulation of triacylglycerol and [14C]triacylglycerol was measured in the incubation medium. Triacylglycerol appearing in the medium was present in a form with properties similar to very-low-density lipoproteins. Triacylglycerol, [14C]triacylglycerol and [14C]phospholipid contents of hepatocytes were also determined. Addition of 10 microM-(-)adrenaline decreased accumulation of glycerolipid in the incubation medium and also decreased cellular [14C]phospholipid content. Prazosin abolished these effects, whereas propranolol did not. The hormone did not affect cellular triacylglycerol content or rates of incorporation of [1-14C]oleate into cell triacylglycerol. The effect of adrenaline on the removal of newly secreted triacylglycerol and the secretion of synthesized glycerolipid was also examined. The catecholamine did not affect rates of removal of newly secreted triacylglycerol. Adrenaline did inhibit the secretion of pre-synthesized lipid by the cells, as assessed by the appearance of radiolabelled triacylglycerol from hepatocytes that had been preincubated with [1,2,3-3H]-glycerol. Adrenaline did not affect rates of fatty acid uptake by hepatocytes, but did stimulate oxidation of [1-14C]oleate, principally to 14CO2.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blair J. B., James M. E., Foster J. L. Adrenergic control of glucose output and adenosine 3':5'-monophosphate levels in hepatocytes from juvenile and adult rats. J Biol Chem. 1979 Aug 25;254(16):7579–7584. [PubMed] [Google Scholar]
- Brindle N. P., Ontko J. A. Suppression of triglyceride secretion by epinephrine in isolated rat hepatocytes. Biochem Biophys Res Commun. 1986 Nov 26;141(1):191–197. doi: 10.1016/s0006-291x(86)80353-9. [DOI] [PubMed] [Google Scholar]
- Burstein M., Scholnick H. R., Morfin R. Rapid method for the isolation of lipoproteins from human serum by precipitation with polyanions. J Lipid Res. 1970 Nov;11(6):583–595. [PubMed] [Google Scholar]
- Carlson L. A., Levi L., Orö L. Plasma lipids and urinary excretion of catecholamines in man during experimentally induced emotional stress, and their modification by nicotinic acid. J Clin Invest. 1968 Aug;47(8):1795–1805. doi: 10.1172/JCI105869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carruthers M., Taggart P. Vagotonicity of violence: biochemical and cardiac responses to violent films and television programmes. Br Med J. 1973 Aug 18;3(5876):384–389. doi: 10.1136/bmj.3.5876.384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chait A., Brunzell J. D., Johnson D. G., Benson J. W., Werner P., Palmer J. P., Albers J. J., Ensinck J. W., Bierman E. L. Reduction of plasma triglyceride concentration by acute stress in man. Metabolism. 1979 May;28(5):553–561. doi: 10.1016/0026-0495(79)90197-5. [DOI] [PubMed] [Google Scholar]
- Chao F. F., Stiers D. L., Ontko J. A. Hepatocellular triglyceride synthesis and transfer to lipid droplets and nascent very low density lipoproteins. J Lipid Res. 1986 Nov;27(11):1174–1181. [PubMed] [Google Scholar]
- Cole T. G., Wilcox H. G., Heimberg M. Effects of adrenalectomy and dexamethasone on hepatic lipid metabolism. J Lipid Res. 1982 Jan;23(1):81–91. [PubMed] [Google Scholar]
- Dickson A. J., Pogson C. I. The metabolic integrity of hepatocytes in sustained incubations. FEBS Lett. 1977 Nov 1;83(1):27–32. doi: 10.1016/0014-5793(77)80634-0. [DOI] [PubMed] [Google Scholar]
- Durrington P. N., Newton R. S., Weinstein D. B., Steinberg D. Effects of insulin and glucose on very low density lipoprotein triglyceride secretion by cultured rat hepatocytes. J Clin Invest. 1982 Jul;70(1):63–73. doi: 10.1172/JCI110604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards P. A., Lemongello D., Fogelman A. M. The effect of glucagon, norepinephrine, and dibutyryl cyclic AMP on cholesterol efflux and on the activity of 3-hydroxy-3-methylglutaryl CoA reductase in rat hepatocytes. J Lipid Res. 1979 Jan;20(1):2–7. [PubMed] [Google Scholar]
- Exton J. H. Mechanisms involved in alpha-adrenergic phenomena. Am J Physiol. 1985 Jun;248(6 Pt 1):E633–E647. doi: 10.1152/ajpendo.1985.248.6.E633. [DOI] [PubMed] [Google Scholar]
- FOLCH J., LEES M., SLOANE STANLEY G. H. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem. 1957 May;226(1):497–509. [PubMed] [Google Scholar]
- Gibbons G. F., Pullinger C. R. Regulation of hepatic very-low-density lipoprotein secretion in rats fed on a diet high in unsaturated fat. Biochem J. 1987 Apr 15;243(2):487–492. doi: 10.1042/bj2430487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HEIMBERG M., FIZETTE N. B. The action of norepinephrine on the transport of fatty acids and triglycerides by the isolated perfused rat liver. Biochem Pharmacol. 1963 Apr;12:392–394. doi: 10.1016/0006-2952(63)90067-4. [DOI] [PubMed] [Google Scholar]
- Haagsman H. P., Van Golde L. M. Synthesis and secretion of very low density lipoproteins by isolated rat hepatocytes in suspension: role of diacylglycerol acyltransferase. Arch Biochem Biophys. 1981 May;208(2):395–402. doi: 10.1016/0003-9861(81)90524-5. [DOI] [PubMed] [Google Scholar]
- Haagsman H. P., van den Heuvel J. M., van Golde L. M., Geelen M. J. Synthesis of phosphatidylcholines in rat hepatocytes. Possible regulation by norepinephrine via an alpha-adrenergic mechanism. J Biol Chem. 1984 Sep 25;259(18):11273–11278. [PubMed] [Google Scholar]
- Heimberg M., Weinstein I., Kohout M. The effects of glucagon, dibutyryl cyclic adenosine 3',5'-monophosphate, and concentration of free fatty acid on hepatic lipid metabolism. J Biol Chem. 1969 Oct 10;244(19):5131–5139. [PubMed] [Google Scholar]
- Ide T., Ontko J. A. Increased secretion of very low density lipoprotein triglyceride following inhibition of long chain fatty acid oxidation in isolated rat liver. J Biol Chem. 1981 Oct 25;256(20):10247–10255. [PubMed] [Google Scholar]
- Jamieson J. D., Palade G. E. Intracellular transport of secretory proteins in the pancreatic exocrine cell. IV. Metabolic requirements. J Cell Biol. 1968 Dec;39(3):589–603. doi: 10.1083/jcb.39.3.589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kempen H. J. Lipoprotein secretion by isolated rat hepatocytes: characterization of the lipid-carrying particles and modulation of their release. J Lipid Res. 1980 Aug;21(6):671–680. [PubMed] [Google Scholar]
- Klausner H. J., Soler-Argilaga C., Heimberg M. Effects of dibutyryl adenosine 3',5'-monophosphate on hepatic metabolism of free fatty acids. Metabolism. 1978 Jan;27(1):13–25. doi: 10.1016/0026-0495(78)90119-1. [DOI] [PubMed] [Google Scholar]
- Klausner H., Heimberg M. Effect of adrenalcortical hormones on release of triglycerides and glucose by liver. Am J Physiol. 1967 Jun;212(6):1236–1246. doi: 10.1152/ajplegacy.1967.212.6.1236. [DOI] [PubMed] [Google Scholar]
- Kosugi K., Harano Y., Nakano T., Suzuki M., Kashiwagi A., Shigeta Y. Mechanism of adrenergic stimulation of hepatic ketogenesis. Metabolism. 1983 Nov;32(11):1081–1087. doi: 10.1016/0026-0495(83)90081-1. [DOI] [PubMed] [Google Scholar]
- Lefkowitz R. J., Caron M. G. Adrenergic receptors: molecular mechanisms of clinically relevant regulation. Clin Res. 1985 Sep;33(3):395–406. [PubMed] [Google Scholar]
- Mangiapane E. H., Brindley D. N. Effects of dexamethasone and insulin on the synthesis of triacylglycerols and phosphatidylcholine and the secretion of very-low-density lipoproteins and lysophosphatidylcholine by monolayer cultures of rat hepatocytes. Biochem J. 1986 Jan 1;233(1):151–160. doi: 10.1042/bj2330151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan N. G., Blackmore P. F., Exton J. H. Age-related changes in the control of hepatic cyclic AMP levels by alpha 1- and beta 2-adrenergic receptors in male rats. J Biol Chem. 1983 Apr 25;258(8):5103–5109. [PubMed] [Google Scholar]
- Nomura T., Tachibana M., Maekawa H., Nomura H., Izuhara K., Hagino Y. Effects of vasopressin, angiotensin II and phenylephrine on hepatic ketogenesis and fatty acid synthesis. Jpn J Pharmacol. 1986 Aug;41(4):525–532. doi: 10.1254/jjp.41.525. [DOI] [PubMed] [Google Scholar]
- Oberhaensli R. D., Schwendimann R., Keller U. Effect of norepinephrine on ketogenesis, fatty acid oxidation, and esterification in isolated rat hepatocytes. Diabetes. 1985 Aug;34(8):774–779. doi: 10.2337/diab.34.8.774. [DOI] [PubMed] [Google Scholar]
- Patsch W., Franz S., Schonfeld G. Role of insulin in lipoprotein secretion by cultured rat hepatocytes. J Clin Invest. 1983 May;71(5):1161–1174. doi: 10.1172/JCI110865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pullinger C. R., Gibbons G. F. Effects of hormones and pyruvate on the rates of secretion of very-low-density lipoprotein triacylglycerol and cholesterol by rat hepatocytes. Biochim Biophys Acta. 1985 Jan 9;833(1):44–51. doi: 10.1016/0005-2760(85)90251-6. [DOI] [PubMed] [Google Scholar]
- Reinhart P. H., Taylor W. M., Bygrave F. L. The role of calcium ions in the mechanism of action of alpha-adrenergic agonists in rat liver. Biochem J. 1984 Oct 1;223(1):1–13. doi: 10.1042/bj2230001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson R. P., Smith P. H. Stress-induced inhibition of triglyceride secretion in vivo sand rats (Psammomys obesus). Metabolism. 1976 Dec;25(12):1583–1590. doi: 10.1016/0026-0495(76)90111-6. [DOI] [PubMed] [Google Scholar]
- Soler-Argilaga C., Russell R. L., Heimberg M. Enzymatic aspects of the reduction of microsomal glycerolipid biosynthesis after perfusion of the liver with dibutyryl adenosine-3',5'-monophosphate. Arch Biochem Biophys. 1978 Oct;190(2):367–372. doi: 10.1016/0003-9861(78)90289-8. [DOI] [PubMed] [Google Scholar]
- Soler-Argilaga C., Russell R. L., Werner H. V., Heimberg M. A possible role of calcium in the action of glucagon, cAMP and dibutyryl cAMP on the metabolism of free fatty acids by rat hepatocytes. Biochem Biophys Res Commun. 1978 Nov 14;85(1):249–256. doi: 10.1016/s0006-291x(78)80036-9. [DOI] [PubMed] [Google Scholar]
- Stanley P. E., Williams S. G. Use of the liquid scintillation spectrometer for determining adenosine triphosphate by the luciferase enzyme. Anal Biochem. 1969 Jun;29(3):381–392. doi: 10.1016/0003-2697(69)90323-6. [DOI] [PubMed] [Google Scholar]
- Sugden M. C., Tordoff A. F., Ilic V., Williamson D. H. Alpha-adrenergic stimulation of [1-14C]oleate oxidation to 14CO2 in isolated rat hepatocytes. FEBS Lett. 1980 Oct 20;120(1):80–84. doi: 10.1016/0014-5793(80)81051-9. [DOI] [PubMed] [Google Scholar]
- Sundler R., Akesson B., Nilsson A. Triacylglycerol secretion in very low density lipoproteins by isolated rat liver parenchymal cells. Biochem Biophys Res Commun. 1973 Dec 10;55(3):961–968. doi: 10.1016/0006-291x(73)91236-9. [DOI] [PubMed] [Google Scholar]
- Taggart P., Carruthers M. Endogenous hyperlipidaemia induced by emotional stress of racing driving. Lancet. 1971 Feb 20;1(7695):363–366. doi: 10.1016/s0140-6736(71)92207-0. [DOI] [PubMed] [Google Scholar]
- Taylor W. M., Reinhart P. H., Bygrave F. L. Stimulation by alpha-adrenergic agonists of Ca2+ fluxes, mitochondrial oxidation and gluconeogenesis in perfused rat liver. Biochem J. 1983 Jun 15;212(3):555–565. doi: 10.1042/bj2120555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaleski J., Ontko J. A. Reciprocal effects of energy utilization on palmitate oxidation and esterification in hepatocytes of fed rats. Biochim Biophys Acta. 1985 Aug 22;836(1):134–142. doi: 10.1016/0005-2760(85)90229-2. [DOI] [PubMed] [Google Scholar]


