Abstract
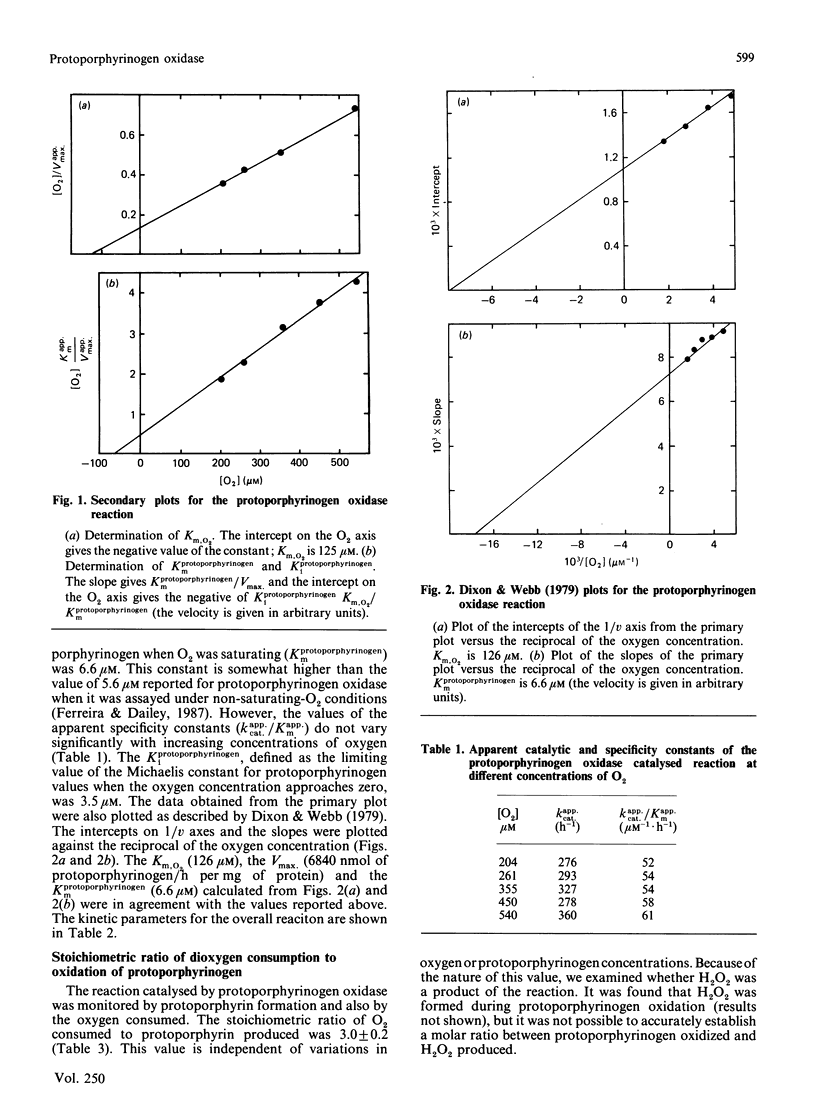
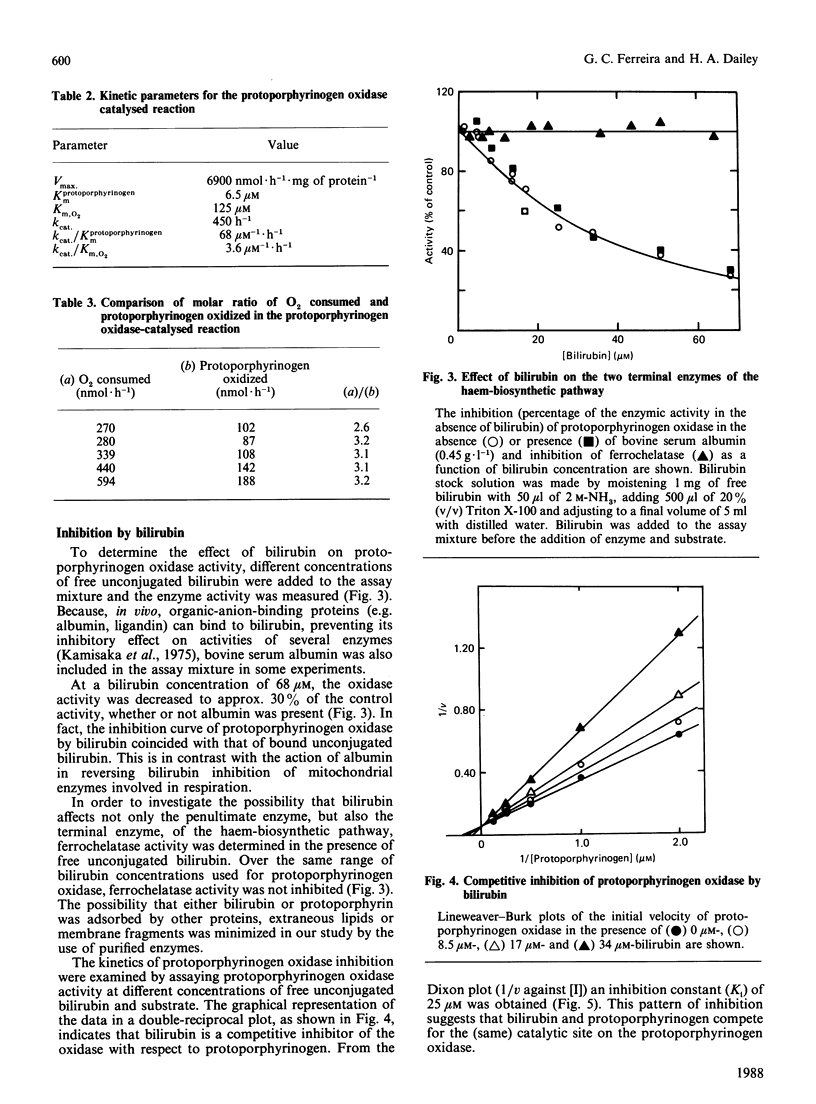
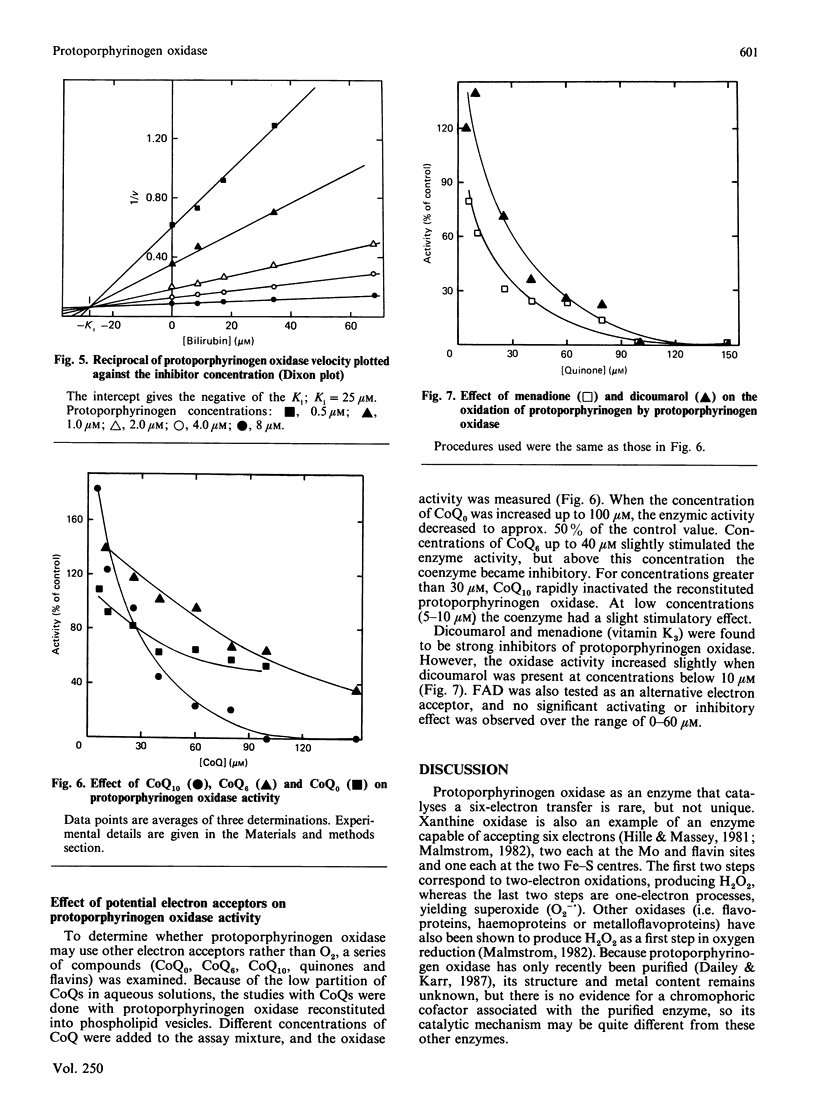
The penultimate step of haem biosynthesis, the oxidation of protoporphyrinogen to protoporphyrin, was examined with purified murine hepatic protoporphyrinogen oxidase (EC 1.3.3.4) in detergent solution. The kinetic parameters for the two-substrate (protoporphyrinogen and oxygen) reaction were determined. The limiting Km for protoporphyrinogen when oxygen is saturating is 6.6 microM, whereas the Km for oxygen with saturating concentrations of protoporphyrinogen is 125 microM. The kcat. for the overall reaction is 447 h-1. The ratio of kcat. to the Km for protoporphyrinogen is approx. 20-fold greater than the kcat./Km,O2 ratio. The ratio of protoporphyrin formed to dioxygen consumed is 1:3. Ubiquinone-6, ubiquinone-10 and dicoumarol stimulate protoporphyrinogen oxidase activity at low concentrations (less than 15 microM), whereas coenzyme Q0 and menadione show no activation at these concentrations. Above 30 microM, all five quinones inhibit the enzyme activity. FAD does not significantly affect the activity of the enzyme. Bilirubin, a product of haem catabolism, is shown to be a competitive inhibitor of the penultimate enzyme of the haem-biosynthetic pathway, protoporphyrinogen oxidase, with a calculated Ki of 25 microM. The terminal enzyme of haem-biosynthetic pathway, namely ferrochelatase, is not inhibited by bilirubin at concentrations over double the Ki value for the oxidase. In contrast with other enzymic systems, the toxicity of bilirubin is not reversed by binding to albumin.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Becker D. M., Viljoen J. D., Katz J., Kramer S. Reduced ferrochelatase activity: a defect common to porphyria variegata and protoporphyria. Br J Haematol. 1977 Jun;36(2):171–179. doi: 10.1111/j.1365-2141.1977.tb00637.x. [DOI] [PubMed] [Google Scholar]
- Berthelot P., Dhumeaux D. New insights into the classification and mechanisms of hereditary, chronic, non-haemolytic hyperbilirubinaemias. Gut. 1978 Jun;19(6):474–480. doi: 10.1136/gut.19.6.474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenner D. A., Bloomer J. R. A fluorometric assay for measurement of protoporphyrinogen oxidase activity in mammalian tissue. Clin Chim Acta. 1980 Jan 31;100(3):259–266. doi: 10.1016/0009-8981(80)90275-2. [DOI] [PubMed] [Google Scholar]
- Dailey H. A., Fleming J. E. Bovine ferrochelatase. Kinetic analysis of inhibition by N-methylprotoporphyrin, manganese, and heme. J Biol Chem. 1983 Oct 10;258(19):11453–11459. [PubMed] [Google Scholar]
- Dailey H. A., Fleming J. E., Harbin B. M. Purification and characterization of mammalian and chicken ferrochelatase. Methods Enzymol. 1986;123:401–408. doi: 10.1016/s0076-6879(86)23049-9. [DOI] [PubMed] [Google Scholar]
- Dailey H. A., Karr S. W. Purification and characterization of murine protoporphyrinogen oxidase. Biochemistry. 1987 May 19;26(10):2697–2701. doi: 10.1021/bi00384a007. [DOI] [PubMed] [Google Scholar]
- Deybach J. C., da Silva V., Grandchamp B., Nordmann Y. The mitochondrial location of protoporphyrinogen oxidase. Eur J Biochem. 1985 Jun 3;149(2):431–435. doi: 10.1111/j.1432-1033.1985.tb08943.x. [DOI] [PubMed] [Google Scholar]
- Deybach J. C., de Verneuil H., Nordmann Y. The inherited enzymatic defect in porphyria variegata. Hum Genet. 1981;58(4):425–428. doi: 10.1007/BF00282829. [DOI] [PubMed] [Google Scholar]
- Ferreira G. C., Dailey H. A. Reconstitution of the two terminal enzymes of the heme biosynthetic pathway into phospholipid vesicles. J Biol Chem. 1987 Mar 25;262(9):4407–4412. [PubMed] [Google Scholar]
- Green T. R., Wu D. E. The NADPH:O2 oxidoreductase of human neutrophils. Stoichiometry of univalent and divalent reduction of O2. J Biol Chem. 1986 May 5;261(13):6010–6015. [PubMed] [Google Scholar]
- Guilbualt G. G., Kramer D. N., Hackley E. A new substrate for fluorometric determination of oxidative enzymes. Anal Chem. 1967 Feb;39(2):271–272. doi: 10.1021/ac60246a029. [DOI] [PubMed] [Google Scholar]
- Hille R., Massey V. Studies on the oxidative half-reaction of xanthine oxidase. J Biol Chem. 1981 Sep 10;256(17):9090–9095. [PubMed] [Google Scholar]
- Jackson A. H., Games D. E., Couch P., Jackson J. R., Belcher R. B., Smith S. G. Conversion of coproporphyrinogen 3 to protoporphyrin IX. Enzyme. 1974;17(1):81–87. doi: 10.1159/000459311. [DOI] [PubMed] [Google Scholar]
- Jacobs N. J., Jacobs J. M. Assay for enzymatic protoporphyrinogen oxidation, a late step in heme synthesis. Enzyme. 1982;28(2-3):206–219. doi: 10.1159/000459103. [DOI] [PubMed] [Google Scholar]
- Jacobs N. J., Jacobs J. M. Fumarate as alternate electron acceptor for the late steps of anaerobic heme synthesis in Escherichia coli. Biochem Biophys Res Commun. 1975 Jul 8;65(1):435–441. doi: 10.1016/s0006-291x(75)80112-4. [DOI] [PubMed] [Google Scholar]
- Jacobs N. J., Jacobs J. M. Nitrate, fumarate, and oxygen as electron acceptors for a late step in microbial heme synthesis. Biochim Biophys Acta. 1976 Oct 13;449(1):1–9. doi: 10.1016/0005-2728(76)90002-5. [DOI] [PubMed] [Google Scholar]
- Kamisaka K., Gatmaitan Z., Moore C. L., Arias I. M. Ligandin reverses bilirubin inhibition of liver mitochondrial respiration in vitro. Pediatr Res. 1975 Dec;9(12):903–905. doi: 10.1203/00006450-197512000-00007. [DOI] [PubMed] [Google Scholar]
- Malmström B. G. Enzymology of oxygen. Annu Rev Biochem. 1982;51:21–59. doi: 10.1146/annurev.bi.51.070182.000321. [DOI] [PubMed] [Google Scholar]
- Poulson R., Polglase W. J. The enzymic conversion of protoporphyrinogen IX to protoporphyrin IX. Protoporphyrinogen oxidase activity in mitochondrial extracts of Saccharomyces cerevisiae. J Biol Chem. 1975 Feb 25;250(4):1269–1274. [PubMed] [Google Scholar]
- Poulson R. The enzymic conversion of protoporphyrinogen IX to protoporphyrin IX in mammalian mitochondria. J Biol Chem. 1976 Jun 25;251(12):3730–3733. [PubMed] [Google Scholar]
- Srivastava D. K., Bernhard S. A. Metabolite transfer via enzyme-enzyme complexes. Science. 1986 Nov 28;234(4780):1081–1086. doi: 10.1126/science.3775377. [DOI] [PubMed] [Google Scholar]
- Viljoen D. J., Cummins R., Alexopoulos J., Kramer S. Protoporphyrinogen oxidase and ferrochelatase in porphyria variegata. Eur J Clin Invest. 1983 Aug;13(4):283–287. doi: 10.1111/j.1365-2362.1983.tb00102.x. [DOI] [PubMed] [Google Scholar]
- Yoshinaga T., Sano S. Coproporphyrinogen oxidase. I. Purification, properties, and activation by phospholipids. J Biol Chem. 1980 May 25;255(10):4722–4726. [PubMed] [Google Scholar]
- Yoshinaga T., Sano S. Coproporphyrinogen oxidase. II. Reaction mechanism and role of tyrosine residues on the activity. J Biol Chem. 1980 May 25;255(10):4727–4731. [PubMed] [Google Scholar]


