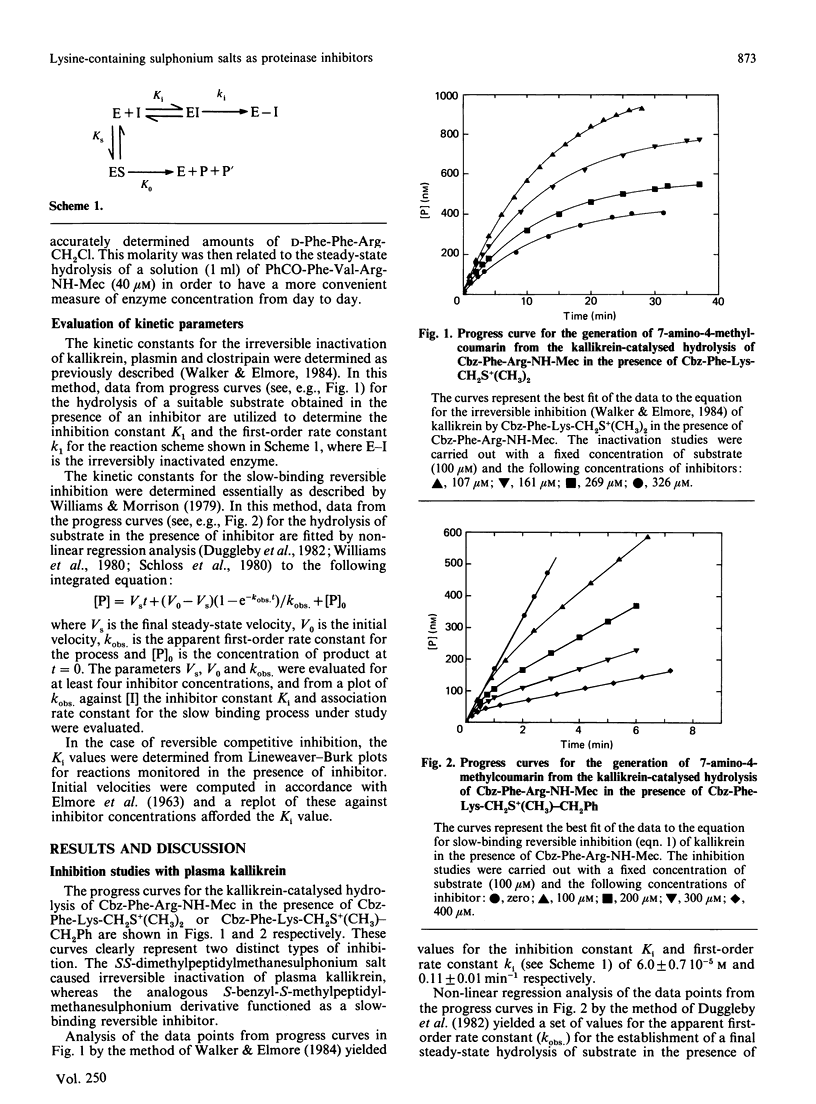
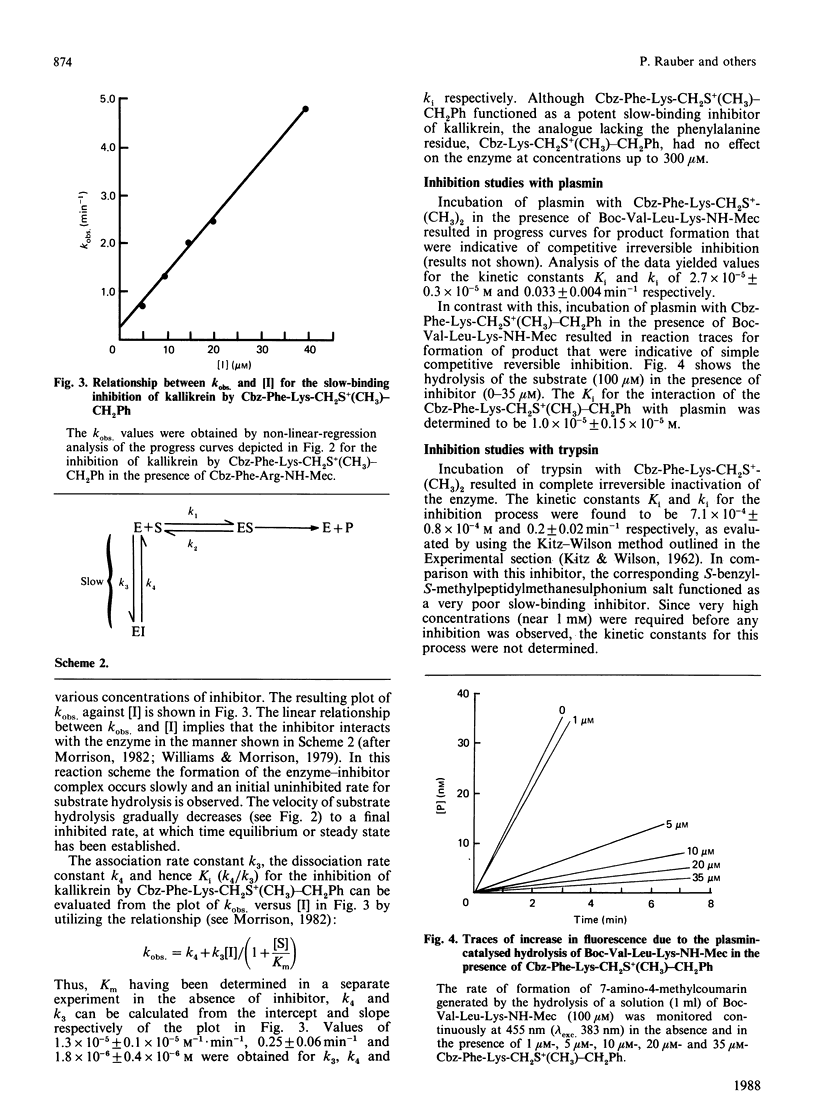
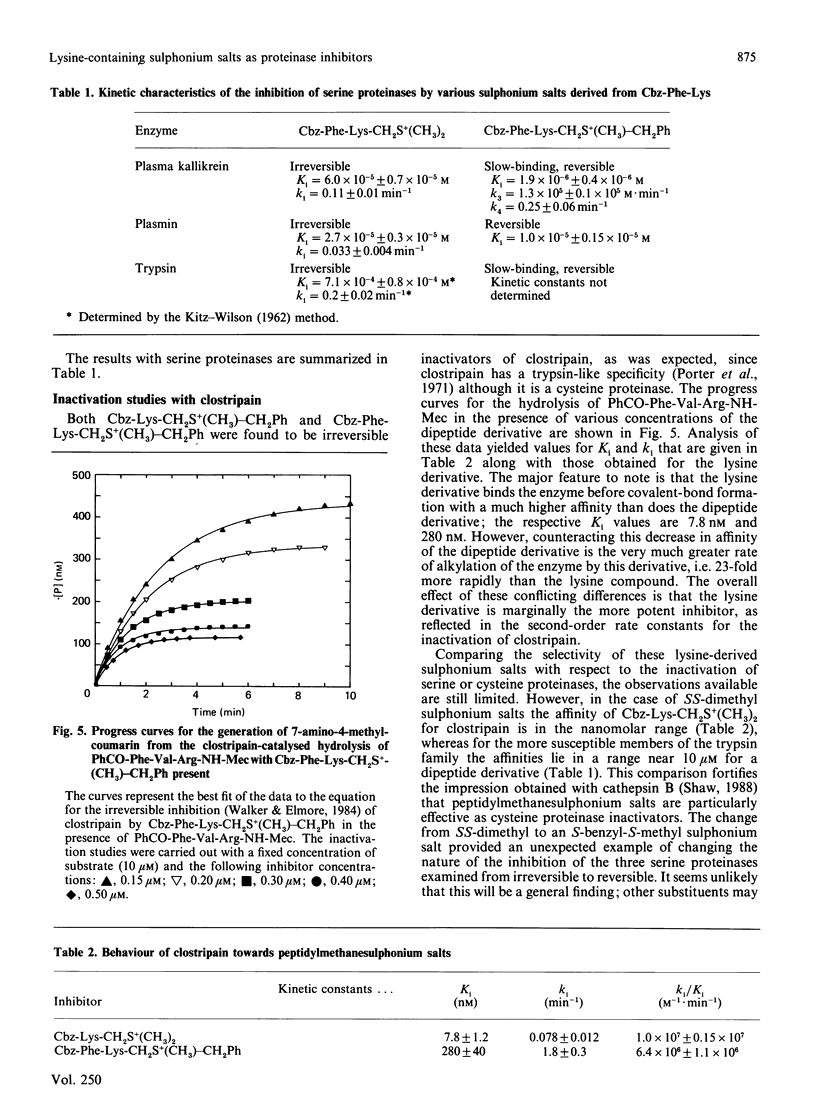
Abstract
Some sulphonium salts derived from lysine were synthesized with the general structure R-Lys-CH2S+-(alkyl)2. They were examined as inhibitors of the cysteine proteinase clostripain, which has a preference for cleaving peptide bonds at the carboxy group of basic amino acids, and of a number of trypsin-related serine proteinases. Clostripain was irreversibly inactivated by all reagents examined, but in the case of the serine proteinases, depending on the reagent structure, irreversible and reversible inhibitions were observed. These were kinetically characterized.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Coggins J. R., Kray W., Shaw E. Affinity labelling of proteinases with tryptic specificity by peptides with C-terminal lysine chloromethyl ketone. Biochem J. 1974 Mar;137(3):579–585. doi: 10.1042/bj1370579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duggleby R. G., Attwood P. V., Wallace J. C., Keech D. B. Avidin is a slow-binding inhibitor of pyruvate carboxylase. Biochemistry. 1982 Jul 6;21(14):3364–3370. doi: 10.1021/bi00257a018. [DOI] [PubMed] [Google Scholar]
- Jameson G. W., Roberts D. V., Adams R. W., Kyle W. S., Elmore D. T. Determination of the operational molarity of solutions of bovine alpha-chymotrypsin, trypsin, thrombin and factor Xa by spectrofluorimetric titration. Biochem J. 1973 Jan;131(1):107–117. doi: 10.1042/bj1310107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KITZ R., WILSON I. B. Esters of methanesulfonic acid as irreversible inhibitors of acetylcholinesterase. J Biol Chem. 1962 Oct;237:3245–3249. [PubMed] [Google Scholar]
- Porter W. H., Cunningham L. W., Mitchell W. M. Studies on the active site of clostripain. The specific inactivation by the chloromethyl ketone derived from -N-tosyl-L-lysine. J Biol Chem. 1971 Dec 25;246(24):7675–7682. [PubMed] [Google Scholar]
- Schloss J. V., Porter D. J., Bright H. J., Cleland W. W. Nitro analogues of citrate and isocitrate as transition-state analogues for aconitase. Biochemistry. 1980 May 27;19(11):2358–2362. doi: 10.1021/bi00552a012. [DOI] [PubMed] [Google Scholar]
- Shaw E., Green G. D. Inactivation of thiol proteases with peptidyl diazomethyl ketones. Methods Enzymol. 1981;80(Pt 100):820–826. doi: 10.1016/s0076-6879(81)80064-x. [DOI] [PubMed] [Google Scholar]
- Walker B., Elmore D. T. The irreversible inhibition of urokinase, kidney-cell plasminogen activator, plasmin and beta-trypsin by 1-(N-6-amino-n-hexyl)carbamoylimidazole. Biochem J. 1984 Jul 1;221(1):277–280. doi: 10.1042/bj2210277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams J. W., Duggleby R. G., Cutler R., Morrison J. F. The inhibition of dihydrofolate reductase by folate analogues: structural requirements for slow- and tight-binding inhibition. Biochem Pharmacol. 1980 Feb 15;29(4):589–595. doi: 10.1016/0006-2952(80)90381-0. [DOI] [PubMed] [Google Scholar]
- Williams J. W., Morrison J. F. The kinetics of reversible tight-binding inhibition. Methods Enzymol. 1979;63:437–467. doi: 10.1016/0076-6879(79)63019-7. [DOI] [PubMed] [Google Scholar]


