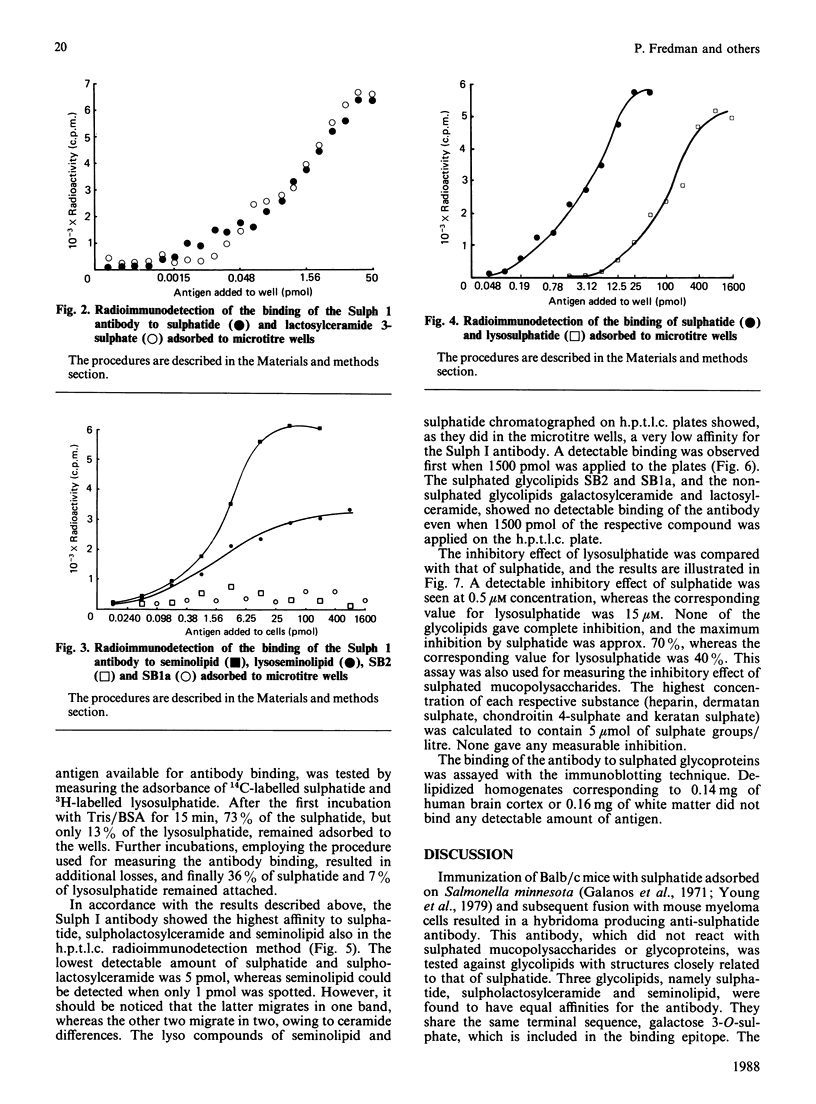
Abstract
An IgG1 monoclonal antibody, Sulph I, reacting with sulphatide (3'-sulphogalactosylceramide), was produced by immunizing Balb/c mice with that glycolipid coated on Salmonella minnesota bacterial membrane. Radioimmunodetection of the binding of the monoclonal antibody to structurally related glycolipids adsorbed to microtitre plates or chromatographed on thin-layer plates was used to determine its binding epitope. The antibody showed similar binding avidity to three sulphated glycolipids: sulphatide, sulpholactosylceramide and seminolipid. Lysosulphatide did bind the antibody, but, compared with sulphatide, 30 times more antigen was needed for half-maximal binding. Bis(sulphogangliotriosyl)ceramide and bis-sulphogangliotetraosylceramide did not bind the antibody. These results suggest that terminal galactose-3-O-sulphate and part of the hydrophobic region of the glycolipid are recognized by the Sulph I antibody.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arnaud P., Wilson G. B., Koistinen J., Fudenberg H. H. Immunofixation after electrofocusing: improved method for specific detection of serum proteins with determination of isoelectric points. I. Immunofixation print technique for detection of alpha-1-protease inhibitor. J Immunol Methods. 1977;16(3):221–231. doi: 10.1016/0022-1759(77)90200-9. [DOI] [PubMed] [Google Scholar]
- Batteiger B., Newhall W. J., 5th, Jones R. B. The use of Tween 20 as a blocking agent in the immunological detection of proteins transferred to nitrocellulose membranes. J Immunol Methods. 1982 Dec 30;55(3):297–307. doi: 10.1016/0022-1759(82)90089-8. [DOI] [PubMed] [Google Scholar]
- Brockhaus M., Magnani J. L., Blaszczyk M., Steplewski Z., Koprowski H., Karlsson K. A., Larson G., Ginsburg V. Monoclonal antibodies directed against the human Leb blood group antigen. J Biol Chem. 1981 Dec 25;256(24):13223–13225. [PubMed] [Google Scholar]
- Fredman P., Nilsson O., Tayot J. L., Svennerholm L. Separation of gangliosides on a new type of anion-exchange resin. Biochim Biophys Acta. 1980 Apr 18;618(1):42–52. doi: 10.1016/0005-2760(80)90052-1. [DOI] [PubMed] [Google Scholar]
- Galanos C., Lüderitz O., Westphal O. Preparation and properties of antisera against the lipid-A component of bacterial lipopolysaccharides. Eur J Biochem. 1971 Dec 22;24(1):116–122. doi: 10.1111/j.1432-1033.1971.tb19661.x. [DOI] [PubMed] [Google Scholar]
- Goujet-Zalc C., Guerci A., Dubois G., Zalc B. Schwann cell marker defined by a monoclonal antibody (224-58) with species cross-reactivity. II. Molecular characterization of the epitope. J Neurochem. 1986 Feb;46(2):435–439. doi: 10.1111/j.1471-4159.1986.tb12987.x. [DOI] [PubMed] [Google Scholar]
- Guerci A., Monge M., Baron-Van Evercooren A., Lubetzki C., Dancea S., Boutry J. M., Goujet-Zalc C., Zalc B. Schwann cell marker defined by a monoclonal antibody (224-58) with species cross-reactivity. I. Cellular localization. J Neurochem. 1986 Feb;46(2):425–434. doi: 10.1111/j.1471-4159.1986.tb12986.x. [DOI] [PubMed] [Google Scholar]
- Hofstetter W., Bologa L., Wetterwald A., Z'graggen A., Blaser K., Herschkowitz N. Production and characterization of monoclonal antibodies to the myelin glycolipid sulfatide. J Neurosci Res. 1984;11(4):341–350. doi: 10.1002/jnr.490110402. [DOI] [PubMed] [Google Scholar]
- Magnani J. L., Nilsson B., Brockhaus M., Zopf D., Steplewski Z., Koprowski H., Ginsburg V. A monoclonal antibody-defined antigen associated with gastrointestinal cancer is a ganglioside containing sialylated lacto-N-fucopentaose II. J Biol Chem. 1982 Dec 10;257(23):14365–14369. [PubMed] [Google Scholar]
- Mancini G., Carbonara A. O., Heremans J. F. Immunochemical quantitation of antigens by single radial immunodiffusion. Immunochemistry. 1965 Sep;2(3):235–254. doi: 10.1016/0019-2791(65)90004-2. [DOI] [PubMed] [Google Scholar]
- Schachner M. Cell type-specific surface antigens in the mammalian nervous system. J Neurochem. 1982 Jul;39(1):1–8. doi: 10.1111/j.1471-4159.1982.tb04694.x. [DOI] [PubMed] [Google Scholar]
- Sjögren-Jansson E., Jeansson S. Large-scale production of monoclonal antibodies in dialysis tubing. J Immunol Methods. 1985 Nov 28;84(1-2):359–364. doi: 10.1016/0022-1759(85)90442-9. [DOI] [PubMed] [Google Scholar]
- Svennerholm L., Vanier M. T., Jungbjer B. Changes in fatty acid composition of human brain myelin lipids during maturation. J Neurochem. 1978 Jun;30(6):1383–1390. doi: 10.1111/j.1471-4159.1978.tb10470.x. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WELLS M. A., DITTMER J. C. THE USE OF SEPHADEX FOR THE REMOVAL OF NONLIPID CONTAMINANTS FROM LIPID EXTRACTS. Biochemistry. 1963 Nov-Dec;2:1259–1263. doi: 10.1021/bi00906a015. [DOI] [PubMed] [Google Scholar]
- Young W. W., Jr, MacDonald E. M., Nowinski R. C., Hakomori S. I. Production of monoclonal antibodies specific for two distinct steric portions of the glycolipid ganglio-N-triosylceramide (asialo GM2). J Exp Med. 1979 Oct 1;150(4):1008–1019. doi: 10.1084/jem.150.4.1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zalc B., Baumann N. Sulfatide: localization by immunohistochemical techniques: relevance to metabolism. Adv Exp Med Biol. 1982;152:439–443. [PubMed] [Google Scholar]




