Abstract
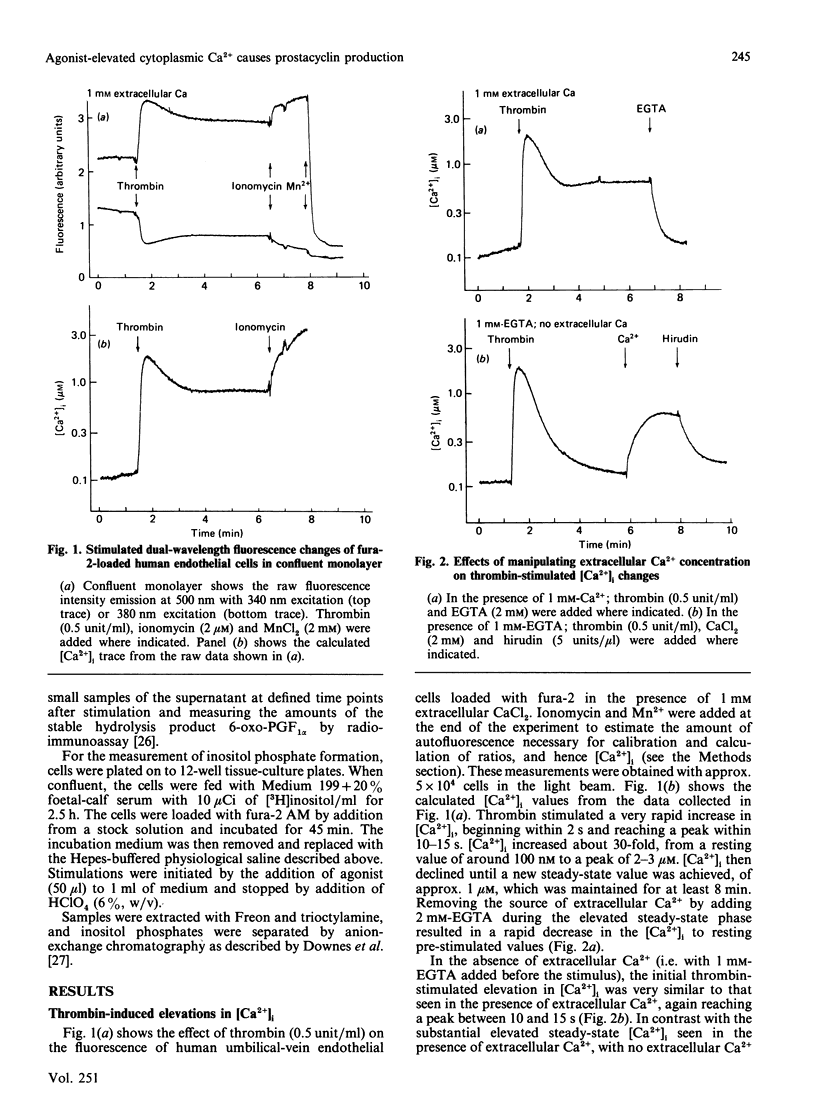
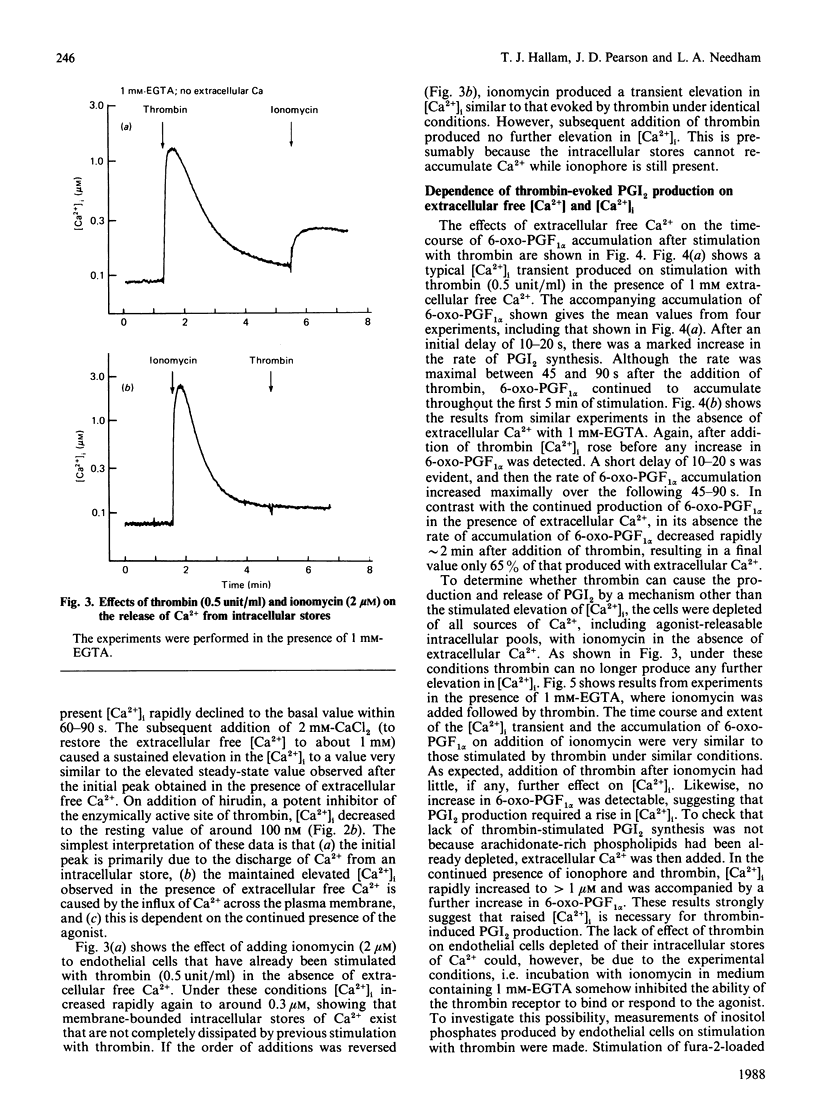
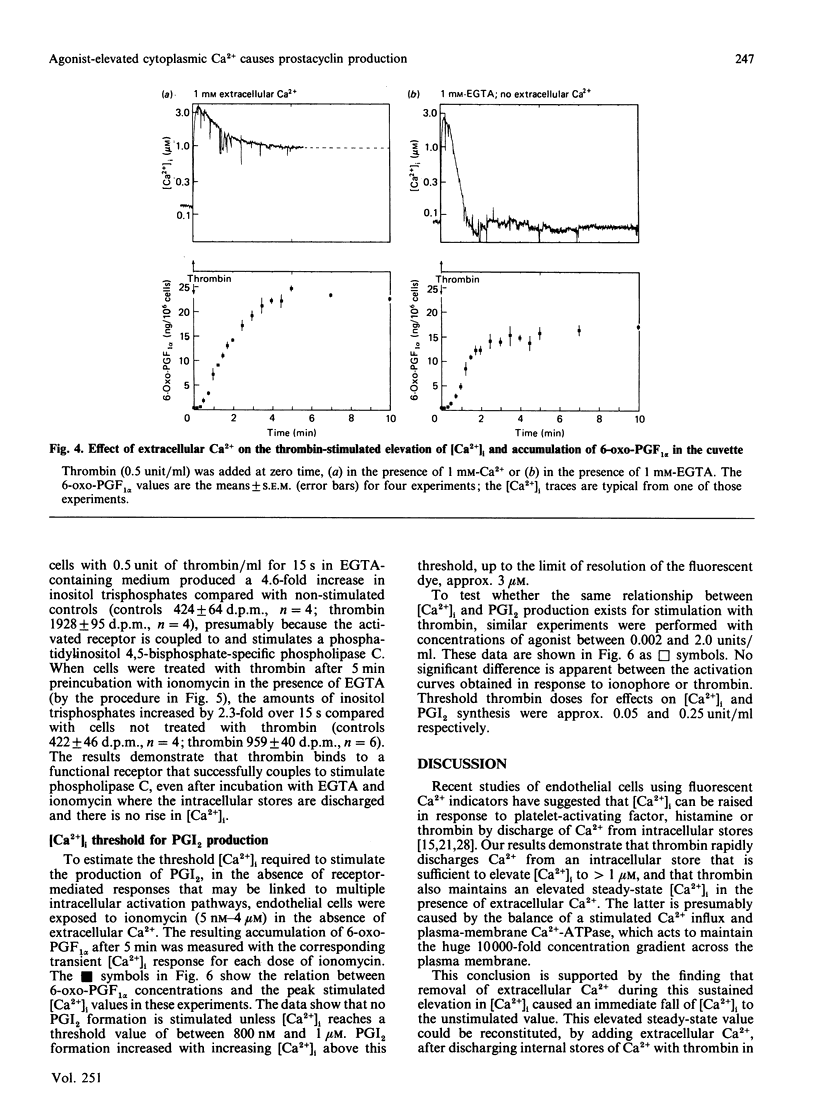
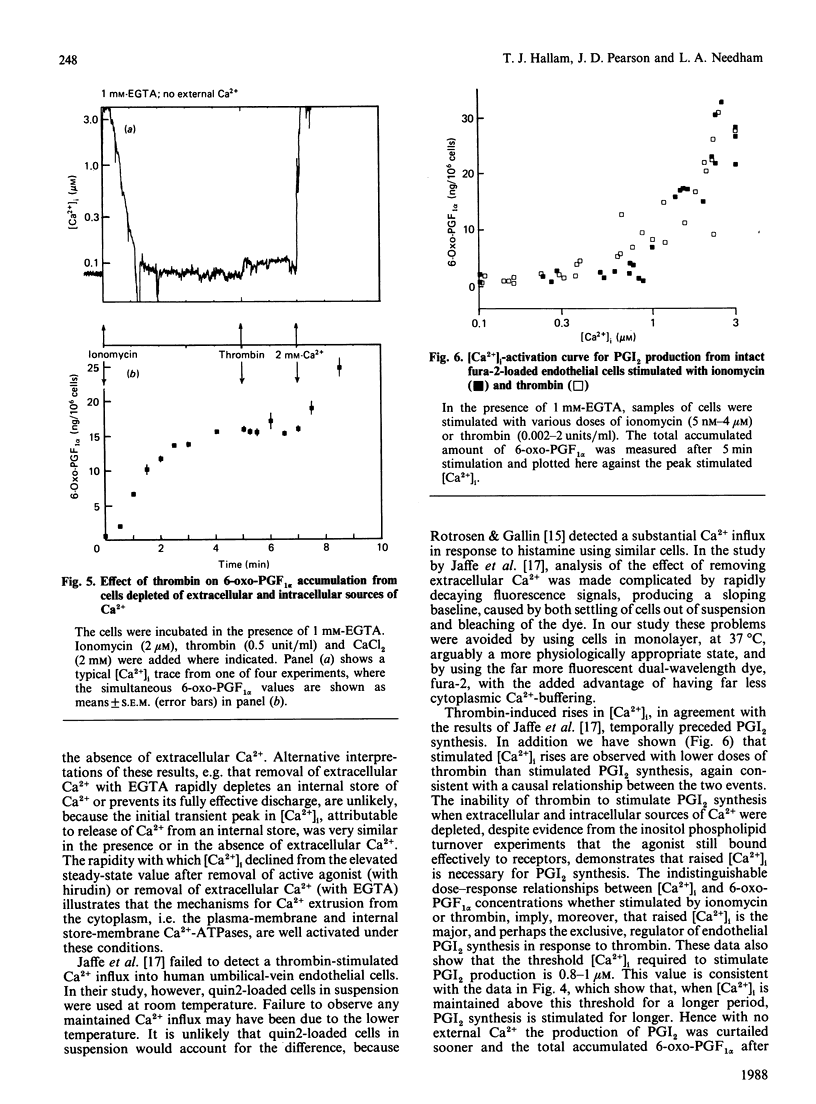
Endothelial cells are known to release prostacyclin (PGI2) in response to agonists, and this has generally been assumed to be caused, at least in part, by activation of a phospholipase A2 by elevated concentrations of cytoplasmic free calcium ([Ca2+]i). However, it has been shown in the blood platelet that agonists can cause arachidonate release without elevating [Ca2+]i. In the present study, rigorous analysis is made of the [Ca2+]i-dependence of PGI2 production in the human umbilical-vein endothelial cell. Thrombin caused a rapid increase in [Ca2+]i from the resting basal value of 0.1 microM to a peak, within 10-15 s, of approx. 2 microM. In the absence of extracellular Ca2+, [Ca2+]i then declined back to the resting value within 2-3 min. In the presence of extracellular Ca2+, [Ca2+]i partly decreased to a new steady-state value of approx. 1 microM. The elevated [Ca2+]i was maintained while the stimulus and the source of extracellular Ca2+ were present, suggesting that it was dependent on influx of Ca2+ across the plasma membrane. Thrombin stimulated the production of PGI2 in the presence or in the absence of extracellular Ca2+. However, the production of PGI2 was more prolonged in the presence of extracellular Ca2+. Total accumulated amounts of 6-oxo-prostaglandin F1 alpha on stimulation with thrombin without extracellular Ca2+ were only 65% of those accumulated with extracellular Ca2+ present. Cells depleted of extracellular and intracellular sources of Ca2+ by incubation with 1 mM extracellular EGTA and exposing them to ionomycin to discharge intracellular stores produced no elevation of [Ca2+]i on stimulation with thrombin or production of PGI2. The threshold [Ca2+]i required to support the production of PGI2 was measured to be 0.8-1.0 microM by using different doses of ionomycin selectively to increase [Ca2+]i. This relationship between [Ca2+]i and PGI2 production was similar to that produced by using different doses of thrombin. Our results show that the major and probably exclusive intracellular stimulus for the production of PGI2 by the vascular endothelial cell in response to thrombin is the elevation of [Ca2+]i.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ager A., Gordon J. L., Moncada S., Pearson J. D., Salmon J. A., Trevethick M. A. Effects of isolation and culture on prostaglandin synthesis by porcine aortic endothelial and smooth muscle cells. J Cell Physiol. 1982 Jan;110(1):9–16. doi: 10.1002/jcp.1041100103. [DOI] [PubMed] [Google Scholar]
- Baenziger N. L., Force L. E., Becherer P. R. Histamine stimulate prostacyclin synthesis in cultured human umbilical vein endothelial cells. Biochem Biophys Res Commun. 1980 Feb 27;92(4):1435–1440. doi: 10.1016/0006-291x(80)90447-7. [DOI] [PubMed] [Google Scholar]
- Boeynaems J. M., Galand N. Stimulation of vascular prostacyclin synthesis by extracellular ADP and ATP. Biochem Biophys Res Commun. 1983 Apr 15;112(1):290–296. doi: 10.1016/0006-291x(83)91829-6. [DOI] [PubMed] [Google Scholar]
- Brock T. A., Gimbrone M. A., Jr Platelet activating factors alters calcium homeostasis in cultured vascular endothelial cells. Am J Physiol. 1986 Jun;250(6 Pt 2):H1086–H1092. doi: 10.1152/ajpheart.1986.250.6.H1086. [DOI] [PubMed] [Google Scholar]
- Brotherton A. F., Hoak J. C. Role of Ca2+ and cyclic AMP in the regulation of the production of prostacyclin by the vascular endothelium. Proc Natl Acad Sci U S A. 1982 Jan;79(2):495–499. doi: 10.1073/pnas.79.2.495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brotherton A. F. Induction of prostacyclin biosynthesis is closely associated with increased guanosine 3',5'-cyclic monophosphate accumulation in cultured human endothelium. J Clin Invest. 1986 Nov;78(5):1253–1260. doi: 10.1172/JCI112709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bussolino F., Aglietta M., Sanavio F., Stacchini A., Lauri D., Camussi G. Alkyl-ether phosphoglycerides influence calcium fluxes into human endothelial cells. J Immunol. 1985 Oct;135(4):2748–2753. [PubMed] [Google Scholar]
- Crutchley D. J., Ryan J. W., Ryan U. S., Fisher G. H. Bradykinin-induced release of prostacyclin and thromboxanes from bovine pulmonary artery endothelial cells. Studies with lower homologs and calcium antagonists. Biochim Biophys Acta. 1983 Mar 22;751(1):99–107. doi: 10.1016/0005-2760(83)90261-8. [DOI] [PubMed] [Google Scholar]
- Downes C. P., Hawkins P. T., Irvine R. F. Inositol 1,3,4,5-tetrakisphosphate and not phosphatidylinositol 3,4-bisphosphate is the probable precursor of inositol 1,3,4-trisphosphate in agonist-stimulated parotid gland. Biochem J. 1986 Sep 1;238(2):501–506. doi: 10.1042/bj2380501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallam T. J., Pearson J. D. Exogenous ATP raises cytoplasmic free calcium in fura-2 loaded piglet aortic endothelial cells. FEBS Lett. 1986 Oct 20;207(1):95–99. doi: 10.1016/0014-5793(86)80019-9. [DOI] [PubMed] [Google Scholar]
- Hamilton K. K., Sims P. J. Changes in cytosolic Ca2+ associated with von Willebrand factor release in human endothelial cells exposed to histamine. Study of microcarrier cell monolayers using the fluorescent probe indo-1. J Clin Invest. 1987 Feb;79(2):600–608. doi: 10.1172/JCI112853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassid A., Oudinet J. P. Relationship between cellular calcium and prostaglandin synthesis in cultured vascular smooth muscle cells. Prostaglandins. 1986 Sep;32(3):457–478. doi: 10.1016/0090-6980(86)90012-2. [DOI] [PubMed] [Google Scholar]
- Hong S. L., Deykin D. Activation of phospholipases A2 and C in pig aortic endothelial cells synthesizing prostacyclin. J Biol Chem. 1982 Jun 25;257(12):7151–7154. [PubMed] [Google Scholar]
- Hong S. L. Effect of bradykinin and thrombin on prostacyclin synthesis in endothelial cells from calf and pig aorta and human umbilical cord vein. Thromb Res. 1980 Jun 15;18(6):787–795. doi: 10.1016/0049-3848(80)90201-7. [DOI] [PubMed] [Google Scholar]
- Hong S. L. Inhibition of prostacyclin synthesis in endothelial cells by methylisobutylxanthine is not mediated through elevated cAMP level. Biochim Biophys Acta. 1983 Dec 20;754(3):258–263. doi: 10.1016/0005-2760(83)90140-6. [DOI] [PubMed] [Google Scholar]
- Jaffe E. A., Grulich J., Weksler B. B., Hampel G., Watanabe K. Correlation between thrombin-induced prostacyclin production and inositol trisphosphate and cytosolic free calcium levels in cultured human endothelial cells. J Biol Chem. 1987 Jun 25;262(18):8557–8565. [PubMed] [Google Scholar]
- Jaffe E. A., Nachman R. L., Becker C. G., Minick C. R. Culture of human endothelial cells derived from umbilical veins. Identification by morphologic and immunologic criteria. J Clin Invest. 1973 Nov;52(11):2745–2756. doi: 10.1172/JCI107470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambert T. L., Kent R. S., Whorton A. R. Bradykinin stimulation of inositol polyphosphate production in porcine aortic endothelial cells. J Biol Chem. 1986 Nov 15;261(32):15288–15293. [PubMed] [Google Scholar]
- Lückhoff A., Busse R. Increased free calcium in endothelial cells under stimulation with adenine nucleotides. J Cell Physiol. 1986 Mar;126(3):414–420. doi: 10.1002/jcp.1041260312. [DOI] [PubMed] [Google Scholar]
- Nishizuka Y. The role of protein kinase C in cell surface signal transduction and tumour promotion. Nature. 1984 Apr 19;308(5961):693–698. doi: 10.1038/308693a0. [DOI] [PubMed] [Google Scholar]
- Pearson J. D., Carleton J. S., Hutchings A., Gordon J. L. Uptake and metabolism of adenosine by pig aortic endothelial and smooth-muscle cells in culture. Biochem J. 1978 Feb 15;170(2):265–271. doi: 10.1042/bj1700265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollock W. K., Irvine R. F., Rink T. J. Free Ca2+ requirements of agonist-induced thromboxane A2 synthesis in human platelets. Eur J Pharmacol. 1986 Dec 16;132(2-3):309–312. doi: 10.1016/0014-2999(86)90622-9. [DOI] [PubMed] [Google Scholar]
- Pollock W. K., Rink T. J., Irvine R. F. Liberation of [3H]arachidonic acid and changes in cytosolic free calcium in fura-2-loaded human platelets stimulated by ionomycin and collagen. Biochem J. 1986 May 1;235(3):869–877. doi: 10.1042/bj2350869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rittenhouse S. E., Horne W. C. Ionomycin can elevate intraplatelet Ca2+ and activate phospholipase A without activating phospholipase C. Biochem Biophys Res Commun. 1984 Aug 30;123(1):393–397. doi: 10.1016/0006-291x(84)90426-1. [DOI] [PubMed] [Google Scholar]
- Rotrosen D., Gallin J. I. Histamine type I receptor occupancy increases endothelial cytosolic calcium, reduces F-actin, and promotes albumin diffusion across cultured endothelial monolayers. J Cell Biol. 1986 Dec;103(6 Pt 1):2379–2387. doi: 10.1083/jcb.103.6.2379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seid J. M., MacNeil S., Tomlinson S. Calcium, calmodulin, and the production of prostacyclin by cultured vascular endothelial cells. Biosci Rep. 1983 Nov;3(11):1007–1015. doi: 10.1007/BF01121027. [DOI] [PubMed] [Google Scholar]
- Simpson A. W., Hallam T. J., Rink T. J. TMB-8 inhibits secretion evoked by phorbol ester at basal cytoplasmic free calcium in quin2-loaded platelets much more effectively than it inhibits thrombin-induced calcium mobilisation. FEBS Lett. 1984 Oct 15;176(1):139–143. doi: 10.1016/0014-5793(84)80928-x. [DOI] [PubMed] [Google Scholar]
- Tsien R. Y., Pozzan T., Rink T. J. Calcium homeostasis in intact lymphocytes: cytoplasmic free calcium monitored with a new, intracellularly trapped fluorescent indicator. J Cell Biol. 1982 Aug;94(2):325–334. doi: 10.1083/jcb.94.2.325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsien R. Y., Rink T. J., Poenie M. Measurement of cytosolic free Ca2+ in individual small cells using fluorescence microscopy with dual excitation wavelengths. Cell Calcium. 1985 Apr;6(1-2):145–157. doi: 10.1016/0143-4160(85)90041-7. [DOI] [PubMed] [Google Scholar]
- Weksler B. B., Ley C. W., Jaffe E. A. Stimulation of endothelial cell prostacyclin production by thrombin, trypsin, and the ionophore A 23187. J Clin Invest. 1978 Nov;62(5):923–930. doi: 10.1172/JCI109220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Bosch H. Intracellular phospholipases A. Biochim Biophys Acta. 1980 Sep 30;604(2):191–246. doi: 10.1016/0005-2736(80)90574-x. [DOI] [PubMed] [Google Scholar]


