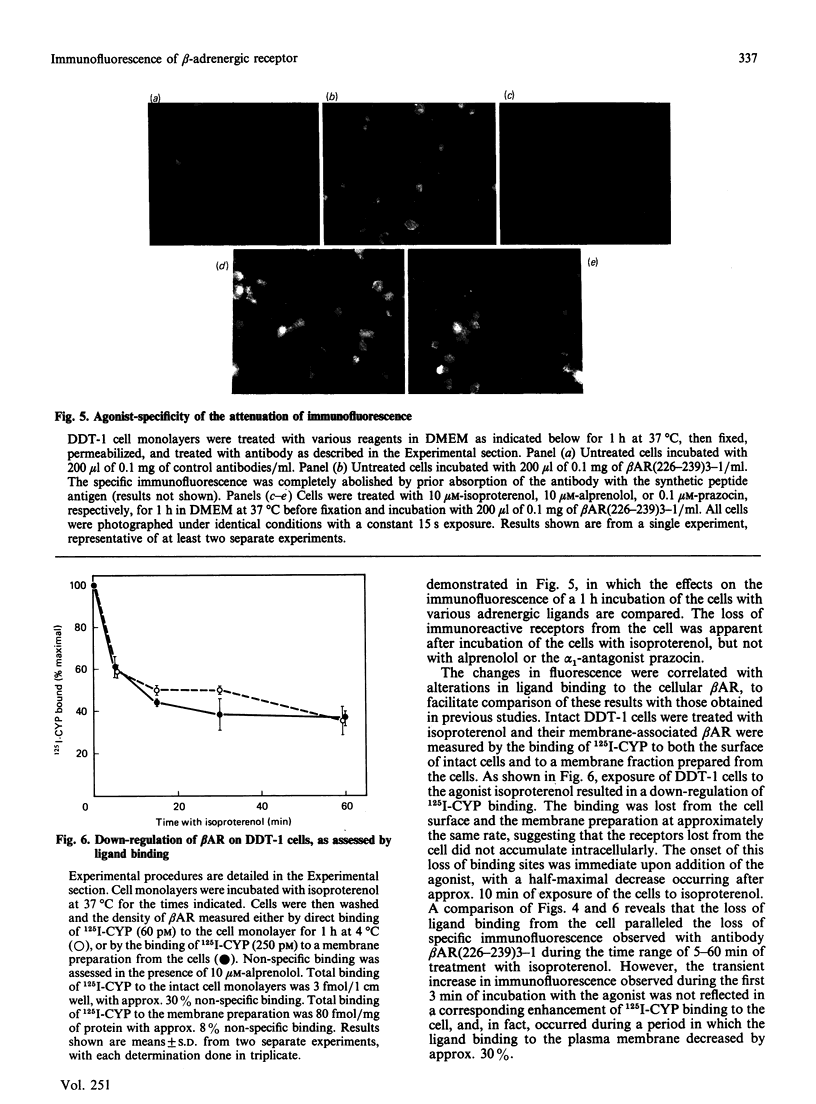
Abstract
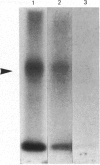
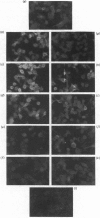
Continuous incubation of cultured cells with beta-adrenergic agonists results in the desensitization of adrenergic responsiveness accompanied by the down-regulation of cell surface beta-adrenergic receptors (beta AR). Previous studies have relied on measurements of ligand binding activity for the detection of the beta AR in the cell. In the present study, we have raised a monoclonal antibody to a synthetic peptide corresponding to amino acid numbers 226-239 of the hamster beta 2AR. This antibody was used to localize the beta AR in hamster smooth-muscle DDT-1 cells by immunofluorescence, without regard for the ability of the receptor to bind ligands. The beta AR was found to be localized primarily at the plasma membrane of these cells, with a nonhomogeneous pattern of distribution. A rapid loss of beta AR-specific immunofluorescence, which paralleled receptor down-regulation as measured by ligand-binding activity, was seen with beta-adrenergic agonists, but not with antagonists. In addition, a transient increase in fluorescence was observed after short times of exposure of the cells to agonists. This fluorescence increase may reflect a ligand-induced conformational change in the receptor.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Benovic J. L., Shorr R. G., Caron M. G., Lefkowitz R. J. The mammalian beta 2-adrenergic receptor: purification and characterization. Biochemistry. 1984 Sep 25;23(20):4510–4518. doi: 10.1021/bi00315a002. [DOI] [PubMed] [Google Scholar]
- Dixon R. A., Kobilka B. K., Strader D. J., Benovic J. L., Dohlman H. G., Frielle T., Bolanowski M. A., Bennett C. D., Rands E., Diehl R. E. Cloning of the gene and cDNA for mammalian beta-adrenergic receptor and homology with rhodopsin. Nature. 1986 May 1;321(6065):75–79. doi: 10.1038/321075a0. [DOI] [PubMed] [Google Scholar]
- Dixon R. A., Sigal I. S., Rands E., Register R. B., Candelore M. R., Blake A. D., Strader C. D. Ligand binding to the beta-adrenergic receptor involves its rhodopsin-like core. Nature. 1987 Mar 5;326(6108):73–77. doi: 10.1038/326073a0. [DOI] [PubMed] [Google Scholar]
- Douillard J. Y., Hoffman T. Enzyme-linked immunosorbent assay for screening monoclonal antibody production using enzyme-labeled second antibody. Methods Enzymol. 1983;92:168–174. doi: 10.1016/0076-6879(83)92016-5. [DOI] [PubMed] [Google Scholar]
- Findlay J. B., Pappin D. J. The opsin family of proteins. Biochem J. 1986 Sep 15;238(3):625–642. doi: 10.1042/bj2380625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kassis S., Sullivan M. Desensitization of the mammalian beta-adrenergic receptor: analysis of receptor redistribution on nonlinear sucrose gradients. J Cyclic Nucleotide Protein Phosphor Res. 1986;11(1):35–46. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Linden J., Patel A., Spanier A. M., Weglicki W. B. Rapid agonist-induced decrease of 125I-pindolol binding to beta-adrenergic receptors. Relationship to desensitization of cyclic AMP accumulation in intact heart cells. J Biol Chem. 1984 Dec 25;259(24):15115–15122. [PubMed] [Google Scholar]
- Mahan L. C., Koachman A. M., Insel P. A. Genetic analysis of beta-adrenergic receptor internalization and down-regulation. Proc Natl Acad Sci U S A. 1985 Jan;82(1):129–133. doi: 10.1073/pnas.82.1.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norris J. S., Brown P., Cohen J., Cornett L. E., Kohler P. O., MacLeod S. L., Popovich K., Robey R. B., Sifford M., Syms A. J. Glucocorticoid induction of beta-adrenergic receptors in the DDT1 MF-2 smooth muscle cell line involves synthesis of new receptor. Mol Cell Biochem. 1987 Mar;74(1):21–27. doi: 10.1007/BF00221909. [DOI] [PubMed] [Google Scholar]
- Sibley D. R., Lefkowitz R. J. Molecular mechanisms of receptor desensitization using the beta-adrenergic receptor-coupled adenylate cyclase system as a model. Nature. 1985 Sep 12;317(6033):124–129. doi: 10.1038/317124a0. [DOI] [PubMed] [Google Scholar]
- Sibley D. R., Peters J. R., Nambi P., Caron M. G., Lefkowitz R. J. Desensitization of turkey erythrocyte adenylate cyclase. Beta-adrenergic receptor phosphorylation is correlated with attenuation of adenylate cyclase activity. J Biol Chem. 1984 Aug 10;259(15):9742–9749. [PubMed] [Google Scholar]
- Sibley D. R., Strasser R. H., Caron M. G., Lefkowitz R. J. Homologous desensitization of adenylate cyclase is associated with phosphorylation of the beta-adrenergic receptor. J Biol Chem. 1985 Apr 10;260(7):3883–3886. [PubMed] [Google Scholar]
- Strader C. D., Pickel V. M., Joh T. H., Strohsacker M. W., Shorr R. G., Lefkowitz R. J., Caron M. G. Antibodies to the beta-adrenergic receptor: attenuation of catecholamine-sensitive adenylate cyclase and demonstration of postsynaptic receptor localization in brain. Proc Natl Acad Sci U S A. 1983 Apr;80(7):1840–1844. doi: 10.1073/pnas.80.7.1840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strader C. D., Sigal I. S., Blake A. D., Cheung A. H., Register R. B., Rands E., Zemcik B. A., Candelore M. R., Dixon R. A. The carboxyl terminus of the hamster beta-adrenergic receptor expressed in mouse L cells is not required for receptor sequestration. Cell. 1987 Jun 19;49(6):855–863. doi: 10.1016/0092-8674(87)90623-4. [DOI] [PubMed] [Google Scholar]
- Strasser R. H., Stiles G. L., Lefkowitz R. J. Translocation and uncoupling of the beta-adrenergic receptor in rat lung after catecholamine promoted desensitization in vivo. Endocrinology. 1984 Oct;115(4):1392–1400. doi: 10.1210/endo-115-4-1392. [DOI] [PubMed] [Google Scholar]
- Su Y. F., Harden T. K., Perkins J. P. Catecholamine-specific desensitization of adenylate cyclase. Evidence for a multistep process. J Biol Chem. 1980 Aug 10;255(15):7410–7419. [PubMed] [Google Scholar]
- Willingham M. C., Yamada S. S., Pastan I. Ultrastructural antibody localization of alpha2-macroglobulin in membrane-limited vesicles in cultured cells. Proc Natl Acad Sci U S A. 1978 Sep;75(9):4359–4363. doi: 10.1073/pnas.75.9.4359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de StGroth S. F., Scheidegger D. Production of monoclonal antibodies: strategy and tactics. J Immunol Methods. 1980;35(1-2):1–21. doi: 10.1016/0022-1759(80)90146-5. [DOI] [PubMed] [Google Scholar]