Abstract
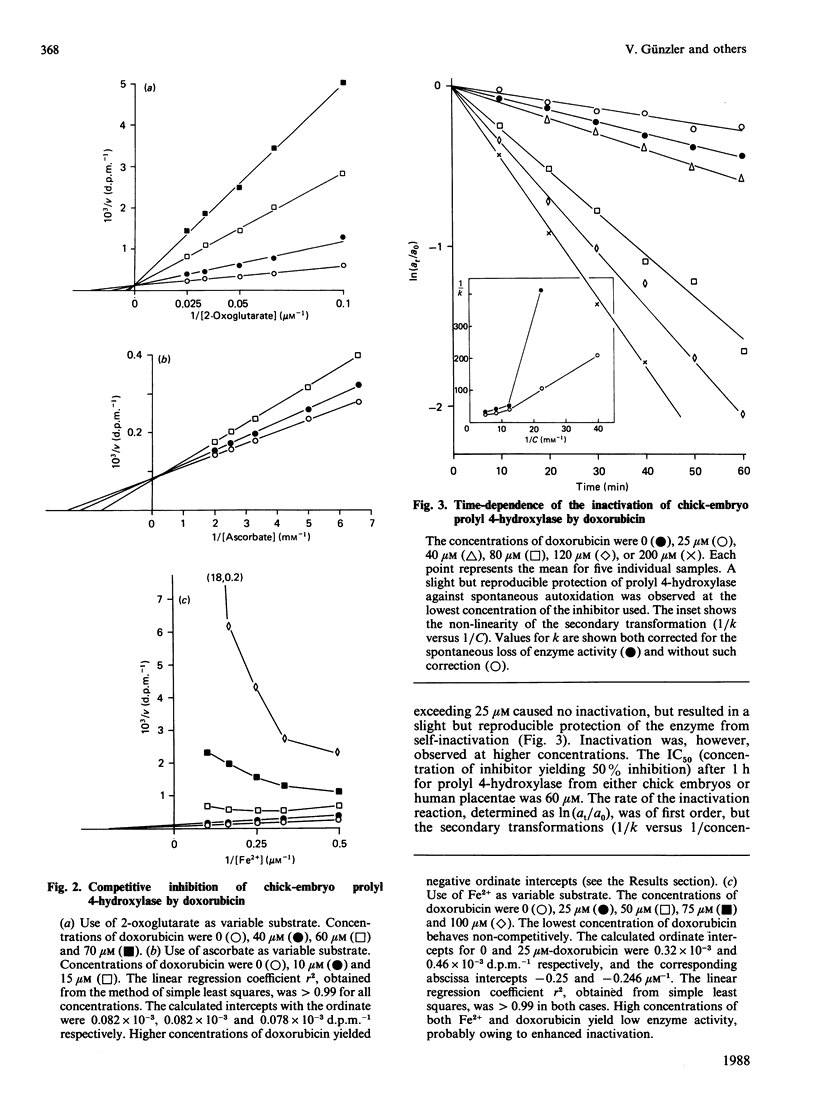
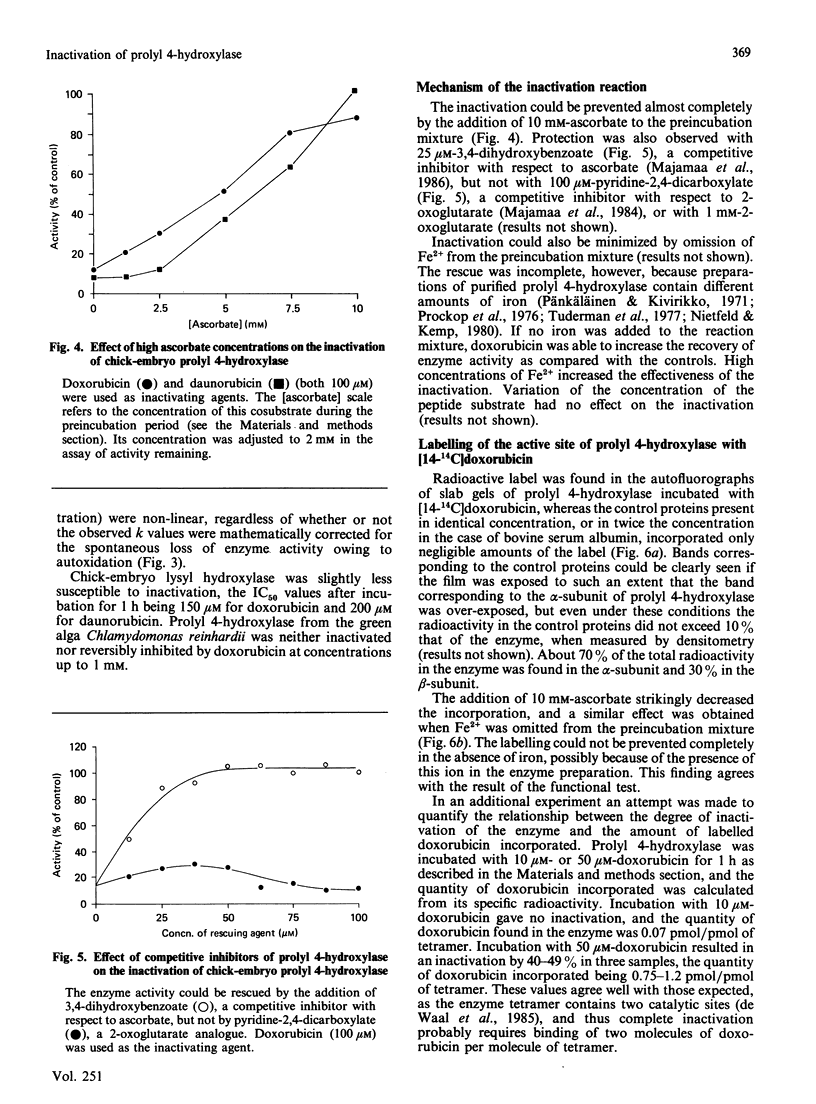
The anthracyclines doxorubicin and daunorubicin were found to act as irreversible inhibitors of prolyl 4-hydroxylase. The reaction rate for enzyme from both chick and human origin was first order, the concentration of inhibitor giving 50% inhibition being 60 microM for both compounds after 1 h. The effect was dependent on the presence of iron ions in the reaction mixture. Inactivation could be prevented by addition of high concentrations of ascorbate, but not 2-oxoglutarate, before the inactivation period. The same results were obtained with competitive analogues of these cosubstrates. Lysyl hydroxylase from chick embryos was also susceptible to inactivation. Its activity was decreased by 50% after incubation for 1 h with a 150 microM concentration of the inhibitors. When chick-embryo prolyl 4-hydroxylase was incubated with [14-14C]doxorubicin, both enzyme subunits were radioactively labelled, about 70% of the total radioactivity being found in the alpha-subunit. Since the anthracyclines are known to undergo a redox reaction generating semiquinone radicals with Fe3+ only, the results suggest that the enzyme-bound iron ion is oxidized to a tervalent intermediate in uncoupled reaction cycles. The data also suggest that both enzyme subunits contribute to the catalytic site of prolyl 4-hydroxylase.
Full text
PDF

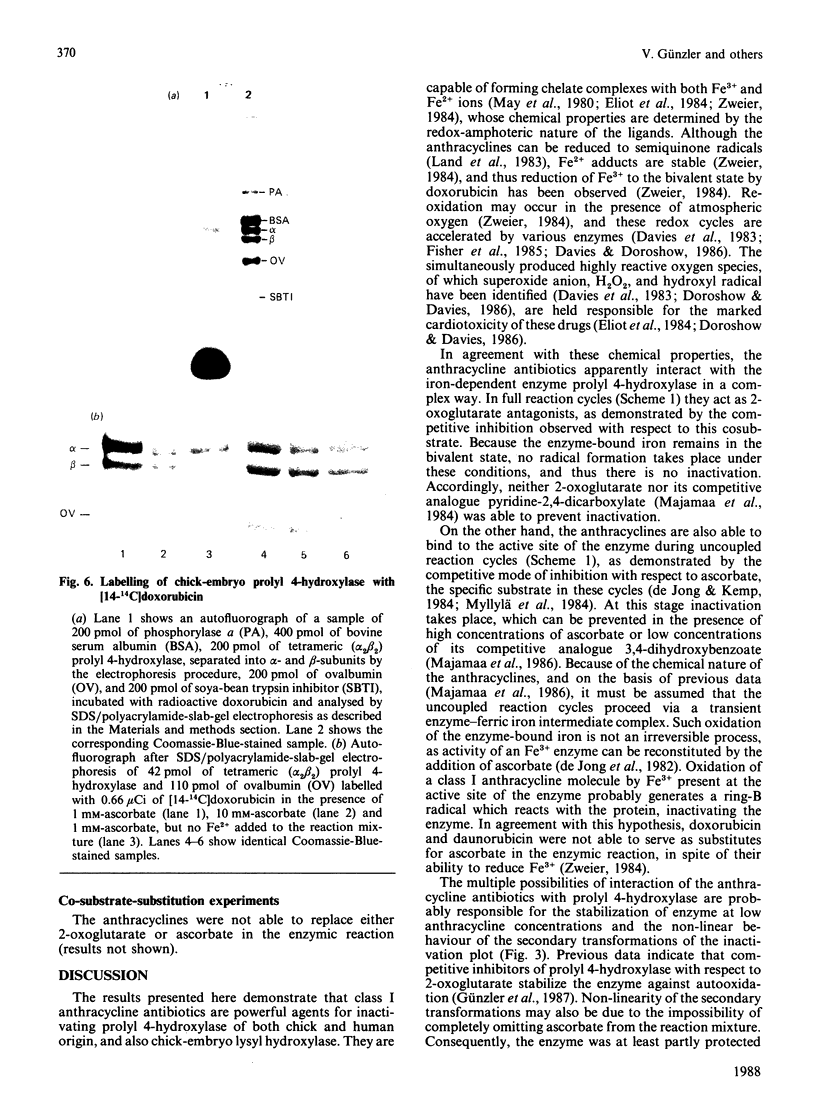


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berg R. A., Prockop D. J. Affinity column purification of protocollagen proline hydroxylase from chick embryos and further characterization of the enzyme. J Biol Chem. 1973 Feb 25;248(4):1175–1182. [PubMed] [Google Scholar]
- Cardinale G. J., Udenfriend S. Prolyl hydroxylase. Adv Enzymol Relat Areas Mol Biol. 1974;41(0):245–300. doi: 10.1002/9780470122860.ch6. [DOI] [PubMed] [Google Scholar]
- Chen-Kiang S., Cardinale G. J., Udenfriend S. Homology between a prolyl hydroxylase subunit and a tissue protein that crossreacts immunologically with the enzyme. Proc Natl Acad Sci U S A. 1977 Oct;74(10):4420–4424. doi: 10.1073/pnas.74.10.4420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Counts D. F., Cardinale G. J., Udenfriend S. Prolyl hydroxylase half reaction: peptidyl prolyl-independent decarboxylation of alpha-ketoglutarate. Proc Natl Acad Sci U S A. 1978 May;75(5):2145–2149. doi: 10.1073/pnas.75.5.2145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies K. J., Doroshow J. H., Hochstein P. Mitochondrial NADH dehydrogenase-catalyzed oxygen radical production by adriamycin, and the relative inactivity of 5-iminodaunorubicin. FEBS Lett. 1983 Mar 7;153(1):227–230. doi: 10.1016/0014-5793(83)80153-7. [DOI] [PubMed] [Google Scholar]
- Davies K. J., Doroshow J. H. Redox cycling of anthracyclines by cardiac mitochondria. I. Anthracycline radical formation by NADH dehydrogenase. J Biol Chem. 1986 Mar 5;261(7):3060–3067. [PubMed] [Google Scholar]
- De Jong L., Kemp A. Stoicheiometry and kinetics of the prolyl 4-hydroxylase partial reaction. Biochim Biophys Acta. 1984 May 31;787(1):105–111. doi: 10.1016/0167-4838(84)90113-4. [DOI] [PubMed] [Google Scholar]
- Devereux D. F., Kent H., Brennan M. F. Time dependent effects of adriamycin and x-ray therapy on wound healing in the rat. Cancer. 1980 Jun 1;45(11):2805–2810. doi: 10.1002/1097-0142(19800601)45:11<2805::aid-cncr2820451115>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- Devereux D. F., Triche T. J., Webber B. L., Thibault L. E., Brennan M. F. A study of adriamycin-reduced wound breaking strenght in rats. An evaluation by light and electron microscopy, induction of collagen maturation, and hydroxyproline content. Cancer. 1980 Jun 1;45(11):2811–2815. doi: 10.1002/1097-0142(19800601)45:11<2811::aid-cncr2820451116>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- Doroshow J. H., Davies K. J. Redox cycling of anthracyclines by cardiac mitochondria. II. Formation of superoxide anion, hydrogen peroxide, and hydroxyl radical. J Biol Chem. 1986 Mar 5;261(7):3068–3074. [PubMed] [Google Scholar]
- Eliot H., Gianni L., Myers C. Oxidative destruction of DNA by the adriamycin-iron complex. Biochemistry. 1984 Feb 28;23(5):928–936. doi: 10.1021/bi00300a021. [DOI] [PubMed] [Google Scholar]
- Fisher J., Abdella B. R., McLane K. E. Anthracycline antibiotic reduction by spinach ferredoxin-NADP+ reductase and ferredoxin. Biochemistry. 1985 Jul 2;24(14):3562–3571. doi: 10.1021/bi00335a026. [DOI] [PubMed] [Google Scholar]
- Günzler V., Hanauske-Abel H. M., Myllylä R., Mohr J., Kivirikko K. I. Time-dependent inactivation of chick-embryo prolyl 4-hydroxylase by coumalic acid. Evidence for a syncatalytic mechanism. Biochem J. 1987 Feb 15;242(1):163–169. doi: 10.1042/bj2420163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanauske-Abel H. M., Günzler V. A stereochemical concept for the catalytic mechanism of prolylhydroxylase: applicability to classification and design of inhibitors. J Theor Biol. 1982 Jan 21;94(2):421–455. doi: 10.1016/0022-5193(82)90320-4. [DOI] [PubMed] [Google Scholar]
- Juva K., Prockop D. J. Modified procedure for the assay of H-3-or C-14-labeled hydroxyproline. Anal Biochem. 1966 Apr;15(1):77–83. doi: 10.1016/0003-2697(66)90249-1. [DOI] [PubMed] [Google Scholar]
- Kaska D. D., Günzler V., Kivirikko K. I., Myllylä R. Characterization of a low-relative-molecular-mass prolyl 4-hydroxylase from the green alga Chlamydomonas reinhardii. Biochem J. 1987 Jan 15;241(2):483–490. doi: 10.1042/bj2410483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kedersha N. L., Berg R. A. An improved method for the purification of vertebrate prolyl hydroxylase by affinity chromatography. Coll Relat Res. 1981 Jul;1(4):345–353. doi: 10.1016/s0174-173x(81)80011-8. [DOI] [PubMed] [Google Scholar]
- Kivirikko K. I., Myllylä R. Posttranslational enzymes in the biosynthesis of collagen: intracellular enzymes. Methods Enzymol. 1982;82(Pt A):245–304. doi: 10.1016/0076-6879(82)82067-3. [DOI] [PubMed] [Google Scholar]
- Kivirikko K. I., Myllylä R. Recent developments in posttranslational modification: intracellular processing. Methods Enzymol. 1987;144:96–114. doi: 10.1016/0076-6879(87)44175-x. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Land E. J., Mukherjee T., Swallow A. J., Bruce J. M. One-electron reduction of adriamycin: properties of the semiquinone. Arch Biochem Biophys. 1983 Aug;225(1):116–121. doi: 10.1016/0003-9861(83)90013-9. [DOI] [PubMed] [Google Scholar]
- Lawrence W. T., Norton J. A., Harvey A. K., Gorschboth C. M., Talbot T. L., Grotendorst G. R. Doxorubicin-induced impairment of wound healing in rats. J Natl Cancer Inst. 1986 Jan;76(1):119–126. [PubMed] [Google Scholar]
- Majamaa K., Günzler V., Hanauske-Abel H. M., Myllylä R., Kivirikko K. I. Partial identity of the 2-oxoglutarate and ascorbate binding sites of prolyl 4-hydroxylase. J Biol Chem. 1986 Jun 15;261(17):7819–7823. [PubMed] [Google Scholar]
- Majamaa K., Hanauske-Abel H. M., Günzler V., Kivirikko K. I. The 2-oxoglutarate binding site of prolyl 4-hydroxylase. Identification of distinct subsites and evidence for 2-oxoglutarate decarboxylation in a ligand reaction at the enzyme-bound ferrous ion. Eur J Biochem. 1984 Jan 16;138(2):239–245. doi: 10.1111/j.1432-1033.1984.tb07907.x. [DOI] [PubMed] [Google Scholar]
- May P. M., Williams G. K., Williams D. R. Solution chemistry studies of adriamycin--iron complexes present in vivo. Eur J Cancer. 1980 Sep;16(9):1275–1276. doi: 10.1016/0014-2964(80)90189-9. [DOI] [PubMed] [Google Scholar]
- Myllylä R., Kuutti-Savolainen E. R., Kivirikko K. I. The role of ascorbate in the prolyl hydroxylase reaction. Biochem Biophys Res Commun. 1978 Jul 28;83(2):441–448. doi: 10.1016/0006-291x(78)91010-0. [DOI] [PubMed] [Google Scholar]
- Myllylä R., Majamaa K., Günzler V., Hanauske-Abel H. M., Kivirikko K. I. Ascorbate is consumed stoichiometrically in the uncoupled reactions catalyzed by prolyl 4-hydroxylase and lysyl hydroxylase. J Biol Chem. 1984 May 10;259(9):5403–5405. [PubMed] [Google Scholar]
- Myllylä R., Tuderman L., Kivirikko K. I. Mechanism of the prolyl hydroxylase reaction. 2. Kinetic analysis of the reaction sequence. Eur J Biochem. 1977 Nov 1;80(2):349–357. doi: 10.1111/j.1432-1033.1977.tb11889.x. [DOI] [PubMed] [Google Scholar]
- Nietfeld J. J., Kemp A. Properties of prolyl 4-hydroxylase containing firmly-bound iron. Biochim Biophys Acta. 1980 Jun 13;613(2):349–358. doi: 10.1016/0005-2744(80)90089-3. [DOI] [PubMed] [Google Scholar]
- Nietfeld J. J., Kemp A. The function of ascorbate with respect to prolyl 4-hydroxylase activity. Biochim Biophys Acta. 1981 Jan 15;657(1):159–167. doi: 10.1016/0005-2744(81)90139-x. [DOI] [PubMed] [Google Scholar]
- Rao N. V., Adams E. Partial reaction of prolyl hydroxylase. (Gly-PRO-Ala)n stimulates alpha-ketoglutarate decarboxylation without prolyl hydroxylation. J Biol Chem. 1978 Sep 25;253(18):6327–6330. [PubMed] [Google Scholar]
- Tuderman L., Kuutti E. R., Kivirikko K. I. An affinity-column procedure using poly(L-proline) for the purification of prolyl hydroxylase. Purification of the enzyme from chick embryos. Eur J Biochem. 1975 Mar 3;52(1):9–16. doi: 10.1111/j.1432-1033.1975.tb03967.x. [DOI] [PubMed] [Google Scholar]
- Tuderman L., Myllylä R., Kivirikko K. I. Mechanism of the prolyl hydroxylase reaction. 1. Role of co-substrates. Eur J Biochem. 1977 Nov 1;80(2):341–348. doi: 10.1111/j.1432-1033.1977.tb11888.x. [DOI] [PubMed] [Google Scholar]
- Turpeenniemi-Hujanen T. M., Puistola U., Kivirikko K. I. Isolation of lysyl hydroxylase, an enzyme of collagen synthesis, from chick embryos as a homogeneous protein. Biochem J. 1980 Aug 1;189(2):247–253. doi: 10.1042/bj1890247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zweier J. L. Reduction of O2 by iron-adriamycin. J Biol Chem. 1984 May 25;259(10):6056–6058. [PubMed] [Google Scholar]
- de Jong L., Albracht S. P., Kemp A. Prolyl 4-hydroxylase activity in relation to the oxidation state of enzyme-bound iron. The role of ascorbate in peptidyl proline hydroxylation. Biochim Biophys Acta. 1982 Jun 4;704(2):326–332. doi: 10.1016/0167-4838(82)90162-5. [DOI] [PubMed] [Google Scholar]
- de Waal A., Hartog A. F., de Jong L. Photoaffinity labelling of the 2-oxoglutarate binding site of prolyl 4-hydroxylase with 5-azidopyridine-2-carboxylic acid. Biochim Biophys Acta. 1987 Mar 18;912(1):151–155. doi: 10.1016/0167-4838(87)90260-3. [DOI] [PubMed] [Google Scholar]
- de Waal A., de Jong L., Hartog A. F., Kemp A. Photoaffinity labeling of peptide binding sites of prolyl 4-hydroxylase with N-(4-azido-2-nitrophenyl)glycyl-(Pro-Pro-Gly)5. Biochemistry. 1985 Nov 5;24(23):6493–6499. doi: 10.1021/bi00344a028. [DOI] [PubMed] [Google Scholar]