Abstract
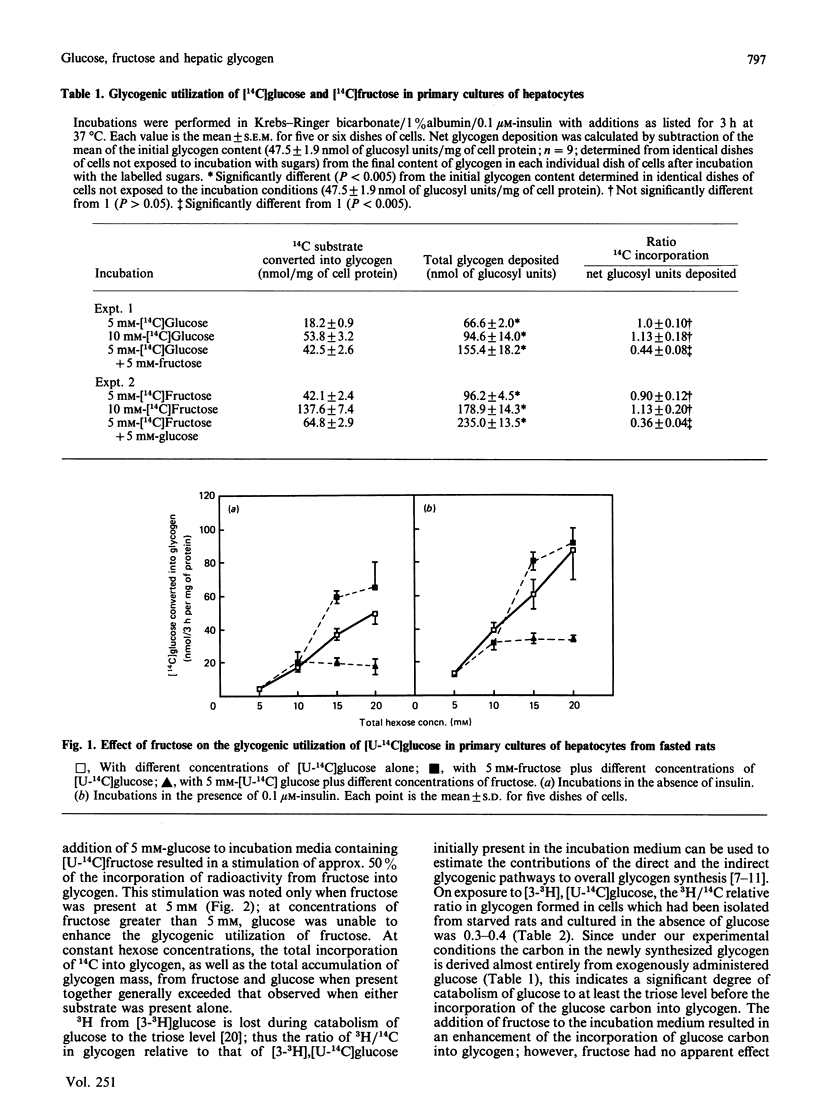
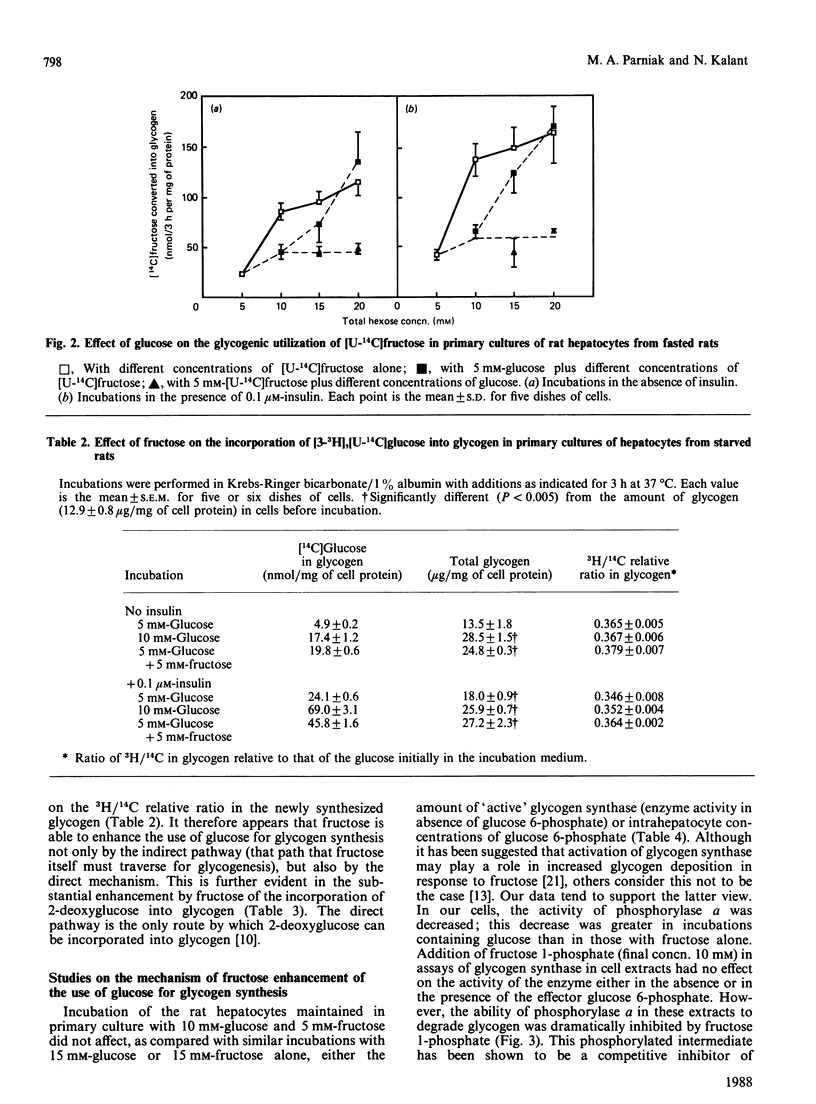
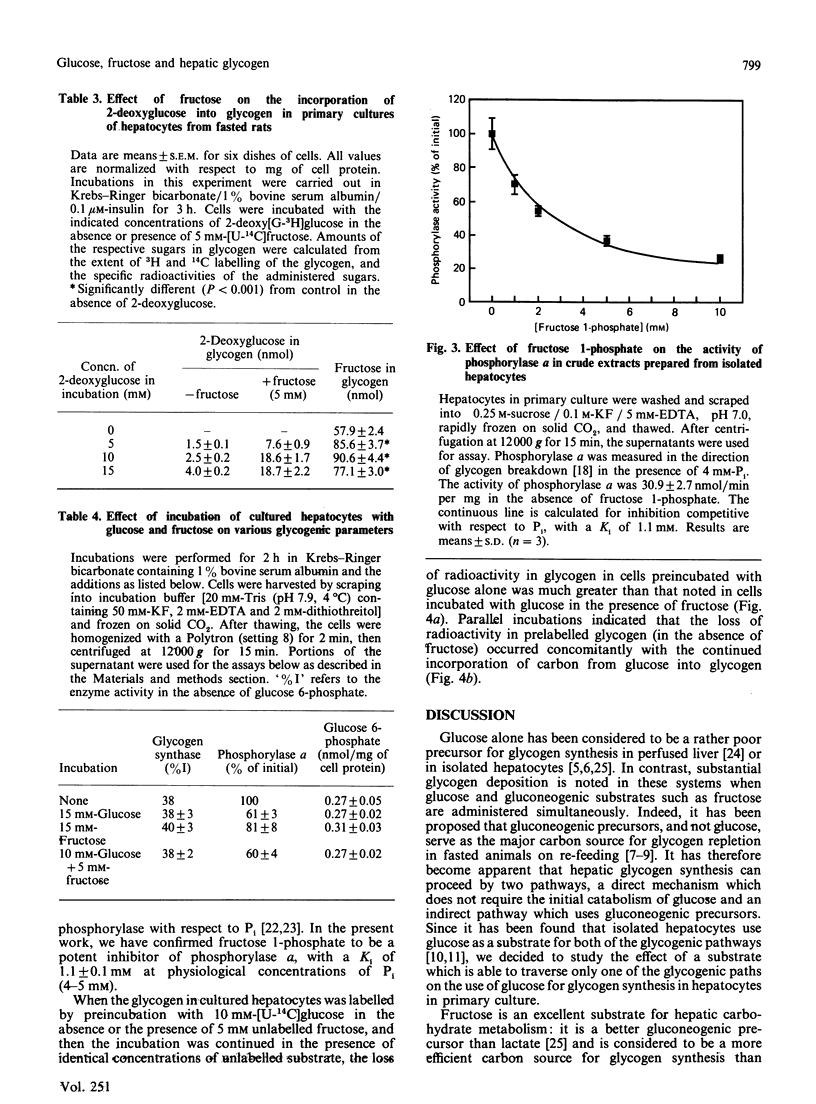
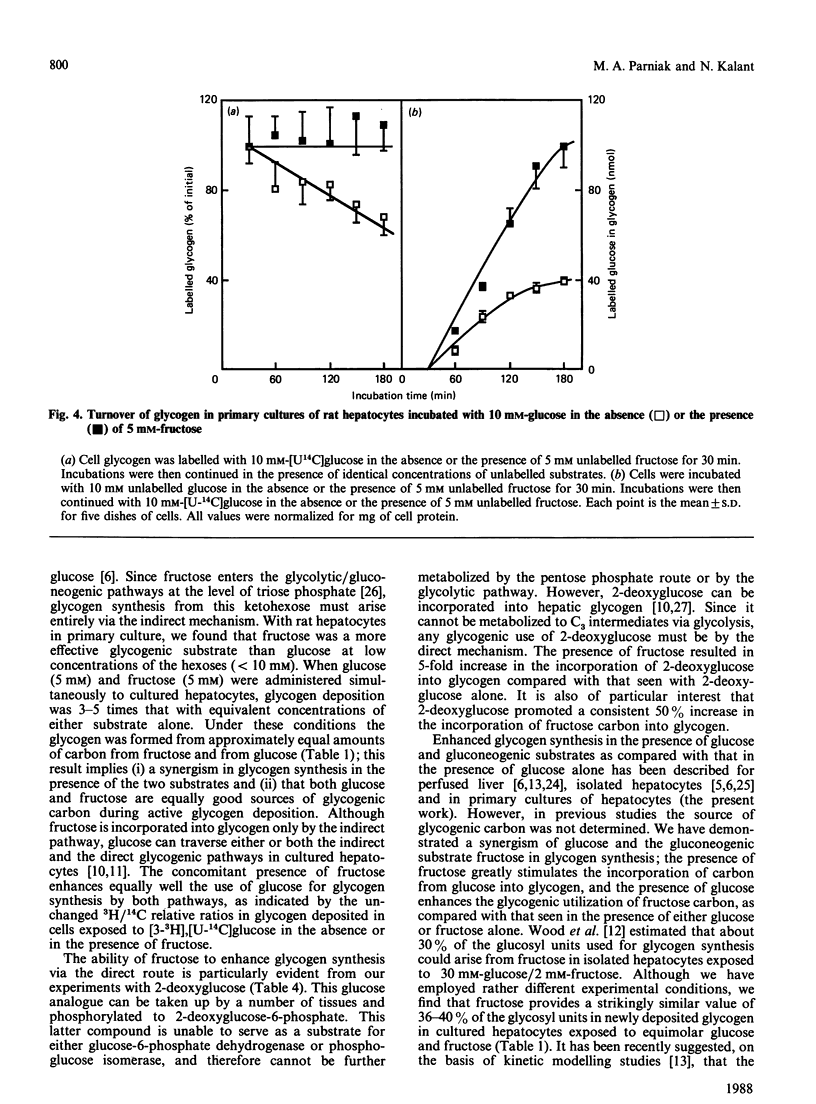
Glycogen synthesis in isolated hepatocytes can occur from glucose both by a direct mechanism and by an indirect process in which glucose is first metabolized to C3 intermediates before use for glycogenesis via gluconeogenesis. We studied the incorporation into glycogen of glucose and the gluconeogenic substrate, fructose, in primary cultures of hepatocytes from fasted rats. In the presence of insulin, both glucose and fructose promoted net deposition of glycogen; however, fructose carbon was incorporated into glycogen to a greater extent than that from glucose. When glucose and fructose were administered simultaneously, the glycogenic utilization of glucose was stimulated 2-3-fold, and that of fructose was increased by about 50%. At constant hexose concentrations, the total incorporation of carbon, and the total accumulation of glycogen mass, from glucose and fructose when present together exceeded that from either substrate alone. Fructose did not change the relative proportion of glucose carbon incorporated into glycogen via the indirect (gluconeogenic) mechanism. The synergism of glucose and fructose in glycogen synthesis in isolated rat hepatocytes in primary culture appears to result from a decrease in the rate of degradation of newly deposited glycogen, owing to (i) decreased amount of phosphorylase a mediated by glucose and (ii) noncovalent inhibition of residual phosphorylase activity by some intermediate arising from the metabolism of fructose, presumably fructose 1-phosphate.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abumrad N. N., Cherrington A. D., Williams P. E., Lacy W. W., Rabin D. Absorption and disposition of a glucose load in the conscious dog. Am J Physiol. 1982 Jun;242(6):E398–E406. doi: 10.1152/ajpendo.1982.242.6.E398. [DOI] [PubMed] [Google Scholar]
- Bergman R. N., Beir J. R., Hourigan P. M. Intraportal glucose infusion matched to oral glucose absorption. Lack of evidence for "gut factor" involvement in hepatic glucose storage. Diabetes. 1982 Jan;31(1):27–35. doi: 10.2337/diab.31.1.27. [DOI] [PubMed] [Google Scholar]
- Boyd M. E., Albright E. B., Foster D. W., McGarry J. D. In vitro reversal of the fasting state of liver metabolism in the rat. Reevaluation of the roles of insulin and glucose. J Clin Invest. 1981 Jul;68(1):142–152. doi: 10.1172/JCI110230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson A. L., Arion W. J. Factors underlying significant underestimations of glucokinase activity in crude liver extracts: physiological implications of higher cellular activity. Arch Biochem Biophys. 1987 Feb 15;253(1):156–167. doi: 10.1016/0003-9861(87)90648-5. [DOI] [PubMed] [Google Scholar]
- DeFronzo R. A., Ferrannini E., Hendler R., Wahren J., Felig P. Influence of hyperinsulinemia, hyperglycemia, and the route of glucose administration on splanchnic glucose exchange. Proc Natl Acad Sci U S A. 1978 Oct;75(10):5173–5177. doi: 10.1073/pnas.75.10.5173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilboe D. P., Larson K. L., Nuttall F. Q. Radioactive method for the assay of glycogen phosphorylases. Anal Biochem. 1972 May;47(1):20–27. doi: 10.1016/0003-2697(72)90274-6. [DOI] [PubMed] [Google Scholar]
- Hems D. A., Whitton P. D., Taylor E. A. Glycogen synthesis in the perfused liver of the starved rat. Biochem J. 1972 Sep;129(3):529–538. doi: 10.1042/bj1290529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hers H. G. The control of glycogen metabolism in the liver. Annu Rev Biochem. 1976;45:167–189. doi: 10.1146/annurev.bi.45.070176.001123. [DOI] [PubMed] [Google Scholar]
- Ishida T., Chap Z., Chou J., Lewis R., Hartley C., Entman M., Field J. B. Differential effects of oral, peripheral intravenous, and intraportal glucose on hepatic glucose uptake and insulin and glucagon extraction in conscious dogs. J Clin Invest. 1983 Aug;72(2):590–601. doi: 10.1172/JCI111007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz J., Golden S., Wals P. A. Glycogen synthesis by rat hepatocytes. Biochem J. 1979 May 15;180(2):389–402. doi: 10.1042/bj1800389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz J., Rognstad R. Futile cycles in the metabolism of glucose. Curr Top Cell Regul. 1976;10:237–289. doi: 10.1016/b978-0-12-152810-2.50013-9. [DOI] [PubMed] [Google Scholar]
- Kuwajima M., Newgard C. B., Foster D. W., McGarry J. D. The glucose-phosphorylating capacity of liver as measured by three independent assays. Implications for the mechanism of hepatic glycogen synthesis. J Biol Chem. 1986 Jul 5;261(19):8849–8853. [PubMed] [Google Scholar]
- LEIBOVITZ A. THE GROWTH AND MAINTENANCE OF TISSUE-CELL CULTURES IN FREE GAS EXCHANGE WITH THE ATMOSPHERE. Am J Hyg. 1963 Sep;78:173–180. doi: 10.1093/oxfordjournals.aje.a120336. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lowry O. H., Schulz D. W., Passonneau J. V. The kinetics of glycogen phosphorylases from brain and muscle. J Biol Chem. 1967 Jan 25;242(2):271–280. [PubMed] [Google Scholar]
- Newgard C. B., Hirsch L. J., Foster D. W., McGarry J. D. Studies on the mechanism by which exogenous glucose is converted into liver glycogen in the rat. A direct or an indirect pathway? J Biol Chem. 1983 Jul 10;258(13):8046–8052. [PubMed] [Google Scholar]
- Newgard C. B., Moore S. V., Foster D. W., McGarry J. D. Efficient hepatic glycogen synthesis in refeeding rats requires continued carbon flow through the gluconeogenic pathway. J Biol Chem. 1984 Jun 10;259(11):6958–6963. [PubMed] [Google Scholar]
- Niewoehner C. B., Nuttall B. Q., Nuttall F. Q. Effects of graded intravenous doses of fructose on glycogen synthase in the liver of fasted rats. Metabolism. 1987 Apr;36(4):338–344. doi: 10.1016/0026-0495(87)90204-6. [DOI] [PubMed] [Google Scholar]
- Parniak M., Kalant N. Incorporation of glucose into glycogen in primary cultures of rat hepatocytes. Can J Biochem Cell Biol. 1985 May;63(5):333–340. doi: 10.1139/o85-049. [DOI] [PubMed] [Google Scholar]
- Pentreath V. W., Seal L. H., Kai-Kai M. A. Incorporation of [3H]2-deoxyglucose into glycogen in nervous tissues. Neuroscience. 1982 Mar;7(3):759–767. doi: 10.1016/0306-4522(82)90081-1. [DOI] [PubMed] [Google Scholar]
- Spence J. T., Koudelka A. P. Pathway of glycogen synthesis from glucose in hepatocytes maintained in primary culture. J Biol Chem. 1985 Feb 10;260(3):1521–1526. [PubMed] [Google Scholar]
- Stalmans W. The role of the liver in the homeostasis of blood glucose. Curr Top Cell Regul. 1976;11:51–97. doi: 10.1016/b978-0-12-152811-9.50009-2. [DOI] [PubMed] [Google Scholar]
- Stanley J. C., Dohm G. L., McManus B. S., Newsholme E. A. Activities of glucokinase and hexokinase in mammalian and avian livers. Biochem J. 1984 Dec 1;224(2):667–671. doi: 10.1042/bj2240667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas J. A., Schlender K. K., Larner J. A rapid filter paper assay for UDPglucose-glycogen glucosyltransferase, including an improved biosynthesis of UDP-14C-glucose. Anal Biochem. 1968 Oct 24;25(1):486–499. doi: 10.1016/0003-2697(68)90127-9. [DOI] [PubMed] [Google Scholar]
- Thurston J. H., Jones E. M., Hauhart R. E. Decrease and inhibition of liver glycogen phosphorylase after fructose. An experimental model for the study of hereditary fructose intolerance. Diabetes. 1974 Jul;23(7):597–604. doi: 10.2337/diab.23.7.597. [DOI] [PubMed] [Google Scholar]
- Van Den Berghe G., Hue L., Hers H. G. Effect of administration of the fructose on the glycogenolytic action of glucagon. An investigation of the pathogeny of hereditary fructose intolerance. Biochem J. 1973 Jun;134(2):637–645. doi: 10.1042/bj1340637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van den Berghe G. Fructose: metabolism and short-term effects on carbohydrate and purine metabolic pathways. Prog Biochem Pharmacol. 1986;21:1–32. [PubMed] [Google Scholar]
- Wood C. L., Babcock C. J., Blum J. J. Effects of vasopressin on fructose and glycogen metabolism in hepatocytes from fed and fasted rats. Arch Biochem Biophys. 1981 Nov;212(1):43–53. doi: 10.1016/0003-9861(81)90341-6. [DOI] [PubMed] [Google Scholar]
- Youn J. H., Kaslow H. R., Bergman R. N. Fructose effect to suppress hepatic glycogen degradation. J Biol Chem. 1987 Aug 25;262(24):11470–11477. [PubMed] [Google Scholar]
- Youn J. H., Youn M. S., Bergman R. N. Synergism of glucose and fructose in net glycogen synthesis in perfused rat livers. J Biol Chem. 1986 Dec 5;261(34):15960–15969. [PubMed] [Google Scholar]


