Summary
Background
Despite significant reductions in mother-to-child HIV-1 transmission risks due to the advancements and scale-up of antiretroviral therapy (ART), the global burden of HIV-1 drug resistance (HIVDR) in treatment-naive and treatment-experienced children and adolescents remains poorly understood. In this study, we conducted a systematic review and meta-analysis to estimate the prevalence of HIVDR in these populations globally, regionally, and at the country level.
Methods
We systematically searched PubMed, Embase, and Web of Science for studies reporting HIVDR in treatment-naive and treatment-experienced children and adolescents from inception to June 28, 2024. Eligible studies reported at least ten successfully genotyped cases. We excluded studies where drug resistance was not reported separately for children and adults or for treatment-naive and treatment-experienced populations. The methodological quality of eligible studies was assessed, and random-effect models were used for meta-analysis to determine the pooled overall and regimen-specific prevalence of one or more HIVDR mutations in these populations globally, regionally, or at the country level. This study is registered with PROSPERO under the number CRD42023424483.
Findings
Of 2282 records identified, 136 studies (28,539 HIV-1-infected children from 52 countries) were included for analysis. The overall prevalence of HIVDR is 26.31% (95% CI, 20.76–32.25) among treatment-naive children and 74.16% (95% CI, 67.74–80.13) among treatment-experienced children (p < 0.0001). HIVDR varied widely across subregion with the highest prevalence in Southern Africa (37.80% [95% CI, 26.24–50.08]) and lowest in South America (11.79% [95% CI, 4.91–20.84]) for treatment-naive children while highest in Asia (80.85% [95% CI, 63.76–93.55]) and lowest in Europe (54.39% [95% CI, 28.61–79.03]) for treatment-experienced children. The proportion of viral failure (VF) presented positive correlation with DR prevalence for treatment-experienced children, which increased from 61.23% (95% CI, 47.98–73.72) in proportion of VF <50%–81.17% (95% CI, 71.57–89.28) in proportion of 100%. Meta-regression analysis for both groups showed that only age (naive: p = 0.0005; treated: p < 0.0001) was the sources of heterogeneity. Non-nucleoside reverse transcriptase inhibitor (NNRTI) resistances were the most seen mutations among the treatment-naive group, with the HIVDR prevalence more than 10% in Southern Africa, Western and Central Africa, Eastern Africa, Asia, and North America. Both nucleoside reverse transcriptase inhibitor (NRTI) and NNRTI resistances were commonly seen among the treatment-experienced group, varying from 36.33% (95% CI, 11.96–64.93) in North America to 77.54% (95% CI, 62.70–89.58) in South America for NRTI and from 39.98% (95% CI, 13.47–69.97) in Europe to 68.86 (95% CI, 43.91–89.17) in Asia for NNRTI, respectively.
Interpretation
This study underscores the significant burden of HIVDR among children and adolescents worldwide, particularly pronounced in sub-Saharan Africa and low-income countries. It emphasizes the critical importance of surveillance in all HIV-1-infected children and advocates for the adoption of dolutegravir (DTG) or other optimal formulations as first-line ART in settings where NNRTI resistance exceeds the WHO's 10% threshold. DTG's high resistance barrier, potent antiviral efficacy, and favorable safety profile makes it a superior choice for managing drug-resistant HIV-1, surpassing traditional antiretroviral therapies.
Funding
This work was supported by the Science and Technology Innovation Committee of Shenzhen Municipality (No. JCYJ20220531102202005) and the Natural Science Foundation of Guangdong Province (No. 2024A1515012118).
Keywords: Global prevalence, HIV drug resistance, Children, Adolescents, Meta-analysis
Research in context.
Evidence before this study
The emergence of HIV-1 drug resistance (HIVDR) among infants, children, and adolescents poses a significant threat to global efforts in combating HIV-1/AIDS, particularly in the context of widespread implementation of a “treat all” approach and the scale-up of prevention of mother-to-child transmission programs. A comprehensive understanding of HIVDR prevalence in this vulnerable population is crucial for informing effective treatment strategies and public health policies. Prior to our study, a systematic search of PubMed, Web of Science, and Embase yielded limited evidence on the global pretreatment drug resistance (PDR) in children with HIV-1. While a meta-analysis conducted in 2016 focused on pretreatment HIVDR in children residing in sub-Saharan Africa, it lacked a comprehensive assessment of HIVDR on a global scale.
Added value of this study
Our systematic review and meta-analysis provides the most comprehensive assessment and robust evidence to date of the HIVDR prevalence in HIV-infected children worldwide. We found that the prevalence of drug resistance (DR) to non-nucleoside reverse transcriptase inhibitor (NNRTI) in treatment-naive children exceeded the WHO's 10% threshold for changing first-line antiretroviral therapy (ART) regimen in Africa, Asia, and North America, and approached this threshold in South America and Europe. Importantly, our study also sheds light on the high prevalence of both nucleoside/nucleotide reverse transcriptase inhibitor and NNRTI resistances among treatment-experienced children, with rates escalating alongside increasing proportions of viral failure.
Implications of all the available evidence
Our results show that routine HIVDR testing or at least periodic nationally representative surveys is imperative for informing the HIVDR prevalence and guiding national programmes on optimal population-level ART regimen selection. In settings where prevalence of PDR to NNRTIs exceeds 10%, or in cases of treatment failure among children on ART, timely transition to dolutegravir and other optimal formulations is paramount. We advocate for global and national support in negotiating better prices for these formulations and for facilitating smooth transitions to optimize treatment outcomes for children living with HIV-1.
Introduction
Over the past two decades, considerable progress has been achieved in expanding and sustaining prevention of mother-to-child HIV-1 transmission (PMTCT) services, as well as in scaling up care and treatment programs for children and adolescents living with HIV-1. Nevertheless, in 2022 alone, there were 130,000 new HIV-1 infections among children aged 0–14 years, with an estimated 1.54 million (IQR: 1.20 million-2.11 million) children worldwide living with HIV-1, of whom only 57% (44–78) were receiving life-saving antiretroviral therapy (ART).1 Compounding this challenge is the emergence of antiretroviral drug resistance (DR), undermining the effectiveness of HIV-1 treatment and jeopardizing global efforts to end the AIDS epidemic in infants and children.
Infants who acquire HIV-1 during pregnancy or delivery are particularly vulnerable to AIDS or death in the first few months of life, underscoring the urgent need to address ART resistance and subsequent treatment failure to prevent unnecessary loss of life. The 2023 guidelines from the European AIDS Clinical Society (EACS) have updated the preferred and alternative first-line ART regimens for children and adolescents, emphasizing the integration of dolutegravir (DTG), an integrase inhibitor (INSTI), in combination with two nucleoside/nucleotide reverse transcriptase inhibitors (NRTIs).2 While these updates provide expanded treatment options, it remains imperative to address the persistently high levels of pretreatment HIV-1 drug resistance (HIVDR) to non-nucleoside reverse transcriptase inhibitors (NNRTIs), particularly in resource-limited settings where access to child-friendly formulations of the most effective ART remains constrained. Moreover, the pill burden, increased vulnerability to adverse drugs effects, and suboptimal adherence to ART contribute to the lower rates of viral load (VL) suppression among children compared to adults.3,4
In response to the escalating prevalence of ART resistance, WHO's Global Action Plan on HIVDR 2017–2021,5 alongside other international initiatives, outlines crucial actions for national and global stakeholders to prevent, monitor, and respond to HIVDR. While the current recommendations place a significant emphasis on the testing of pretreatment drug resistance (PDR), particularly to NNRTIs due to their high prevalence, they also advocate for comprehensive surveillance and strategic responses to manage emerging resistance patterns across all antiretroviral drug classes, ensuring a holistic approach to addressing HIVDR on a global scale.6 Despite Sub-Saharan Africa bearing the brunt of the pediatric HIV-1 epidemic, individual-level HIVDR testing remains prohibitively expensive and largely inaccessible in resource-limited settings. Therefore, the synthesis of globally and nationally representative HIVDR data becomes imperative for informing targeted public health interventions. To date, trends in prevalence and patterns of resistance in adult people living with HIV-1 (PLWH) have been comprehensively assessed,7 but such systematic analysis in children and adolescents living with HIV-1 has not yet been investigated globally.
Therefore, we conducted a systematic review and meta-analysis to provide a comprehensive assessment of HIVDR among children and adolescents living with HIV-1 worldwide. Specifically, our study aims to estimate the prevalence of both pretreatment and treatment-experienced HIVDR, as well as to investigate the frequencies of drug resistance mutations of public health significance. By synthesizing available evidence, our analysis seeks to inform evidence-based interventions and guide policy efforts to optimize pediatric HIV-1 care and treatment outcomes globally.
Methods
Search strategy and selection criteria
This study constituted a systematic review and meta-regression analysis aimed at assessing the global prevalence of treatment-naive and treatment-experienced DR among children and adolescents. We conducted searches in PubMed, Web of Science, and Embase for studies published in English up to June 28, 2024. Our search strategy employed the following terms: “resistan∗” AND (“`HIV” OR “human immunodeficiency virus” OR “AIDS” OR “acquired immunodeficiency syndrome”) AND (“child∗” OR “adolecen∗” OR “infant∗” OR “newborn∗” OR “pediatri∗”). Detailed search strategies for each database and the full list of search terms are provided in the appendix (Table S1).
We included cross-sectional, case–control, or cohort studies involving children and adolescents (aged <19 years) with or without prior ART exposure history. Studies were eligible if they reported at least ten genotypes successfully tested using standard population sequencing methods (such as ViroSeq/TrueGene/In-house genotyping), single-genome sequencing, allele-specific real-time polymerase chain reaction (AS-PCR), and ultra-deep sequencing. Studies reporting data on PMTCT were included if they provided information on ART exposure in infants. We excluded studies that did not separately report HIVDR for children and adults or for treatment-naive and treatment-experienced populations. We include full length articles and excluded letters, meeting abstracts, case reports, case-series, comments, abstracts, notes, conference proceedings, short communications, reviews and posters due to the limited information they provided.
All titles and abstracts retrieved from the literature search were carefully examined by two independent reviewers (GLY and LYS). The same reviewers assessed the full texts of potentially eligible articles, with any disagreements resolved through consensus by two additional authors (LJY and LXR).
This systematic review and meta-analysis was conducted in accordance with The Cochrane Handbook for Systematic Reviews of Interventions and adhered to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines (Supplementary PRISMA Checklist).8 The study protocol was registered in the International Prospective Register of Systematic Reviews (PROSPERO) (No. CRD42023424483).
Data extraction and quality assessment
Two main study groups, treatment-naive and treatment-experienced, were established for data extraction. Studies were categorized into one of these groups based on the ART history of PLWH. A preconceived and standardized data extraction form was utilized to collect information on various parameters, including study details (author, publication year, country/region, study design, period of sample collection, number of participants and genotypes successfully sequenced, HIV-1 subtypes, genotypic resistance sequencing assay), participant characteristics (age, sex, HIV-1 VL, US CDC or WHO clinical stage), number of patients with more than one drug-resistance mutation, with one or more NRTI/NNRTI/protease inhibitor (PI)/thymidine analogue mutation (TAM), and with multiple-class of HIVDR. We also extracted the drug resistance mutations database used to determine specific drug resistance. If an article met the inclusion criteria but had missing data, such as age and sex information, it was still included in the overall analysis. However, for specific analyses where age and sex were essential variables, those studies were excluded. Most studies reported drug-resistance mutations according to the Stanford Drug Resistance Database list,9 while others used lists such as the WHO Surveillance Drug Resistance Mutations List,10 the ANRS (French National Agency for AIDS Research)11 or the IAS-USA (International AIDS Society-USA Drug Mutation list.12 Additionally, data on PMTCT history, prophylaxis drugs for treatment-naive individuals, antiretroviral regimen, and duration of drug exposure for treatment-experienced individuals were extracted.
We assessed the quality of the included studies using an adapted version of the risk of bias tool specifically designed for prevalence studies, as developed by Hoy et al.13 This tool was selected due to its strong reliability and validity, having been rigorously tested and demonstrated high interrater agreement (overall Kappa statistic of 0.82). We chose this tool over the initially planned tool from our PROSPERO registration to better address the specific needs of prevalence studies, ensuring methodological rigor and the reliability of our findings. The tool addresses key domains of bias, including selection bias, measurement bias, and analysis bias, making it particularly well-suited for our systematic review. Studies were categorized as good quality (low risk of bias), fair quality (moderate risk of bias), or poor quality (high risk of bias) based on this assessment.
For studies reporting resistance data for both treatment-naive and treatment-experienced populations, multiple datasets were extracted and subjected to analysis based on ART history. In cases where studies reported characteristics and resistance mutations across multiple nations or over several sampling years, data were segmented to yield estimates tailored to individual countries and sampling years. When a study includes aggregate data from multiple regions without separate regional reporting (e.g., data on 10 out of 100 patients with inseparable resistance data from Africa and Asia), the collective results will be attributed to each involved region for regional analyses (e.g., reporting 1 resistance in 10 out of 100 patients in both Africa and Asia). Studies were categorized by region—Asia, Europe, North and South America, Eastern Africa, Southern Africa, Western and Central Africa when analyzed in regional level due to the data availability that two thirds of the datasets were from Africa (Table S4 and Fig. S1).
Statistics
The main outcome was the overall prevalence of HIV-1 ART-resistant mutations. Following an exploratory analysis, we employed Freeman-Tukey double arcsine transformation to stabilize the variances of raw prevalence data. The prevalence of ART-resistant mutations was estimated by pooling data using a random-effects model. We estimated heterogeneity between studies using Cochran's Q statistic (p < 0.05 indicates moderate heterogeneity) and the I2 statistic (≥50% or higher indicates moderate heterogeneity). We conducted similar analyses to estimate the prevalence of drug-class-specific mutations (NRTI mutations, NNRTI, and PI) by region, as well as prevalence of selected major individual mutation within these three classes.
Subgroup analyses considered multiple variables, including country, region (Asia, Europe, North and South America, Eastern Africa, Southern Africa, Western and Central Africa), level of country income (low, lower middle, upper middle, high income), sampling year (before 2015 vs after 2015), age (≤2 years vs >2 years for treatment-naive participants; ≤7 years vs >7 years for treatment-experienced participants), PMTCT experience (yes vs no), proportion of the male (≤50% vs >50%) considering that the birth ratio of males to females is approximately 1:1, CD4+ T cell count (<500 vs ≥500 cells/mL), HIV-1 plasma VL (<5 log copies/mL vs ≥5 log copies/mL) given that the median VL across these studies was approximately 5 log copies/mL for both treatment-naive and treatment-experienced children, proportion of WHO Stage 3/4 or CDC stage C (<50% vs ≥50%), exposure period of antiretroviral treatment (<3 years vs ≥3 years), proportion of participants with viral failure (VF) (100%, 50%–99%, <50%) given the distribution of datasets number across the VF categories, ART regimen (NRTI, NRTI + NNRTI, NRTI + NNRTI/PI, NRTI + PI, NRTI + NNRTI + PI, NNRTI + PI, NRTI + NNRTI/PI/INSTI). We reanalyzed the data using datasets from 2015 onward to provide a more up-to-date and precise understanding of resistance dynamics.
Sensitivity analysis was performed by systematically omitting one study at a time to assess the stability of results and explore potential sources of heterogeneity.
Univariable and multivariable meta-regression analyses were employed to assess the impact of study and participant characteristics on the prevalence estimate. A significance level of p < 0.05 was utilized to determine statistical significance. The meta-regression models revealed a significant association between the age of participants and the proportion of drug-resistant mutations (p < 0.05). Consequently, we conducted further analyses to investigate the relationship between participant age and the prevalence of mutations in both treatment-naive and treatment-experienced children and adolescents.
Data were analyzed using the meta and metafor packages of the statistical software R, version 4.2.1.
Ethics
Ethical approval was not required for this study since it used only secondary data from existing published studies.
Role of funding source
This work was supported by the Science and Technology Innovation Committee of Shenzhen Municipality (No. JCYJ20220531102202005) and the Natural Science Foundation of Guangdong Province (No. 2024A1515012118). The funders of the study had no role in study design, data collection, data analyses, interpretation, or writing of report. All authors had full access to the data in the study and had final responsibility for the decision to submit this manuscript for publication.
Results
A total of 2282 records were initially identified, of which 1100 remained after duplicate removal. After screening titles and abstracts, 714 irrelevant records were excluded. Subsequently, the full texts of 386 papers were scrutinized for eligibility, resulting in the exclusion of 250 studies. Ultimately, 136 full texts (comprising 162 datasets)14, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39, 40, 41, 42, 43, 44, 45, 46, 47, 48, 49, 50, 51, 52, 53, 54, 55, 56, 57, 58, 59, 60, 61, 62, 63, 64, 65, 66, 67, 68, 69, 70, 71, 72, 73, 74, 75, 76, 77, 78, 79, 80, 81, 82, 83, 84, 85, 86, 87, 88, 89, 90, 91, 92, 93, 94, 95, 96, 97, 98, 99, 100, 101, 102, 103, 104, 105, 106, 107, 108, 109, 110, 111, 112, 113, 114, 115, 116, 117, 118, 119, 120, 121, 122, 123, 124, 125, 126, 127, 128, 129, 130, 131, 132, 133, 134, 135, 136, 137, 138, 139, 140, 141, 142, 143, 144, 145, 146, 147, 148, 149 were included in the meta-analysis (Fig. 1). Among the included studies, 69 (48%) were assessed as being of good quality, while 75 (52%) were rated as fair quality; none were deemed to be of poor quality. The appendix summarizes the quality assessment and characteristics of the included studies (Tables S2 and S3). A comprehensive table outlining the Population, Intervention, Comparison, and Outcome (PICO) elements for all included studies is provided in the supplementary materials (Table S2).
Fig. 1.
PRISMA Flowchart for study selection.
In total, we analyzed 162 datasets from 136 individual reports spanning 52 countries. The study-level data encompassed 29,015 patients, of whom 16,812 individuals reporting HIVDR. A comparable number of studies reported drug resistance data for treatment-naive and treatment-experienced individuals across regions, with the majority of data originating from Africa (10,951/16,812, 65.1%, Table 1). The median age was 6 months (IQR 3.62–36.9) for treatment-naive children and 94.8 months (IQR 60.0–121.5) for treated children. Approximately 64.4% (47/73) of datasets involving treatment-naive drug resistance experienced PMTCT. Among treated individuals, approximately 55.2% (8323/15,090) experienced VF during ART, with the therapy duration ranging from 1995 to 2024 and a median duration of 26.5 months (IQR 12.7–50.1, Table 1).
Table 1.
Characteristics of included studies, by region.
| Region | Number of datasets | Number of patients | Number of genotypes | Sampling yeara | Age (months)a | PMTCT experience (number of datasets) | Proportion of viral failure (%)a | ART treatment time (years)a | Regime (%) |
|---|---|---|---|---|---|---|---|---|---|
| Treatment-naive | |||||||||
| Eastern Africa | 12 | 1182 | 975 | 2008 (2000–2018) | 6.0 (1.4–108.0) | 8 | NA | NA | NA |
| Southern Africa | 19 | 4956 | 2610 | 2008 (2002–2017) | 4.7 (1.2–25.2) | 15 | NA | NA | NA |
| Western and Central Africa | 10 | 933 | 898 | 2014 (2006–2019) | 44.4 (3–76.8) | 6 | NA | NA | NA |
| Asia | 10 | 1250 | 893 | 2008 (2002–2019) | 21.0 (0.7–144.0) | 6 | NA | NA | NA |
| South America | 8 | 715 | 580 | 2009 (2002–2013) | 21.5 (2.3–108) | 5 | NA | NA | NA |
| North America | 8 | 4018 | 747 | 2003 (1998–2013) | 5.5 (2.6–180) | 4 | NA | NA | NA |
| Europe | 6 | 871 | 783 | 2001 (1995–2012) | 6.0 (1.0–26) | 3 | NA | NA | NA |
| Treatment-experienced | |||||||||
| Eastern Africa | 18 | 4220 | 2163 | 2010 (2003–2020) | 66.0 (6–144) | NA | 53.8 | 2.2 (0.8–5.0) | NRTI + NNRTI (66.6) NRTI + NNRTI/PI (16.6) NRTI (11.1) NNRTI + PI (5.5) |
| Southern Africa | 19 | 5424 | 3425 | 2009 (2004–2018) | 94.8 (7.3–154.8) | NA | 45.8 | 2.9 (1.0–7.6) | NRTI + NNRTI/PI (33.3) NRTI + NNRTI (26.6) NRTI + PI (20.0) NRTI (13.3) NRTI + NNRTI + PI (6.6) |
| Western and Central Africa | 17 | 1776 | 880 | 2012 (2001–2021) | 84.0 (4.2–192) | NA | 60.9 | 2.3 (0.5–9.8) | NRTI + NNRTI/PI (41.1) NRTI + NNRTI (23.5) NRTI (11.7) NRTI + NNRTI + PI (23.5) |
| Asia | 13 | 2056 | 1494 | 2010 (2004–2024) | 96.0 (36–166.8) | NA | 66.5 | 2.0 (0.1–3.9) | NRTI + NNRTI (53.8) NRTI + NNRTI/PI (46.1) |
| South America | 6 | 412 | 403 | 2006 (1999–2018) | 85.2 (7.6–144) | NA | 93.7 | 2.5 (1.7–11.0) | NRTI + NNRTI + PI (33.3) NRTI + NNRTI/PI (16.6) NRTI (16.6) NRTI + PI (16.6) NRTI + NNRTI (16.6) |
| North America | 8 | 563 | 447 | 2007 (2001–2015) | 85.2 (7.6–144) | NA | 58.7 | 0.9 (0.5–10.1) | NRTI + NNRTI/PI (50.0) NRTI + NNRTI (25.0) NRTI + PI (25.0) |
| Europe | 8 | 639 | 514 | 2002 (1999–2020) | 111.0 (2.5–182.4) | NA | 63.1 | 2.1 (2.1–1.9) | NRTI + PI (40.0) NRTI + NNRTI/PI (20.0) NRTI + NNRT + PI (20.0) NRTI + NNRTI/PI/INSTI (20.0) |
NA, not applicable; ART, antiretroviral therapy; PMTCT, the prevention of mother-to-child transmission; NRTI, nucleoside reverse-transcriptase inhibitor; NNRTI, non-nucleoside reverse-transcriptase inhibitor; PI, protease inhibitor; INSTI, integrase inhibitor.
Several datasets are generated from the same study. If multiple regions are involved in the study, statistics will be repeated.
16 datasets out of 8 studies were included in both treatment-naive and treatment-experienced datasets; 12 datasets out of 4 studies were included in treatment-experienced datasets; 18 datasets out of 7 studies were included in treatment-naive datasets; 45 datasets out of 20 studies were included in total.
Statistics for the treatment-naive group (6 datasets) and the treatment-experienced group (4 datasets) were repeated to the presence of multiple regions.
Median (range).
We present overall and subgroup analyses on the prevalence of one or more drug-resistance mutations among treatment-naive and treatment-experienced children across various characteristics (Tables 2 and 3). The overall HIVDR prevalence is 26.31% (95% CI, 20.76–32.25) among treatment-naive children and 74.16% (95% CI, 67.74–80.13) among treatment-experienced children (p < 0.0001) (Fig. S2). HIVDR prevalence varied widely across subregions, with the highest prevalence in Southern Africa (37.80% [95% CI, 26.24–50.08]) and the lowest in South America (11.79% [95% CI, 4.91–20.84]) for treatment-naive children; it was highest in Asia (80.85% [95% CI, 63.76–93.55]) and lowest in Europe (54.39% [95% CI, 28.61–79.03]) for treatment-experienced children. By country, HIVDR prevalence was highest in Haiti (71.38% [95% CI, 65.94–76.40]) and lowest in Mali and Benin (0% [95% CI, 0.00–8.04]) for treatment-naive children; it was highest in Belgium and Puerto Rico (100% [95% CI, 96.90–100.00]) and lowest in Mexico and Portugal (3.70% [95% CI, 0.45–12.75]) for treatment-experienced children (Fig. 2). Sensitivity analysis indicated that the main summary prevalence did not significantly change among treatment-naive and treatment-experienced HIV-1-infected children (Fig. S3).
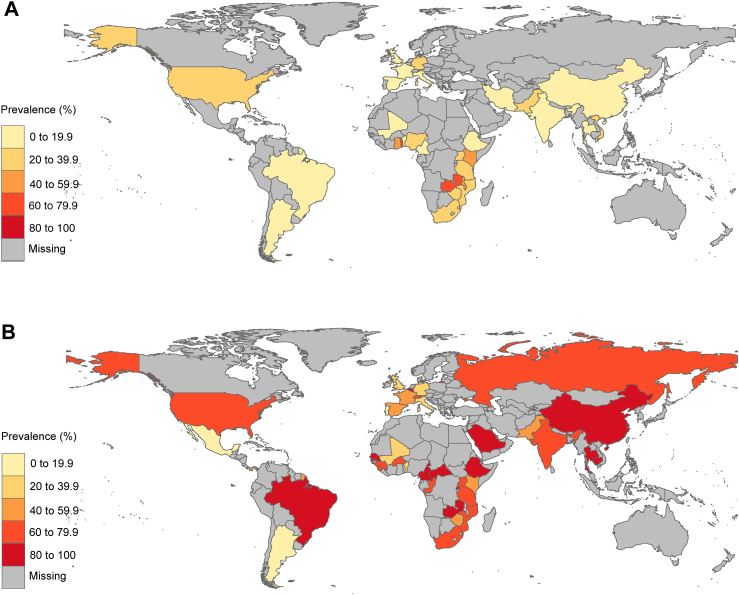
Table 2.
Pooled prevalence of HIV-1 drug resistance among treatment-naive children.
| Number of datasets | Number of HIV-infected individuals | Number of individuals with DR | Prevalence of DR (95% Confidence Interval) | Heterogeneity |
p value for subgroup difference | ||
|---|---|---|---|---|---|---|---|
| I2 | p value | ||||||
| Overall | 73 | 6914 | 2464 | 26.31% (20.76–32.25) | 96% | 0 | |
| Regiona | <0.01 | ||||||
| South America | 8 | 580 | 91 | 11.79% (4.91–20.84) | 84% | <0.01 | |
| North America | 8 | 747 | 435 | 34.21% (15.35–55.93) | 97% | <0.01 | |
| Europe | 6 | 783 | 83 | 14.31% (7.19–23.19) | 83% | <0.01 | |
| Asia | 10 | 893 | 140 | 18.30% (7.32–32.32) | 95% | <0.01 | |
| Eastern Africa | 12 | 975 | 268 | 31.35% (18.40–45.98) | 94% | <0.01 | |
| Southern Africa | 19 | 2610 | 1144 | 37.80% (26.24–50.08) | 95% | <0.01 | |
| Western and Central Africa | 10 | 898 | 366 | 21.60% (8.44–38.26) | 95% | <0.01 | |
| World Bank Income Level | 0.44 | ||||||
| Low income | 15 | 1218 | 421 | 33.89% (19.15–50.33) | 97% | <0.01 | |
| Lower middle income | 20 | 1539 | 663 | 30.14% (19.35–42.07) | 96% | <0.01 | |
| Upper middle income | 17 | 1532 | 463 | 22.8% (14.47–32.28) | 93% | <0.01 | |
| High income | 11 | 730 | 252 | 21.07% (10.81–33.45) | 97% | <0.01 | |
| Age group (years) | <0.01 | ||||||
| <2 | 44 | 4398 | 1984 | 35.39% (28.11–43.00) | 94% | <0.01 | |
| ≥2 | 29 | 2516 | 480 | 14.48% (8.68–21.34) | 95% | <0.01 | |
| Sampling Year | 0.86 | ||||||
| <2015 | 62 | 5943 | 2103 | 27.04% (20.95–33.56) | 97% | <0.01 | |
| ≥2015 | 11 | 971 | 361 | 25.40% (11.80–41.79) | 94% | <0.01 | |
| PMTCT experience | <0.01 | ||||||
| Yes | 50 | 4818 | 2014 | 32.58% (25.18–40.42) | 96% | <0.01 | |
| No | 23 | 2031 | 450 | 16.52% (10.26–23.82) | 96% | <0.01 | |
| Proportion of male | 0.19 | ||||||
| <50% | 23 | 4024 | 1593 | 28.71% (19.52–38.84) | 97% | <0.01 | |
| ≥50% | 14 | 1000 | 186 | 18.97% (9.75–30.21) | 93% | <0.01 | |
| HIV plasma viral load (log10 copies/mL)a | 0.16 | ||||||
| <5 | 9 | 672 | 314 | 33.59% (17.61–51.65) | 97% | <0.01 | |
| ≥5 | 18 | 1676 | 265 | 19.73% (11.1–29.95) | 93% | <0.01 | |
| Proportion of WHO Stage 3/4 or CDC stage C | 0.67 | ||||||
| <50% | 6 | 563 | 101 | 16.93% (10.68–24.20) | 76% | <0.01 | |
| ≥50% | 4 | 728 | 127 | 14.61% (5.57–26.85) | 95% | <0.01 | |
CDC, National Centers for Disease Control; DR, drug resistance; PMTCT, the prevention of mother-to-child transmission; WHO, World Health Organization.
Several datasets are generated from the same study.
The median extracted from the original literature was used for grouping.
Table 3.
Pooled prevalence of HIV-1 drug resistance among treatment-experienced children.
| Number of datasets | Number of HIV-infected individuals | Number of Individuals with DR | Prevalence of DR (95% Confidence Interval) | Heterogeneity |
p value for subgroup difference | ||
|---|---|---|---|---|---|---|---|
| I2 | p value | ||||||
| Overall | 89 | 7656 | 5256 | 74.16% (67.74–80.13) | 97% | 0 | |
| Region | 0.63 | ||||||
| South America | 6 | 403 | 274 | 75.72% (53.81–92.37) | 96% | <0.01 | |
| North America | 8 | 447 | 258 | 56.88% (27.45–83.98) | 98% | <0.01 | |
| Europe | 8 | 514 | 273 | 54.39% (28.61–79.03) | 96% | <0.01 | |
| Asia | 13 | 1494 | 982 | 80.85% (63.76–93.55) | 98% | <0.01 | |
| Eastern Africa | 18 | 2163 | 1189 | 68.68% (54.83–81.09) | 96% | <0.01 | |
| Southern Africa | 19 | 3425 | 2366 | 69.68% (53.17–84.02) | 99% | <0.01 | |
| Western and Central Africa | 17 | 880 | 618 | 72.02% (58.23–84.04) | 95% | <0.01 | |
| Income level | 0.17 | ||||||
| Low income | 18 | 1258 | 868 | 75.70% (60.91–88.03) | 98% | <0.01 | |
| Lower middle income | 24 | 1776 | 1160 | 72.24% (61.77–81.66) | 96% | <0.01 | |
| Upper middle income | 22 | 2679 | 2181 | 85.03% (76.51–92.00) | 96% | <0.01 | |
| High income | 11 | 649 | 415 | 70.20% (50.92–86.51) | 96% | <0.01 | |
| Age group (years) | <0.01 | ||||||
| <7 | 33 | 3223 | 1657 | 60.54% (49.58–71.01) | 96% | <0.01 | |
| ≥7 | 45 | 4076 | 3360 | 81.80% (74.48–88.17) | 96% | <0.01 | |
| Sampling Year | 0.30 | ||||||
| <2015 | 61 | 5089 | 3448 | 75.42% (67.66–82.46) | 97% | <0.01 | |
| ≥2015 | 20 | 2644 | 1808 | 67.93% (55.59–79.15) | 97% | <0.01 | |
| Proportion of male | 0.10 | ||||||
| <50% | 20 | 2222 | 1253 | 65.52% (51.32–78.49) | 97% | <0.01 | |
| ≥50% | 52 | 4791 | 3686 | 78.17% (70.89–84.71) | 96% | <0.01 | |
| CD4 cell count (cells/mL)a | 0.66 | ||||||
| <500 | 18 | 998 | 768 | 73.63% (56.27–88.01) | 96% | <0.01 | |
| ≥500 | 18 | 1066 | 800 | 78.2% (63.95–89.79) | 96% | <0.01 | |
| HIV plasma viral load (log10 copies/mL)a | 0.37 | ||||||
| <5 | 24 | 2208 | 1762 | 78.71% (67.16–88.41) | 95% | <0.01 | |
| ≥5 | 17 | 2034 | 1146 | 69.45% (51.03–85.23) | 98% | <0.01 | |
| Proportion of WHO Stage 3/4 or CDC stage C | 0.54 | ||||||
| <50% | 18 | 1609 | 1102 | 75.27% (61.45–86.94) | 97% | <0.01 | |
| ≥50% | 17 | 1902 | 1040 | 69.38% (53.81–83.07) | 97% | <0.01 | |
| Antiretroviral treatment time (years) | 0.44 | ||||||
| <3 | 46 | 3570 | 2338 | 70.88% (61.52–79.46) | 97% | <0.01 | |
| ≥3 | 26 | 2770 | 1790 | 76.38% (64.75–86.37) | 97% | <0.01 | |
| Proportion of viral failure | 0.05 | ||||||
| 100% | 28 | 3318 | 2278 | 81.17% (71.57–89.28) | 97% | <0.01 | |
| 50%–99% | 15 | 1379 | 1109 | 73.16% (61.73–83.29) | 92% | <0.01 | |
| <50% | 28 | 2097 | 1229 | 61.23% (47.98–73.72) | 98% | <0.01 | |
| ART regimenb | <0.01 | ||||||
| NRTI | 7 | 636 | 575 | 84.53% (75.12–92.14) | 85% | <0.01 | |
| NRTI + NNRTI | 30 | 3292 | 2010 | 73.54% (60.80–84.63) | 98% | <0.01 | |
| NRTI + NNRTI/PI | 24 | 2636 | 1977 | 78.09% (70.47–84.90) | 95% | <0.01 | |
| NRTI + PI | 9 | 336 | 206 | 66.06% (36.83–90.17) | 97% | <0.01 | |
| NRTI + NNRTI + PI | 8 | 516 | 308 | 61.14% (32.40–86.32) | 97% | <0.01 | |
| NNRTI + PI | 1 | 199 | 93 | 73.96% (64.00–82.38) | |||
| NRTI + NNRTI/PI/INSTI | 1 | 96 | 71 | 73.60% (66.95–79.78) | |||
CDC, National Centers for Disease Control; DR, drug resistance; PMTCT, the prevention of mother-to-child transmission; WHO, World Health Organization; ART, antiretroviral therapy; NNRTI, non-nucleoside reverse-transcriptase inhibitor; NRTI, nucleoside reverse-transcriptase inhibitor; PI, protease inhibitor; INSTI, integrase inhibitor.
Several datasets are generated from the same study.
The median extracted from the original literature was used for grouping.
ART regimes are defined as the maximum proportion of all treatment among each dataset.
Fig. 2.
Prevalence of HIV-1 drug resistance among treatment-naive children (A) and treatment-experienced children (B) by countries. Global distribution of HIV-1 drug resistant prevalence demonstrated by different color. The gray area represents countries with unavailable resistance data.
The PDR tended to decline with an increase in World Bank income level among treatment-naive children, with low-income countries showing a PDR prevalence of 33.89% (95% CI, 19.15–50.33) and high-income countries showing a PDR prevalence of 21.07% (95% CI, 10.81–33.45) (Table 2). However, among treated patients, upper-middle-income countries exhibited the highest DR prevalence of 85.03% (95% CI, 76.51–92.00) (Table 3, Table S6). HIVDR prevalence decrease over the sampling years (naive: 27.04% [95% CI, 20.95–33.56] before 2015 vs. 25.40% [95% CI, 11.80–41.79] after 2015; treated: 75.42% [95% CI, 67.66–82.46] before 2015 vs. 67.93% [95% CI, 55.59–79.15] after 2015), while no significant differences in resistance prevalence across different sampling times were observed (both p values > 0.05). The results of the subgroup analyses conducted using datasets from 2015 onward are presented in the appendix (Table S5).
Children exclusively exposed to NRTI regimens showed the highest DR prevalence of 84.53% (95% CI, 75.12–92.14). Additionally, a positive correlation was observed between the proportion of VF and DR prevalence, increasing from 61.23% (95% CI, 47.98–73.72) when VF was less than 50% to 81.17% (95% CI, 71.57–89.28) when VF was 100% (Tables 2 and 3).
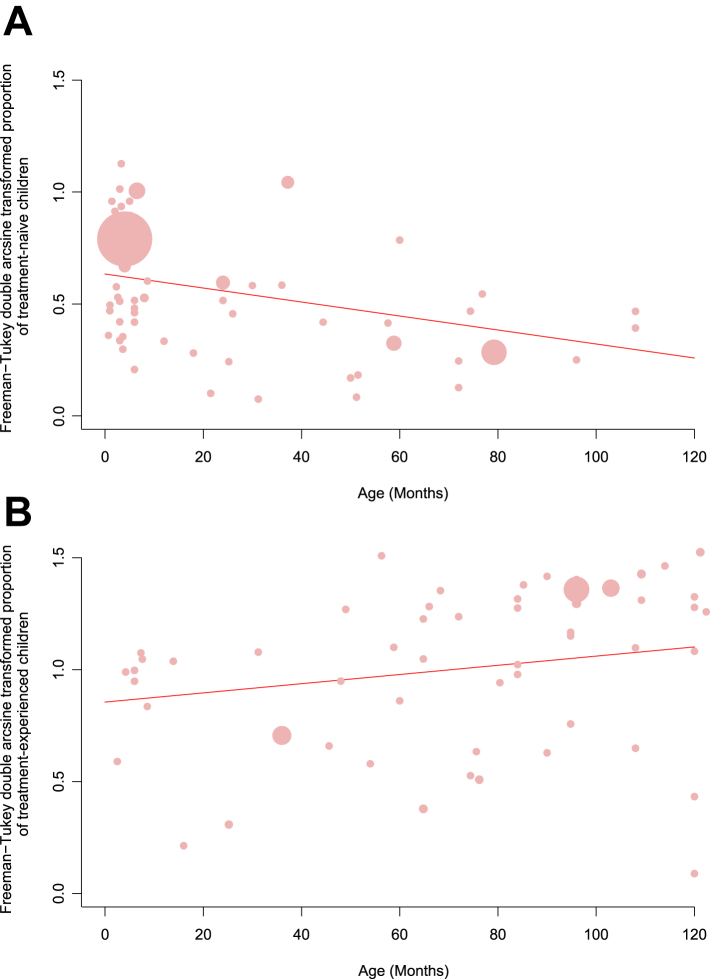
Considerable heterogeneity was observed between HIVDR prevalence estimates; therefore, we did a meta regression to explore sources of variation. Univariate random-effects meta-regression analyses indicated that region (p = 0.0319), history of PMTCT (p = 0.0045), and age (p = 0.0001) could be potential sources of heterogeneity among treatment-naive children, while no significant differences were observed in sampling year (p = 0.1352), income level (p = 0.4238), or CD4 count (p = 0.6686). Among treated patients, univariate random-effects meta-regression analyses revealed that age (p = 0.0198) and VF (p = 0.0307) could be a source of heterogeneity. Multivariate random-effects meta-regression analysis for both groups indicated that only age (naive: p = 0.0005; treated: p ≤ 0.0001) constituted a source of heterogeneity (Table S6). Thus, we analyzed associations between age and HIVDR prevalence for treatment-naive and treatment-experienced HIV-1-infected children. We found that PDR prevalence declined with increasing age among treatment-naive children, ranging from 35.39% (95% CI, 28.11–43.00) in children under 2 years to 14.48% (95% CI, 8.68–21.34) in those aged 2 years and older. In contrast, a positive correlation between HIVDR and age persisted in the treated group, ranging from 60.54% (95% CI, 49.58–71.01) in children under 7 years to 81.80% (95% CI, 74.48–88.17) in those aged 7 years and older (Tables 2 and 3 and Fig. 3).
Fig. 3.
Prevalence of HIV-1 resistance among treatment-naive children (A) and treatment-experienced children (B) to any drug mutations by age of sampling. Each bubble represents a study and the size of the bubble is proportional to the size of the study. The trend line is predicted prevalence.
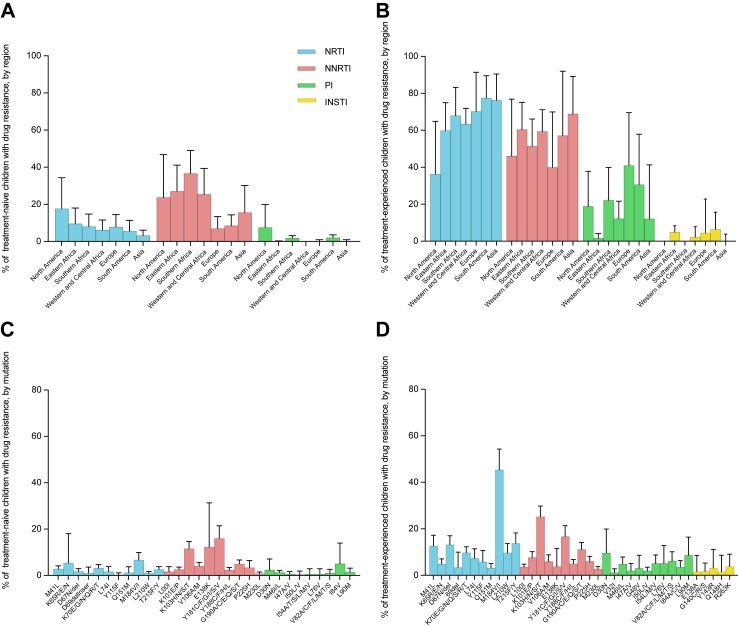
Regarding the class of ART drugs, NNRTI resistances were the most commonly observed mutations among the treatment-naive group, occurring in 23.64% (95% CI, 18.26–29.45) of patients with one or more drug-resistance mutations. Southern Africa exhibiting the highest PDR prevalence at 36.57% (95% CI, 24.94–49.01), followed by Eastern Africa (27.03%, [95% CI, 14.85–41.16]), while Europe had the lowest PDR at 6.95% (95% CI, 2.34–13.39). For NNRTI resistance, the most common mutation observed was Y181 C/F/G/I/S/V with a PDR prevalence of 16.01% (95% CI, 11.20–21.45), followed by K103 N/S at 11.56% (95% CI, 8.82–14.59) (Fig. 3 and Fig. S4). Given the mutations potentially associated with rilpivirine (RPV), the PDR prevalence is 7.72% (95% CI, 5.51–10.23) for K101P, 16.66% (95% CI, 12.42–21.35) for Y181I/V, and 5.02% (95% CI, 3.49–6.78) for Y188L.
NRTI resistances were the most commonly observed mutations among the treatment-experienced group, occurring in 65.19% (95% CI, 58.48–71.62) of patients with one or more drug-resistance mutations, with Asia exhibiting the highest DR prevalence of 76.23% (95% CI, 58.24–90.47) and North America the lowest DR prevalence of 36.33% (95% CI, 11.96–64.93). For NRTI resistance, the most common mutation observed was M184V/I, with a DR prevalence of 45.40% (95% CI, 36.63–54.31). It is worth noting that NNRTI DR prevalence were also high, ranging from 39.98% to 68.86% across various regions in the treatment-experienced group. The top two mutations were consistent with those observed in the treatment-naive group (Fig. 4 and Fig. S5).
Fig. 4.
Prevalence of drug-resistance mutations in children with any mutation stratified by region and drug class. Pooled estimation with 95% confidence interval error bars of NRTI (blue), NNRTI (red), PI (green) and INSTI (yellow) resistance prevalence of treatment-naive (A and C) and treatment-experienced (B and D) children. NRTI, nucleoside reverse transcriptase inhibitors; NNRTI, non-nucleoside reverse transcriptase inhibitor; PI, protease inhibitor; INSTI, integrase inhibitor.
Regarding PI, the most prevalent mutations among the treatment-naive group were I84A/C/V (5.00%, [95% CI, 1.04–13.92]) and D30N (2.27%, [95% CI, 0.00–7.02]); for the treatment-experienced, the most common mutation was D30N, observed in 9.65% (95% CI, 2.59–19.90) of cases (Fig. S6). INSTI drug resistance was observed in the treatment-experienced group across 13 datasets, with an overall prevalence of 2.82% (95% CI, 0.75–5.80). The highest prevalence was reported in South America, at 6.45% (95% CI, 0.12–15.70). The most common INSTI mutation was R263K, with a prevalence of 3.79% (95% CI, 0.49–9.09) (Fig. 4 and Fig. S7).
The most prevalent drug resistance among dual-therapy were NRTI + PI among the treatment-naive group (13.51% [95% CI, 0.00–50.17]) and NRTI + NNRTI in the treatment-experienced group (46.55% [95% CI, 36.22–57.03]) (Fig. S8). Regarding triple class therapy, the drug resistance prevalence were 0.94% (95% CI, 0.00–4.04) among the treatment-naive group and 6.69% (95% CI, 3.67–10.39) among the treatment-experienced group (Table S3).
Discussion
Our findings indicate that drug resistance both in treatment-naive and treatment-experienced children and adolescents has become a worldwide issue, with the overall HIVDR prevalence being 26.31% among treatment-naive children and 74.16% among treatment-experienced children. Drug resistance in treatment-naive children is primarily concentrated in Southern Africa, East Africa, Central and West Africa, and North America, predominantly due to NNRTI mutations. Children younger than 2 years or received PMTCT exhibited higher PDR. Furthermore, the drug resistance among treatment-experienced children was more severe, with higher DR prevalence in various regions involving regimens of NRTI, NNRTI, and PI. Children older than 7 years or those who experienced VF had higher post-treatment DR prevalence. The different epidemics of drug resistance in treatment-naive and treatment-experienced children underscore the need for targeted decision-making in developing effective strategies to monitor for, prevent and respond to the emergence of HIVDR.
The risk of HIV-1 transmission to infant substantially decreases when mother receives ART during pregnancy and delivery, along with ART given to the infant postpartum.150 However, an unintended consequence of ART use for PMTCT is the potential development of drug resistance in a small number of infected infants. HIVDR in infants and children with perinatal HIV-1 infection may stem from either a drug-resistant strain transmitted from the mother, or the administration of pediatric ART or ART used for PMTCT, or maternal ART.151 This study revealed that children who underwent PMTCT exhibited a higher PDR (32.58%) compared to those who did not (16.52%), indicating that prior ART drug exposure is a significant risk factor for pretreatment HIVDR. The 2010 WHO guidelines on PMTCT recommended a preferred first-line ART regimen during pregnancy, consisting of an AZT + 3TC backbone combined with an NNRTI, along with daily administration of NVP or twice-daily AZT to infants from birth or as soon as possible thereafter until 4–6 weeks of age. Most studies included in our analysis adhered to these recommendations, resulting in a notable prevalence of NNRTI and NRTI drug resistance across various regions.
The 2016 WHO consolidated ART guidelines recommended that countries where PDR to NNRTIs exceeds 10% among individuals starting first-line ART should promptly consider alternative regimens without NNRTIs. Our analysis revealed that NNRTI DR prevalence exceeded 10% in Southern Africa, Western and Central Africa, Eastern Africa, and North America. A mathematical model projected that in sub-Saharan Africa, where NNRTI DR prevalence surpasses 10% and NNRTI-based ART remains the first-line option, this resistance could contribute to a cumulative 16% increase in AIDS-related deaths (890,000 deaths) and a 9% rise in new HIV-1 infections (450,000) over the next 15 years.152 Conversely, PI-based second-line ART remains a viable option for treatment-naive children, with PDR for PIs remaining below 10% across various regions. However, access to PI-based regimens remains limited, with less than 5% of ART recipients in most LMICs receiving them. Moreover, the cost of second-line regimens in LMICs averages three times higher than first-line regimens, amounting to US$ 263 per patient per year compared to US$ 85.153
The development of HIVDR in children on ART is commonly associated with factors such as poor adherence, utilization of suboptimal regimens, and issues related to drug absorption or pharmacokinetics.154 These factors contribute to subtherapeutic drug levels and the rebound of viremia with resistant viruses. Given that most included studies focused on treatment-experienced children with varying degrees of treatment failure, the DR prevalence ranged from 54.39% to 80.85% across various regions. Notably, even among children from studies with a proportion of VF less than 50%, the pooled prevalence of HIVDR remained high at 61.23%. HIVDR in treatment-experienced children necessitates more expensive second-line regimens, increasing costs associated with further transmission of highly resistant and potentially untreatable viruses. The WHO estimated that when DR prevalence to NNRTI reaches 10%, an additional 2510 individuals per 100,000 starting ART fail to achieve and maintain VL suppression below 1000 copies/mL, requiring second-line ART. This translates to an annual increase of US$ 502,000 for the purchase of second-line drugs per 100,000 people initiating ART.6 It is noteworthy that DR prevalence for NNRTI and NRTI in children on ART exceeded 20% in each region. As a prominent member of the NNRTI class, RPV has garnered significant attention not only for its role in contemporary treatment regimens but also for the potential application of its long-acting formulation, which has been increasingly advocated for use in adolescents. Our findings reveal that the DR prevalence associated with RPV in treatment-experienced children is notably elevated for the Y181I/V mutation, with a prevalence of 16.66%. This observation is particularly critical in regions with high levels of NNRTI resistance, where the availability of alternative therapeutic options may be constrained. Furthermore, the DR prevalence for PI was over 10% in each region except for the Eastern Africa in our analysis. Particularly striking was the finding that dual DR prevalence to NNRTIs and NRTIs was observed in 46.55% of treatment-experienced children, highlighting the limited availability of alternative regimens.
In 2019, WHO updated interim guidelines recommending DTG in combination with a NRTI backbone as the preferred first-line regimen for infants and children with approved DTG dosing. A raltegravir (RAL)-based regimen may be suggested as an alternative first-line option for infants and children lacking access to approved DTG dosing, and for neonates, a RAL-based regimen may be the preferred first-line choice.155 Compared to efavirenz (EFV)-based regimens, mathematical modeling predicts that initiating first-line ART with DTG would increase the prevalence of VL suppression (from a mean of 77% to 86%), reduce mortality (from 4.5 to 3.5 persons per 1000 person-years), and decrease HIV-1 incidence (from 0.79 to 0.72 new HIV-1 infections per 100 person-years).156 This modeling also suggests that in sub-Saharan Africa and similar settings where the cost of a DTG-containing regimen is comparable to that of an EFV-based regimen, adopting DTG as the first-line treatment would be cost-effective and could potentially lead to cost savings. Therefore, reducing the prices of new first-line drugs like DTG or RAL, as well as second-line drugs such as PI, could prove to be a promising and cost-effective strategy in combating drug resistance in children.6
Despite global HIV-1 treatment guidelines recommending DTG-based ART for first-, second-, and third-line treatment as the most promising strategy to mitigate increasing drug resistance levels, fewer than half of sites in low- and middle-income countries had fully implemented or planned to implement these recommendations.155 On World AIDS Day, UNAIDS and CHAI announced a groundbreaking agreement that achieved a significant 75% reduction in the cost of HIV-1 treatment for children in low- and middle-income countries. This agreement ensured that DTG 10 mg dispersible tablets would be available at a cost of US$ 4.50 for a 90-count bottle.157 This development makes the WHO-recommended, preferred first-line DTG-based antiretroviral treatment more accessible in affordable and child-friendly generic formulations for young children and infants as young as four weeks of age, weighing more than 3 kg. A rapid transition to this optimal treatment regimen, coupled with improved HIV-1 diagnosis and other supportive measures, is crucial to urgently reduce the 95,000 preventable AIDS-related deaths in children.158 Therefore, prior to widespread use of DTG in children, early monitoring of HIVDR is essential to prevent its development. Since HIVDR testing is not routinely offered to all individuals starting ART in most low- and middle-income countries, periodic nationally representative surveys are the gold standard to inform the DR prevalence and guide a country's choice of recommended regimens for first-line ART. Furthermore, WHO-recommended ART site-based HIVDR early warning indicators (EWIs) could be employed to prevent the emergence of HIVDR.159,160 These indicators include ART prescribing practices, rates of loss to follow-up, patient retention on first-line ART, adherence to on-time drug pick-up, attendance of on-time ART clinic appointments, and continuity of ART drug supply.
Our study has several limitations that warrant consideration. Firstly, our search strategy was limited to articles published in English, potentially overlooking relevant studies published in other languages. Moreover, the geographical distribution of the included studies was skewed, with a predominant focus on the African region and less representation from South America, North America, Europe, and Asia. This discrepancy in geographic coverage might affect the overall representativeness of our findings. Additionally, some including studies conducted at non-national levels introduces the possibility of bias in estimating national resistance rates. Furthermore, the use of various genotyping methods across the included studies poses a challenge in standardizing the data for meta-analysis, potentially affecting the accuracy of our results. Despite attempts to explore sources of heterogeneity through meta-analyses, considerable unexplained heterogeneity persisted, potentially limiting our ability to accurately identify specific epidemic trends. Lastly, our study may not fully capture the resistance prevalence in treatment-experienced children with a high proportion of VF, thereby limiting the generalizability of our findings to the broader population of children undergoing treatment. In settings where drug resistance surveillance is unfeasible, conducting nationally representative surveys is recommended to assess whether resistance rates surpass established thresholds, thereby guiding adjustments to first-line regimens.
In conclusion, our global meta-analysis highlights the significant burden of HIVDR among both treatment-naive and treatment-experienced HIV-1-infected children and adolescents, especially in sub-Saharan Africa and low-income countries. The prevalence of PDR to NNRTIs exceeded the critical threshold of 10% across the entire Africa region and approached 10% in Europe and South America. These findings underscore the urgent need for a comprehensive, coordinated, and integrated approach to address HIVDR. Implementing routine HIVDR testing or conducting periodic nationally representative surveys is crucial for accurately assessing the DR prevalence and informing national programs on the optimal selection of population-level ART regimens. In regions where DR prevalence to NNRTIs exceeds 10% or in cases of treatment failure among children on ART therapy, prioritizing the transition to DTG and other optimal formulations is imperative. Efforts to facilitate this transition, including guidance on the best practices for transitioning to optimal formulations and advocating for improved pricing through negotiation, should receive robust support at both global and national levels. Collaboration among key stakeholders, including countries, non-governmental organizations, PLWH and their communities, civil society organizations, United Nations programs and agencies, and international implementing partners and donors, is essential in achieving these objectives.
Contributors
JL conceptualized and supervised the study. JL, LG, LS, FB and DS designed the study. JL, LG and LS designed study search terms. JL, LG, DS and FB identified studies for inclusion. LG, YL, XL and YH collected data. LG, YL, XL and YH extracted data and assessed data quality. JL, LG, YL and XL analyzed or interpreted data. JL and LG drafted the manuscript. YL, XL and YH have verified the underlying data. All authors critically reviewed or revised the manuscript and approved the final version of the manuscript.
Data sharing statement
This meta-analysis did not require the collection of new data, but rather the analysis of previously published data. The datasets that were used and evaluated in this study can be obtained from the corresponding author upon making a reasonable request.
Editor note
The Lancet Group takes a neutral position with respect to territorial claims in published maps and institutional affiliations.
Declaration of interests
All authors declare no competing interests.
Acknowledgements
This work was supported by the Science and Technology Innovation Committee of Shenzhen Municipality (No. JCYJ20220531102202005) and the Natural Science Foundation of Guangdong Province (No. 2024A1515012118).
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.eclinm.2024.102859.
Appendix A. Supplementary data
References
- 1.UNICF HIV burden in children. Fast Fact. https://data.unicef.org/topic/hivaids/paediatric-treatment-and-care/#:∼:text=In%202022%2C%20130%2C000%20%5B90%2C000%2D,of%20AIDS%2Drelated%20causes%20globally Available at:
- 2.European AIDS Clinical Society European guidelines for the treatment of HIV-positive adults in Europe, version 12.0, 2023. https://www.eacsociety.org/media/final2021eacsguidelinesv11.0_oct2021.pdf Available at:
- 3.Davies C., Johnson L., Sawry S., et al. Effect of antiretroviral therapy care interruptions on mortality in children living with HIV. AIDS. 2022;36(5):729–737. doi: 10.1097/QAD.0000000000003194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ray S., Seth A., Singh S., et al. Short-term adverse drug reactions to antiretroviral therapy in children with HIV: a cohort study. Indian J Pediatr. 2023;90(1):9–15. doi: 10.1007/s12098-021-04045-4. [DOI] [PubMed] [Google Scholar]
- 5.World Health Organization Global action plan on HIV drug resistance 2017–2021. https://www.who.int/publications/i/item/978-92-4-151284-8
- 6.World Health Organization Guidelines on the public health response to pretreatment HIV drug resistance. 2017. https://iris.who.int/handle/10665/255880 Available at:
- 7.Gupta R.K., Gregson J., Parkin N., et al. HIV-1 drug resistance before initiation or re-initiation of first-line antiretroviral therapy in low-income and middle-income countries: a systematic review and meta-regression analysis. Lancet Infect Dis. 2018;18(3):346–355. doi: 10.1016/S1473-3099(17)30702-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.S Page M.J., McKenzie J.E., Bossuyt P.M., et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. Syst Rev. 2021;10(1):89. doi: 10.1186/s13643-021-01626-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stanford University Stanford HIV drug resistance database. https://hivdb.stanford.edu Available at:
- 10.World Health Organization WHO surveillance drug resistance mutations list. https://iris.who.int/bitstream/handle/10665/343175/9789240030565-eng.pdf?sequence=1 Available at:
- 11.ANRS HIV-1 genotypic drug resistance interpretation's algorithms (French National Agency for AIDS Research) https://hivfrenchresistance.org
- 12.IAS-USA Update of the drug resistance mutations in HIV-1. 2022. https://www.iasusa.org/wp-content/uploads/2022/09/30-4-muta.pdf Available at:
- 13.Hoy D., Brooks P., Woolf A., et al. Assessing risk of bias in prevalence studies: modification of an existing tool and evidence of interrater agreement. J Clin Epidemiol. 2012;65(9):934–939. doi: 10.1016/j.jclinepi.2011.11.014. [DOI] [PubMed] [Google Scholar]
- 14.Boender T.S., Kityo C.M., Boerma R.S., et al. Accumulation of HIV-1 drug resistance after continued virological failure on first-line ART in adults and children in sub-Saharan Africa. J Antimicrob Chemother. 2016;71(10):2918–2927. doi: 10.1093/jac/dkw218. [DOI] [PubMed] [Google Scholar]
- 15.Lidström J., Li Q., Hoover D.R., et al. Addition of extended zidovudine to extended nevirapine prophylaxis reduces nevirapine resistance in infants who were HIV-infected in utero. AIDS. 2010;24(3):381–386. doi: 10.1097/QAD.0b013e3283352ef1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Towler W.I., Barlow-Mosha L., Church J.D., et al. Analysis of drug resistance in children receiving antiretroviral therapy for treatment of HIV-1 infection in Uganda. AIDS Res Hum Retroviruses. 2010;26(5):563–568. doi: 10.1089/aid.2009.0164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Church J.D., Omer S.B., Guay L.A., et al. Analysis of nevirapine (NVP) resistance in Ugandan infants who were HIV infected despite receiving single-Dose (SD) NVP versus SD NVP plus daily NVP up to 6 weeks of age to prevent HIV vertical transmission. J Infect Dis. 2008;198(7):1075–1082. doi: 10.1086/591503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fogel J., Hoover D.R., Sun J., et al. Analysis of nevirapine resistance in HIV-infected infants who received extended nevirapine or nevirapine/zidovudine prophylaxis. AIDS. 2011;25(7):911–917. doi: 10.1097/QAD.0b013e328344fedc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ramkissoon A.P., Amarakoon I.I., Hamilton C.L., et al. Analysis of reverse transcriptase and protease genes of HIV for antiretroviral drug resistance in treatment-exposed Jamaican pediatrics. AIDS Res Hum Retrovir. 2015;31(9):932–937. doi: 10.1089/AID.2015.0122. [DOI] [PubMed] [Google Scholar]
- 20.Lwembe R., Ochieng W., Panikulam A., et al. Anti-retroviral drug resistance-associated mutations among non-subtype B HIV-1-infected Kenyan children with treatment failure. J Med Virol. 2007;79(7):865–872. doi: 10.1002/jmv.20912. [DOI] [PubMed] [Google Scholar]
- 21.Van Dyke R.B., Patel K., Kagan R.M., et al. Antiretroviral drug resistance among children and youth in the United States with perinatal HIV. Clin Infect Dis. 2016;63(1):133–137. doi: 10.1093/cid/ciw213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bismara B.A., Anjos E.B., Andrade P., et al. Antiretroviral drug resistance in Brazilian children infected by human immunodeficiency virus type 1. J Antivir Antiretrovir. 2012;4(4):66–74. [Google Scholar]
- 23.Al Hajjar S.H., Frayha H., Althawadi S. Antiretroviral resistance in HIV-infected Saudi children failing first-line highly active antiretroviral therapy. Ann Saudi Med. 2012;32(6):565–569. doi: 10.5144/0256-4947.2012.565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chalermchockcharoenkit A., Culnane M., Chotpitayasunondh T., et al. Antiretroviral resistance patterns and HIV-1 subtype in mother-infant pairs after the administration of combination short-course zidovudine plus single-dose nevirapine for the prevention of mother-to-child transmission of HIV. Clin Infect Dis. 2009;49(2):299–305. doi: 10.1086/599612. [DOI] [PubMed] [Google Scholar]
- 25.Rogo T., DeLong A.K., Chan P., et al. Antiretroviral treatment failure, drug resistance, and subtype diversity in the only pediatric HIV clinic in Rhode Island. Clin Infect Dis. 2015;60(9):1426–1435. doi: 10.1093/cid/civ058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tambuyzer L., Thys K., Hoogstoel A., et al. Assessment of etravirine resistance in HIV-1-infected paediatric patients using population and deep sequencing: final results of the PIANO study. Antivir Ther. 2016;21(4):317–327. doi: 10.3851/IMP3011. [DOI] [PubMed] [Google Scholar]
- 27.Makatini Z., Mda S., Towobola O., et al. Characterisation of protease resistance mutations in a South African paediatric cohort with virological failure, 2011 - 2017. S Afr Med J. 2019;109(7):511–515. doi: 10.7196/SAMJ.2019.v109i7.13705. [DOI] [PubMed] [Google Scholar]
- 28.Brice J., Sylla M., Desire N., et al. Characterization of drug resistance and the defective HIV reservoir in virally suppressed vertically infected children in Mali. J Antimicrob Chemother. 2020;75(5):1272–1279. doi: 10.1093/jac/dkaa002. [DOI] [PubMed] [Google Scholar]
- 29.Phung T.T.B., Phan C.T.T., Khuc H.T.R., et al. Characterization of drug resistance mutations in ART-naïve HIV-1 infected children in Northern Vietnam. Asian Pac J Trop Dis. 2015;5(9):691–694. [Google Scholar]
- 30.Toni T.D., Masquelier B., Lazaro E., et al. Characterization of nevirapine (NVP) resistance mutations and HIV type 1 subtype in women from Abidjan (Cote d'Ivoire) after NVP single-dose prophylaxis of HIV type 1 mother-to-child transmission. AIDS Res Hum Retrovir. 2005;21(12):1031–1034. doi: 10.1089/aid.2005.21.1031. [DOI] [PubMed] [Google Scholar]
- 31.Yendewa G.A., Lakoh S., Yendewa S.A., et al. Characterizing HIV-1 genetic subtypes and drug resistance mutations among children, adolescents and pregnant women in Sierra Leone. Genes. 2021;12(9):1314. doi: 10.3390/genes12091314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Makadzange A.T., Higgins-Biddle M., Chimukangara B., et al. Clinical, virologic, immunologic outcomes and emerging HIV drug resistance patterns in children and adolescents in public ART care in Zimbabwe. PLoS One. 2015;10(12) doi: 10.1371/journal.pone.0144057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vaz P., Buck W.C., Bhatt N., et al. Compromise of second-line antiretroviral therapy due to high rates of human immunodeficiency virus drug resistance in Mozambican treatment-experienced children with virologic failure. J Pediatric Infect Dis Soc. 2020;9(1):6–13. doi: 10.1093/jpids/piy102. [DOI] [PubMed] [Google Scholar]
- 34.Camara-Cisse M., Djohan Y.F., Toni T.D., et al. Determination of reverse transcriptase inhibitor resistance mutations in HIV-1 infected children in Côte d'Ivoire. Genome. 2021;64(4):347–354. doi: 10.1139/gen-2020-0112. [DOI] [PubMed] [Google Scholar]
- 35.Muri L., Gamell A., Ntamatungiro A.J., et al. Development of HIV drug resistance and therapeutic failure in children and adolescents in rural Tanzania: an emerging public health concern. AIDS. 2017;31(1):61–70. doi: 10.1097/QAD.0000000000001273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Antunes F., Zindoga P., Gomes P., et al. Development of nevirapine resistance in children exposed to the prevention of mother-to-child HIV-1 transmission programme in Maputo, Mozambique. PLoS One. 2015;10(7) doi: 10.1371/journal.pone.0131994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dumans A.T., Barreto C.C., Santos A.F., et al. Distinct resistance mutation and polymorphism acquisition in HIV-1 protease of subtypes B and F1 from children and adult patients under virological failure. Infect Genet Evol. 2009;9(1):62–70. doi: 10.1016/j.meegid.2008.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Almeida F.J., Berezin E.N., Rodrigues R., et al. Diversity and prevalence of antiretroviral genotypic resistance mutations among HIV-1-infected children. J Pediatr. 2009;85(2):104–109. doi: 10.2223/JPED.1877. [DOI] [PubMed] [Google Scholar]
- 39.Fokam J., Salpini R., Santoro M.M., et al. Drug resistance among drug-naive and first-line antiretroviral treatment-failing children in Cameroon. Pediatr Infect Dis J. 2011;30(12):1062–1068. doi: 10.1097/INF.0b013e31822db54c. [DOI] [PubMed] [Google Scholar]
- 40.Kuhn L., Hunt G., Technau K.G., et al. Drug resistance among newly diagnosed HIV-infected children in the era of more efficacious antiretroviral prophylaxis. AIDS. 2014;28(11):1673–1678. doi: 10.1097/QAD.0000000000000261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Green T.N., Archary M., Gordon M.L., et al. Drug resistance and coreceptor usage in HIV type 1 subtype C-infected children initiating or failing highly active antiretroviral therapy in South Africa. AIDS Res Hum Retrovir. 2012;28(4):324–332. doi: 10.1089/aid.2011.0106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Andrade S.D., Sabidó M., Monteiro W.M., et al. Drug resistance in antiretroviral-naive children newly diagnosed with HIV-1 in Manaus, Amazonas. J Antimicrob Chemother. 2017;72(6):1774–1783. doi: 10.1093/jac/dkx025. [DOI] [PubMed] [Google Scholar]
- 43.Ventosa-Cubillo J., Pinzón R., González-Alba J.M., et al. Drug resistance in children and adolescents with HIV in Panama. J Antimicrob Chemother. 2023;78(2):423–435. doi: 10.1093/jac/dkac407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pillay S., Bland R.M., Lessells R.J., et al. Drug resistance in children at virological failure in a rural KwaZulu-Natal, South Africa, cohort. AIDS Res Ther. 2014;11(1):3. doi: 10.1186/1742-6405-11-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Beghin J.C., Ruelle J., Goubau P., et al. Drug resistance in HIV-infected children living in rural South Africa: implications of an antiretroviral therapy initiated during the first year of life. J Clin Virol. 2020;129 doi: 10.1016/j.jcv.2020.104547. [DOI] [PubMed] [Google Scholar]
- 46.Gupta R.K., Ford D., Mulenga V., et al. Drug resistance in human immunodeficiency virus type-1 infected Zambian children using adult fixed dose combination stavudine, lamivudine, and nevirapine. Pediatr Infect Dis J. 2010;29(8):e57–e62. doi: 10.1097/INF.0b013e3181e47609. [DOI] [PubMed] [Google Scholar]
- 47.Bratholm C., Johannessen A., Naman E., et al. Drug resistance is widespread among children who receive long-term antiretroviral treatment at a rural Tanzanian hospital. J Antimicrob Chemother. 2010;65(9):1996–2000. doi: 10.1093/jac/dkq234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.de Mulder M., Yebra G., Martín L., et al. Drug resistance prevalence and HIV-1 variant characterization in the naive and pretreated HIV-1-infected paediatric population in Madrid, Spain. J Antimicrob Chemother. 2011;66(10):2362–2371. doi: 10.1093/jac/dkr305. [DOI] [PubMed] [Google Scholar]
- 49.Zhao Y., Mu W., Harwell J., et al. Drug resistance profiles among HIV-1-infected children experiencing delayed switch and 12-month efficacy after using second-line antiretroviral therapy: an observational cohort study in rural China. J Acquir Immune Defic Syndr. 2011;58(1):47–53. doi: 10.1097/QAI.0b013e318229f2a2. [DOI] [PubMed] [Google Scholar]
- 50.Yan L., Yu F., Liang J., et al. Drug resistance profiles and influencing factors among HIV-infected children and adolescents receiving long-term ART: a multicentre observational study in China. J Antimicrob Chemother. 2022;77(3):727–734. doi: 10.1093/jac/dkab430. [DOI] [PubMed] [Google Scholar]
- 51.Ruel T.D., Kamya M.R., Li P., et al. Early virologic failure and the development of antiretroviral drug resistance mutations in HIV-infected Ugandan children. J Acquir Immune Defic Syndr. 2011;56(1):44–50. doi: 10.1097/QAI.0b013e3181fbcbf7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Shet A., Neogi U., Sahoo P.N., et al. Effectiveness of first-line antiretroviral therapy and acquired drug resistance among HIV-1-infected children in India. Pediatr Infect Dis J. 2013;32(5):e227–e229. doi: 10.1097/INF.0b013e31827fb2d1. [DOI] [PubMed] [Google Scholar]
- 53.Fitzgibbon J.E., Gaur S., Walsman S.M., et al. Emergence of drug resistance mutations in a group of HIV-infected children taking nelfinavir-containing regimens. AIDS Res Hum Retrovir. 2001;17(14):1321–1328. doi: 10.1089/08892220152596579. [DOI] [PubMed] [Google Scholar]
- 54.Pang X., Lu H., He Q., et al. Emergence of HIV-1 drug resistance mutations among children and adolescents undergoing prolonged antiretroviral therapy in Guangxi. J Glob Antimicrob Resist. 2024;37:208–213. doi: 10.1016/j.jgar.2024.03.013. [DOI] [PubMed] [Google Scholar]
- 55.Kurle S.N., Gangakhedkar R.R., Sen S., et al. Emergence of NNRTI drug resistance mutations after single-dose nevirapine exposure in HIV type 1 subtype C-infected infants in India. AIDS Res Hum Retrovir. 2007;23(5):682–685. doi: 10.1089/aid.2006.0167. [DOI] [PubMed] [Google Scholar]
- 56.Kamori D., Barabona G., Rugemalila J., et al. Emerging integrase strand transfer inhibitor drug resistance mutations among children and adults on ART in Tanzania: findings from a national representative HIV drug resistance survey. J Antimicrob Chemother. 2023;78(3):779–787. doi: 10.1093/jac/dkad010. [DOI] [PubMed] [Google Scholar]
- 57.Saravanan S., Kausalya B., Gomathi S., et al. Etravirine and rilpivirine drug resistance among HIV-1 subtype C infected children failing non-nucleoside reverse transcriptase inhibitor-based regimens in South India. AIDS Res Hum Retrovir. 2017;33(6):567–574. doi: 10.1089/aid.2016.0133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Vignoles M., Barboni G., Agosti M.R., et al. Evaluation of minority populations of HIV type-1 with K103N and M184V drug resistance mutations among children in Argentina. Antivir Ther. 2009;14(8):1175–1181. doi: 10.3851/IMP1461. [DOI] [PubMed] [Google Scholar]
- 59.Gibb D.M., Walker A.S., Kaye S., et al. Evolution of antiretroviral phenotypic and genotypic drug resistance in antiretroviral-naive HIV-1-infected children treated with abacavir/lamivudine, zidovudine/lamivudine or abacavir/zidovudine, with or without nelfinavir (the PENTA 5 trial) Antivir Ther. 2002;7(4):293–303. [PubMed] [Google Scholar]
- 60.Coetzer M., Westley B., Delong A., et al. Extensive drug resistance in HIV-infected Cambodian children who are undetected as failing first-line antiretroviral therapy by WHO 2010 guidelines. AIDS Res Hum Retrovir. 2013;29(7):985–992. doi: 10.1089/aid.2013.0025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Rossouw T.M., Feucht U.D., Melikian G., et al. Factors associated with the development of drug resistance mutations in HIV-1 infected children failing protease inhibitor-based antiretroviral therapy in South Africa. PLoS One. 2015;10(7) doi: 10.1371/journal.pone.0133452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Khamadi S.A., Bahemana E., Dear N., et al. Factors associated with viral suppression and drug resistance in children and adolescents living with HIV in care and treatment programs in southern Tanzania. J Pediatric Infect Dis Soc. 2023;12(6):353–363. doi: 10.1093/jpids/piad040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Jarchi M., Bokharaei-Salim F., Esghaei M., et al. The frequency of HIV-1 infection in Iranian children and determination of the transmitted drug resistance in treatment-naïve children. Curr HIV Res. 2019;17(6):397–407. doi: 10.2174/1570162X17666191106111211. [DOI] [PubMed] [Google Scholar]
- 64.Nelson J.A., Fokar A., Hudgens M.G., et al. Frequent nevirapine resistance in infants infected by HIV-1 via breastfeeding while on nevirapine prophylaxis. AIDS. 2015;29(16):2131–2138. doi: 10.1097/QAD.0000000000000814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Chaix M.L., Rouet F., Kouakoussui K.A., et al. Genotypic human immunodeficiency virus type 1 drug resistance in highly active antiretroviral therapy-treated children in Abidjan, Côte d'Ivoire. Pediatr Infect Dis J. 2005;24(12):1072–1076. doi: 10.1097/01.inf.0000190413.88671.92. [DOI] [PubMed] [Google Scholar]
- 66.Machado E.S., Lambert J.S., Watson D.C., et al. Genotypic resistance and HIV-1 subtype in Brazilian children on dual and triple combination therapy. J Clin Virol. 2004;30(1):24–31. doi: 10.1016/j.jcv.2003.08.001. [DOI] [PubMed] [Google Scholar]
- 67.Rodríguez-Galet A., Ventosa-Cubillo J., Bendomo V., et al. High drug resistance levels compromise the control of HIV infection in pediatric and adult populations in Bata, Equatorial Guinea. Viruses. 2022;15(1):27. doi: 10.3390/v15010027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Rubio-Garrido M., Reina G., Ndarabu A., et al. High drug resistance levels could compromise the control of HIV infection in paediatric and adolescent population in Kinshasa, the democratic republic of Congo. PLoS One. 2021;16(4) doi: 10.1371/journal.pone.0248835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Tadesse B.T., Kinloch N.N., Baraki B., et al. High levels of dual-class drug resistance in HIV-infected children failing first-line antiretroviral therapy in southern Ethiopia. Viruses. 2018;10(2):60. doi: 10.3390/v10020060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Louis F.J., Segaren N., Desinor O., et al. High levels of HIV-1 drug resistance in children who acquired HIV infection through mother to child transmission in the era of option B+, Haiti, 2013 to 2014. Pediatr Infect Dis J. 2019;38(5):503–507. doi: 10.1097/INF.0000000000002270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Boerma R.S., Boender T.S., Sigaloff K.C., et al. High levels of pre-treatment HIV drug resistance and treatment failure in Nigerian children. J Int AIDS Soc. 2016;19(1) doi: 10.7448/IAS.19.1.21140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Mossoro-Kpinde C.D., Gody J.C., Mboumba Bouassa R.S., et al. High levels of virological failure with major genotypic resistance mutations in HIV-1-infected children after 5 years of care according to WHO-recommended 1st-line and 2nd-line antiretroviral regimens in the Central African Republic: a cross-sectional study. Medicine (Baltimore) 2017;96(10) doi: 10.1097/MD.0000000000006282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Bennett S.J., Chunda-Liyoka C., Poppe L.K., et al. High nonnucleoside reverse transcriptase inhibitor resistance levels in HIV-1-infected Zambian mother-infant pairs. AIDS. 2020;34(12):1833–1842. doi: 10.1097/QAD.0000000000002614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Mboumba Bouassa R.S., Mossoro-Kpinde C.D., Gody J.C., et al. High predictive efficacy of integrase strand transfer inhibitors in perinatally HIV-1-infected African children in therapeutic failure of first- and second-line antiretroviral drug regimens recommended by the WHO. J Antimicrob Chemother. 2019;74(7):2030–2038. doi: 10.1093/jac/dkz099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Inzaule S.C., Osi S.J., Akinbiyi G., et al. High prevalence of HIV drug resistance among newly diagnosed infants aged <18 months: results from a nationwide surveillance in Nigeria. J Acquir Immune Defic Syndr. 2018;77(1):e1–e7. doi: 10.1097/QAI.0000000000001553. [DOI] [PubMed] [Google Scholar]
- 76.Kebe K., Thiam M., Diagne Gueye N.R., et al. High rate of antiretroviral drug resistance mutations in HIV type 1-infected Senegalese children in virological failure on first-line treatment according to the world health organization guidelines. AIDS Res Hum Retrovir. 2013;29(2):242–249. doi: 10.1089/aid.2011.0300. [DOI] [PubMed] [Google Scholar]
- 77.Crowell C.S., Maiga A.I., Sylla M., et al. High rates of baseline drug resistance and Virologic failure among ART-naive HIV-infected children in Mali. Pediatr Infect Dis J. 2017;36(11):e258–e263. doi: 10.1097/INF.0000000000001575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Salou M., Butel C., Konou A.A., et al. High rates of drug resistance among newly diagnosed HIV-infected children in the national prevention of mother-to-child transmission program in Togo. Pediatr Infect Dis J. 2016;35(8):879–885. doi: 10.1097/INF.0000000000001203. [DOI] [PubMed] [Google Scholar]
- 79.Neogi U., Sahoo P.N., De Costa A., et al. High viremia and low level of transmitted drug resistance in anti-retroviral therapy-naïve perinatally-infected children and adolescents with HIV-1 subtype C infection. BMC Infect Dis. 2012;12:317. doi: 10.1186/1471-2334-12-317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Steegen K., Levin L., Ketseoglou I., et al. High-level cross-resistance to didanosine observed in South African children failing an abacavir- or stavudine-based 1st-line regimen. PLoS One. 2014;9(5) doi: 10.1371/journal.pone.0097067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Aboulker J.P., Babiker A., Chaix M.L., et al. Highly active antiretroviral therapy started in infants under 3 months of age: 72-week follow-up for CD4 cell count, viral load and drug resistance outcome. AIDS. 2004;18(2):237–245. doi: 10.1097/00002030-200401230-00013. [DOI] [PubMed] [Google Scholar]
- 82.Kityo C., Sigaloff K.C., Sonia Boender T., et al. HIV drug resistance among children initiating first-line antiretroviral treatment in Uganda. AIDS Res Hum Retrovir. 2016;32(7):628–635. doi: 10.1089/aid.2015.0215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Sivay M.V., Maksimenko L.V., Nalimova T.M., et al. HIV drug resistance among patients experiencing antiretroviral therapy failure in Russia, 2019-2021. Int J Antimicrob Agents. 2024;63(2) doi: 10.1016/j.ijantimicag.2023.107074. [DOI] [PubMed] [Google Scholar]
- 84.Abuogi L., Oyaro P., Wakjira G., et al. HIV drug resistance patterns and characteristics associated with clinically significant drug resistance among children with virologic failure on antiretroviral treatment in Kenya: findings from the Opt4Kids randomized controlled trial. Viruses. 2023;15(10):2083. doi: 10.3390/v15102083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Dow D.E., Schimana W., Nyombi B.M., et al. HIV resistance and prevention of mother-to-child transmission regimen in HIV-infected infants in northern Tanzania. AIDS Res Hum Retrovir. 2017;33(11):1107–1113. doi: 10.1089/aid.2017.0025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Servais J., Lambert C., Karita E., et al. HIV type 1 pol gene diversity and archived nevirapine resistance mutation in pregnant women in Rwanda. AIDS Res Hum Retrovir. 2004;20(3):279–283. doi: 10.1089/088922204322996518. [DOI] [PubMed] [Google Scholar]
- 87.de Azevedo S.S.D., Delatorre E., Gaido C.M., et al. HIV-1 diversity and drug resistance in treatment-naïve children and adolescents from Rio de Janeiro, Brazil. Viruses. 2022;14(8):1761. doi: 10.3390/v14081761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Hunt G.M., Coovadia A., Abrams E.J., et al. HIV-1 drug resistance at antiretroviral treatment initiation in children previously exposed to single-dose nevirapine. AIDS. 2011;25(12):1461–1469. doi: 10.1097/QAD.0b013e3283492180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Zeh C., Weidle P.J., Nafisa L., et al. HIV-1 drug resistance emergence among breastfeeding infants born to HIV-infected mothers during a single-arm trial of triple-antiretroviral prophylaxis for prevention of mother-to-child transmission: a secondary analysis. PLoS Med. 2011;8(3) doi: 10.1371/journal.pmed.1000430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Puthanakit T., Jourdain G., Hongsiriwon S., et al. HIV-1 drug resistance mutations in children after failure of first-line nonnucleoside reverse transcriptase inhibitor-based antiretroviral therapy. HIV Med. 2010;11(9):565–572. doi: 10.1111/j.1468-1293.2010.00828.x. [DOI] [PubMed] [Google Scholar]
- 91.Nii-Trebi N.I., Ibe S., Barnor J.S., et al. HIV-1 drug-resistance surveillance among treatment-experienced and -naïve patients after the implementation of antiretroviral therapy in Ghana. PLoS One. 2013;8(8) doi: 10.1371/journal.pone.0071972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Neubert J., Michalsky N., Laws H.J., et al. HIV-1 subtype diversity and prevalence of primary drug resistance in a single-center pediatric cohort in Germany. Intervirology. 2016;59(5-6):301–306. doi: 10.1159/000477811. [DOI] [PubMed] [Google Scholar]
- 93.Nyandiko W., Holland S., Vreeman R., et al. HIV-1 treatment failure, drug resistance, and clinical outcomes in perinatally infected children and adolescents failing first-line antiretroviral therapy in western Kenya. J Acquir Immune Defic Syndr. 2022;89(2):231–239. doi: 10.1097/QAI.0000000000002850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Jordan M.R., Penazzato M., Cournil A., et al. Human immunodeficiency virus (HIV) drug resistance in african infants and young children newly diagnosed with HIV: a multicountry analysis. Clin Infect Dis. 2017;65(12):2018–2025. doi: 10.1093/cid/cix698. [DOI] [PubMed] [Google Scholar]
- 95.Yeganeh N., Kerin T., Ank B., et al. Human immunodeficiency virus antiretroviral resistance and transmission in mother-infant pairs enrolled in a large perinatal study. Clin Infect Dis. 2018;66(11):1770–1777. doi: 10.1093/cid/cix1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Fogel J.M., Mwatha A., Richardson P., et al. Impact of maternal and infant antiretroviral drug regimens on drug resistance in HIV-infected breastfeeding infants. Pediatr Infect Dis J. 2013;32(4):e164–e169. doi: 10.1097/INF.0b013e31827f44ee. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Chaix M.L., Ekouevi D.K., Peytavin G., et al. Impact of nevirapine (NVP) plasma concentration on selection of resistant virus in mothers who received single-dose NVP to prevent perinatal human immunodeficiency virus type 1 transmission and persistence of resistant virus in their infected children. Antimicrob Agents Chemother. 2007;51(3):896–901. doi: 10.1128/AAC.00910-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Mutwa P.R., Boer K.R., Rusine J., et al. Long-term effectiveness of combination antiretroviral therapy and prevalence of HIV drug resistance in HIV-1-infected children and adolescents in Rwanda. Pediatr Infect Dis J. 2014;33(1):63–69. doi: 10.1097/INF.0b013e31829e6b9f. [DOI] [PubMed] [Google Scholar]
- 99.Lehman D.A., Wamalwa D.C., McCoy C.O., et al. Low-frequency nevirapine resistance at multiple sites may predict treatment failure in infants on nevirapine-based treatment. J Acquir Immune Defic Syndr. 2012;60(3):225–233. doi: 10.1097/QAI.0b013e3182515730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Chohan B.H., Tapia K., Benki-Nugent S., et al. Nevirapine resistance in previously nevirapine-#nexposed HIV-1-Infected Kenyan infants initiating early antiretroviral therapy. AIDS Res Hum Retrovir. 2015;31(8):783–791. doi: 10.1089/aid.2014.0370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Fisher R.G., Smith D.M., Murrell B., et al. Next generation sequencing improves detection of drug resistance mutations in infants after PMTCT failure. J Clin Virol. 2015;62:48–53. doi: 10.1016/j.jcv.2014.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Fokam J., Bellocchi M.C., Armenia D., et al. Next-generation sequencing provides an added value in determining drug resistance and viral tropism in Cameroonian HIV-1 vertically infected children. Medicine (Baltimore) 2018;97(13) doi: 10.1097/MD.0000000000010176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Ikomey G.M., Assoumou M.C.O., Gichana J.O., et al. Observed HIV drug resistance associated mutations amongst naïve immunocompetent children in Yaoundé, Cameroon. Germs. 2017;7(4):178–185. doi: 10.18683/germs.2017.1124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Delaugerre C., Chaix M.L., Blanche S., et al. Perinatal acquisition of drug-resistant HIV-1 infection: mechanisms and long-term outcome. Retrovirology. 2009;6:85. doi: 10.1186/1742-4690-6-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Kovacs A., Cowles M.K., Britto P., et al. Pharmacokinetics of didanosine and drug resistance mutations in infants exposed to zidovudine during gestation or postnatally and treated with didanosine or zidovudine in the first three months of life. Pediatr Infect Dis J. 2005;24(6):503–509. doi: 10.1097/01.inf.0000164787.63237.0b. [DOI] [PubMed] [Google Scholar]
- 106.Abidi S.H., Nduva G.M., Siddiqui D., et al. Phylogenetic and drug-resistance analysis of HIV-1 sequences from an extensive paediatric HIV-1 outbreak in Larkana, Pakistan. Front Microbiol. 2021;12 doi: 10.3389/fmicb.2021.658186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Aulicino P.C., Zapiola I., Kademian S., et al. Pre-treatment drug resistance and HIV-1 subtypes in infants from Argentina with and without exposure to antiretroviral drugs for prevention of mother-to-child transmission. J Antimicrob Chemother. 2019;74(3):722–730. doi: 10.1093/jac/dky486. [DOI] [PubMed] [Google Scholar]
- 108.Jittamala P., Puthanakit T., Chaiinseeard S., et al. Predictors of virologic failure and genotypic resistance mutation patterns in Thai children receiving non-nucleoside reverse transcriptase inhibitor-based antiretroviral therapy. Pediatr Infect Dis J. 2009;28(9):826–830. doi: 10.1097/INF.0b013e3181a458f9. [DOI] [PubMed] [Google Scholar]
- 109.Soeria-Atmadja S., Amuge P., Nanzigu S., et al. Pretreatment HIV drug resistance predicts accumulation of new mutations in ART-naive Ugandan children. Acta Paediatr. 2020;109(12):2706–2716. doi: 10.1111/apa.15320. [DOI] [PubMed] [Google Scholar]
- 110.Kityo C., Boerma R.S., Sigaloff K.C.E., et al. Pretreatment HIV drug resistance results in virological failure and accumulation of additional resistance mutations in Ugandan children. J Antimicrob Chemother. 2017;72(9):2587–2595. doi: 10.1093/jac/dkx188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Jordan M.R., Bikinesi L., Ashipala L., et al. Pretreatment human immunodeficiency virus (HIV) drug resistance among treatment-naive infants newly diagnosed with HIV in 2016 in Namibia: results of a nationally representative study. Open Forum Infect Dis. 2022;9(5) doi: 10.1093/ofid/ofac102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Tadesse B.T., Tsai O., Chala A., et al. Prevalence and correlates of pre-treatment HIV drug resistance among HIV-infected children in Ethiopia. Viruses. 2019;11(9):877. doi: 10.3390/v11090877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Inzaule S.C., Weidle P.J., Yang C., et al. Prevalence and dynamics of the K65R drug resistance mutation in HIV-1-infected infants exposed to maternal therapy with lamivudine, zidovudine and either nevirapine or nelfinavir in breast milk. J Antimicrob Chemother. 2016;71(6):1619–1626. doi: 10.1093/jac/dkw039. [DOI] [PubMed] [Google Scholar]
- 114.Ngo-Giang-Huong N., Wittkop L., Judd A., et al. Prevalence and effect of pre-treatment drug resistance on the virological response to antiretroviral treatment initiated in HIV-infected children - a EuroCoord-CHAIN-EPPICC joint project. BMC Infect Dis. 2016;16(1):654. doi: 10.1186/s12879-016-1968-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Agwu A.L., Chang J.Y., Wiegand R.E., et al. Prevalence and outcomes of recycling NNRTIs despite documented NNRTI resistance in HIV-infected children and youth. AIDS Patient Care STDS. 2014;28(1):10–14. doi: 10.1089/apc.2013.0308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Delaugerre C., Warszawski J., Chaix M.L., et al. Prevalence and risk factors associated with antiretroviral resistance in HIV-1-infected children. J Med Virol. 2007;79(9):1261–1269. doi: 10.1002/jmv.20940. [DOI] [PubMed] [Google Scholar]
- 117.Contreras G.A., Bell C.S., Del Bianco G.P., et al. Prevalence and risk factors associated with resistance-associated mutations to etravirine in a cohort of perinatally HIV-infected children. J Antimicrob Chemother. 2013;68(10):2344–2348. doi: 10.1093/jac/dkt198. [DOI] [PubMed] [Google Scholar]
- 118.Frange P., Avettand-Fenoel V., Veber F., et al. Prevalence of drug resistance in children recently diagnosed with HIV-1 infection in France (2006-17): impact on susceptibility to first-line strategies. J Antimicrob Chemother. 2018;73(9):2475–2479. doi: 10.1093/jac/dky203. [DOI] [PubMed] [Google Scholar]
- 119.Karchava M., Pulver W., Smith L., et al. Prevalence of drug-resistance mutations and non-subtype B strains among HIV-infected infants from New York State. J Acquir Immune Defic Syndr. 2006;42(5):614–619. doi: 10.1097/01.qai.0000225871.87456.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Parker M.M., Wade N., Lloyd R.M., Jr., et al. Prevalence of genotypic drug resistance among a cohort of HIV-infected newborns. J Acquir Immune Defic Syndr. 2003;32(3):292–297. doi: 10.1097/00126334-200303010-00008. [DOI] [PubMed] [Google Scholar]
- 121.Djiyou A.B.D., Penda C.I., Madec Y., et al. Prevalence of HIV drug resistance among adolescents receiving ART in Cameroon with low- or high-level viraemia. J Antimicrob Chemother. 2023;78(12):2938–2942. doi: 10.1093/jac/dkad334. [DOI] [PubMed] [Google Scholar]
- 122.Hunt G.M., Ledwaba J., Salimo A., et al. Prevalence of HIV-1 drug resistance amongst newly diagnosed HIV-infected infants age 4-8 weeks, enrolled in three nationally representative PMTCT effectiveness surveys, South Africa: 2010, 2011-12 and 2012-13. BMC Infect Dis. 2019;19(Suppl 1):787. doi: 10.1186/s12879-019-4339-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Fofana D.B., Diarra H., Guindo I., et al. Prevalence of HIV-1 natural polymorphisms and integrase-resistance-associated mutations in African children. Viruses. 2023;15(2):547. doi: 10.3390/v15020546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Green H., Gibb D.M., Compagnucci A., et al. A randomized controlled trial of genotypic HIV drug resistance testing in HIV-1-infected children: the PERA (PENTA 8) trial. Antivir Ther. 2006;11(7):857–867. [PubMed] [Google Scholar]
- 125.Taylor B.S., Hunt G., Abrams E.J., et al. Rapid development of antiretroviral drug resistance mutations in HIV-infected children less than two years of age initiating protease inhibitor-based therapy in South Africa. AIDS Res Hum Retrovir. 2011;27(9):945–956. doi: 10.1089/aid.2010.0205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Compagno F., Naegele K., Kahlert C.R., et al. The rate of mother-to-child transmission of antiretroviral drug-resistant HIV strains is low in the Swiss Mother and Child HIV Cohort Study. Swiss Med Wkly. 2019;149 doi: 10.4414/smw.2019.20059. [DOI] [PubMed] [Google Scholar]
- 127.Hunt G.M., Yousif M., Levin L., et al. Resistance is common in paediatric patients failing ART in South Africa. J Antimicrob Chemother. 2023;78(5):1160–1167. doi: 10.1093/jac/dkac443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Thu H.H.K., Schemelev A.N., Ostankova Y.V., et al. Resistance mutation patterns among HIV-1-Infected children and features of the program for prevention of mother-to-child transmission in Vietnam's central highlands and southern regions, 2017-2021. Viruses. 2024;16(5):696. doi: 10.3390/v16050696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Han J., Wang L., Jiang Y., et al. Resistance mutations in HIV-1 infected pregnant women and their infants receiving antiretrovirals to prevent HIV-1 vertical transmission in China. Int J STD AIDS. 2009;20(4):249–254. doi: 10.1258/ijsa.2008.008480. [DOI] [PubMed] [Google Scholar]
- 130.Fofana D.B., d'Almeida M., Lambert-Niclot S., et al. Resistance profile and treatment outcomes in HIV-infected children at virological failure in Benin, West Africa. J Antimicrob Chemother. 2018;73(11):3143–3147. doi: 10.1093/jac/dky300. [DOI] [PubMed] [Google Scholar]
- 131.Servais J., Hainaut M., Schmitz V., et al. Resistance testing in children changing human immunodeficiency virus type 1 protease inhibitor. Pediatr Infect Dis J. 2002;21(3):214–220. doi: 10.1097/00006454-200203000-00011. [DOI] [PubMed] [Google Scholar]
- 132.Vaz P., Chaix M.L., Jani I., et al. Risk of extended viral resistance in human immunodeficiency virus-1-infected Mozambican children after first-line treatment failure. Pediatr Infect Dis J. 2009;28(12):e283–e287. doi: 10.1097/INF.0b013e3181ba6c92. [DOI] [PubMed] [Google Scholar]
- 133.Sylla M., Dolo O., Maiga A.I., et al. Second-line antiretroviral therapy failure and characterization of HIV-1 drug resistance patterns in children in Mali. Arch Pediatr. 2019;26(5):254–258. doi: 10.1016/j.arcped.2019.06.002. [DOI] [PubMed] [Google Scholar]
- 134.Eshleman S.H., Mracna M., Guay L.A., et al. Selection and fading of resistance mutations in women and infants receiving nevirapine to prevent HIV-1 vertical transmission (HIVNET 012) AIDS. 2001;15(15):1951–1957. doi: 10.1097/00002030-200110190-00006. [DOI] [PubMed] [Google Scholar]
- 135.Martinson N.A., Morris L., Gray G., et al. Selection and persistence of viral resistance in HIV-infected children after exposure to single-dose nevirapine. J Acquir Immune Defic Syndr. 2007;44(2):148–153. doi: 10.1097/QAI.0b013e31802b920e. [DOI] [PubMed] [Google Scholar]
- 136.Lange C.M., Hué S., Violari A., et al. Single genome analysis for the detection of linked multiclass drug resistance mutations in HIV-1-Infected children after failure of protease inhibitor-based first-line therapy. J Acquir Immune Defic Syndr. 2015;69(2):138–144. doi: 10.1097/QAI.0000000000000568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Persaud D., Bedri A., Ziemniak C., et al. Slower clearance of nevirapine resistant virus in infants failing extended nevirapine prophylaxis for prevention of mother-to-child HIV transmission. AIDS Res Hum Retrovir. 2011;27(8):823–829. doi: 10.1089/aid.2010.0346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Vaz P., Augusto O., Bila D., et al. Surveillance of HIV drug resistance in children receiving antiretroviral therapy: a pilot study of the World Health Organization's generic protocol in Maputo, Mozambique. Clin Infect Dis. 2012;54(Suppl 4):S369–S374. doi: 10.1093/cid/cis006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Olusola F.I., Olusola B.A., Oladokun R., et al. Surveillance of pretreatment drug resistance among HIV-infected children in Ibadan, Nigeria. AIDS Res Hum Retrovir. 2021;37(12):922–929. doi: 10.1089/AID.2020.0272. [DOI] [PubMed] [Google Scholar]
- 140.Brindeiro P.A., Brindeiro R.M., Mortensen C., et al. Testing genotypic and phenotypic resistance in human immunodeficiency virus type 1 isolates of clade B and other clades from children failing antiretroviral therapy. J Clin Microbiol. 2002;40(12):4512–4519. doi: 10.1128/JCM.40.12.4512-4519.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Guimarães P.M., Ferreira J.L., Coelho L.P., et al. Transmitted drug resistance among recently diagnosed adults and children in São Paulo, Brazil. AIDS Res Hum Retrovir. 2015;31(12):1219–1224. doi: 10.1089/AID.2014.0354. [DOI] [PubMed] [Google Scholar]
- 142.Ross L.L., Cotton M.F., Cassim H., et al. Treatment-emergent mutations and resistance in HIV-infected children treated with Fosamprenavir-containing antiretroviral regimens. Open AIDS J. 2015;9:38–44. doi: 10.2174/1874613601509010038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Gopalan B.P., D'Souza R.R., Rajnala N., et al. Viral evolution in the cell-associated HIV-1 DNA during early ART can lead to drug resistance and virological failure in children. J Med Virol. 2019;91(6):1036–1047. doi: 10.1002/jmv.25413. [DOI] [PubMed] [Google Scholar]
- 144.Adjé-Touré C., Hanson D.L., Talla-Nzussouo N., et al. Virologic and immunologic response to antiretroviral therapy and predictors of HIV type 1 drug resistance in children receiving treatment in Abidjan, Côte d'Ivoire. AIDS Res Hum Retrovir. 2008;24(7):911–917. doi: 10.1089/aid.2007.0264. [DOI] [PubMed] [Google Scholar]
- 145.Tagnouokam-Ngoupo P.A., Penda I.C., Tchatchueng Mbougua J.B., et al. Virological failure and antiretroviral resistance among HIV-infected children after five years follow-up in the ANRS 12225-PEDIACAM cohort in Cameroon. PLoS One. 2021;16(3) doi: 10.1371/journal.pone.0248642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Szubert A.J., Prendergast A.J., Spyer M.J., et al. Virological response and resistance among HIV-infected children receiving long-term antiretroviral therapy without virological monitoring in Uganda and Zimbabwe: observational analyses within the randomised ARROW trial. PLoS Med. 2017;14(11) doi: 10.1371/journal.pmed.1002432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Charpentier C., Gody J.C., Mbitikon O., et al. Virological response and resistance profiles after 18 to 30 months of first- or second-/third-line antiretroviral treatment: a cross-sectional evaluation in HIV type 1-infected children living in the Central African Republic. AIDS Res Hum Retrovir. 2012;28(1):87–94. doi: 10.1089/aid.2011.0035. [DOI] [PubMed] [Google Scholar]
- 148.Amani-Bosse C., Dahourou D.L., Malateste K., et al. Virological response and resistances over 12 months among HIV-infected children less than two years receiving first-line lopinavir/ritonavir-based antiretroviral therapy in Cote d'Ivoire and Burkina Faso: the MONOD ANRS 12206 cohort. J Int AIDS Soc. 2017;20(1) doi: 10.7448/IAS.20.01.21362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Masquelier B., Chaix M.L., Burgard M., et al. Zidovudine genotypic resistance in HIV-1-infected newborns in the French perinatal cohort. J Acquir Immune Defic Syndr. 2001;27(2):99–104. doi: 10.1097/00126334-200106010-00001. [DOI] [PubMed] [Google Scholar]
- 150.World Health Organization Antiretroviral therapy for HIV infection in infants and children: towards universal access: recommendations for a public health approach: 2010 revision. https://www.who.int/publications/i/item/9789241599801 Available at: [PubMed]
- 151.Koay W.L.A., Kose-Otieno J., Rakhmanina N. HIV drug resistance in children and adolescents: always a challenge? Curr Epidemiol Rep. 2021;8(3):97–107. doi: 10.1007/s40471-021-00268-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Phillips A.N., Stover J., Cambiano V., et al. Impact of HIV drug resistance on HIV/AIDS-associated mortality, new infections, and antiretroviral therapy program costs in Sub-Saharan Africa. J Infect Dis. 2017;215(9):1362–1365. doi: 10.1093/infdis/jix089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.World Health Organization Global price reporting mechanism [online database] http://apps.who.int/hiv/amds/price/hdd
- 154.SeyedAlinaghi S., Afsahi A.M., Moradi A., et al. Current ART, determinants for virologic failure and implications for HIV drug resistance: an umbrella review. AIDS Res Ther. 2023;20(1):74. doi: 10.1186/s12981-023-00572-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.World Health Organization Updated recommendations on first-line and second-line antiretroviral regimens and post-exposure prophylaxis and recommendations on early infant diagnosis of HIV: interim guidelines. https://www.who.int/publications/i/item/WHO-CDS-HIV-18.51
- 156.Phillips A.N., Cambiano V., Jordan M., et al. Cost effectiveness of policy options when pretreatment NNRTI drug resistance is high. Conference on retroviruses and opportunistic infections (CROI) 2017, 13–16 February 2017. https://www.croiconference.org/abstract/cost-effectiveness-policy-options-when-pretreatment-nnrti-drug-resistance-high/ Seattle, WA, USA; Abstract 112; Available at:
- 157.UNITAID press release Groundbreaking agreement reduces by 75% the cost of HIV treatment for children in low-and middle-income countries. https://www.clintonhealthaccess.org/news/groundbreaking-agreement-reduces-by-75-the-cost-of-hiv-treatment-for-children-in-low-and-middle-income-countries/ Available at:
- 158.UNAIDS Start free stay free AIDS free - 2020 report. https://www.unaids.org/en/resources/documents/2020/start-free-stay-free-aids-free-2020-progress-report Available at:
- 159.Sigaloff K.C., Hamers R.L., Menke J., et al. Early warning indicators for population-based monitoring of HIV drug resistance in 6 African countries. Clin Infect Dis. 2012;54(Suppl 4):S294–S299. doi: 10.1093/cid/cir1015. [DOI] [PubMed] [Google Scholar]
- 160.Getaneh Y., Zealyas K., Adugna F., et al. HIV drug resistance early warning indicators in Ethiopia: variability at regional and health facility levels and trend over time. Int J Infect Dis. 2020;95:90–97. doi: 10.1016/j.ijid.2020.02.031. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.