Summary
Background
Guidelines recommend low-dose colchicine for secondary prevention in cardiovascular disease, but uncertainty remains concerning its efficacy for stroke, efficacy in key subgroups and about uncommon but serious safety outcomes.
Methods
In this trial-level meta-analysis, we searched bibliographic databases and trial registries form inception to May 16, 2024. We included randomised trials of colchicine for secondary prevention of ischaemic stroke and major adverse cardiovascular events (MACE: ischaemic stroke, myocardial infarction, coronary revascularisation, or cardiovascular death). Secondary outcomes were serious safety outcomes and mortality. A fixed-effect inverse-variance model was used to generate a pooled estimate of relative risk (RR) with 95% confidence intervals (CI). This study is registered with PROSPERO, CRD42024540320.
Findings
Six trials involving 14,934 patients with prior stroke or coronary disease were included. In all patients, colchicine compared with placebo or no colchicine reduced the risk for ischaemic stroke by 27% (132 [1.8%] events versus 186 [2.5%] events, RR 0.73 [95% CI 0.58–0.90]) and MACE by 27% (505 [6.8%] events versus 693 [9.4%] events, with RR 0.73 [0.65–0.81]). Efficacy was consistent in key subgroups (females versus males, age below versus above 70, with versus without diabetes, statin versus non-statin users). Colchicine was not associated with an increase in serious safety outcomes: hospitalisation for pneumonia (109 [1.5%] versus 106 [1.5%], RR 0.99 [0.76–1.30]), cancer (247 [3.5%] versus 255 [3.6%], RR 0.97 [0.82–1.15]), and gastro-intestinal events (153 [2.1%] versus 135 [1.9%]), RR 1.15 [0.91–1.44]. There was no difference in all-cause death (201 [2.7%] versus 181 [2.4%], RR 1.09 [0.89–1.33]), cardiovascular death (70 [0.9%] versus 80 [1.1%], RR 0.89 [0.65–1.23]), or non-cardiovascular death (131 [1.8%] versus 101 [1.4%], RR 1.26 [0.98–1.64]).
Interpretation
In patients with prior stroke or coronary disease, colchicine reduced ischaemic stroke and MACE, with consistent treatment effect in key subgroups, and did not increase serious safety events or death.
Funding
There was no funding source for this study.
Keywords: Colchicine, Meta-analysis, Stroke, MACE, Safety
Research in context.
Evidence before this study
Low-dose colchicine (0.5 mg once daily) prevents major adverse cardiovascular events (MACE) in patients with coronary disease and is recommended by treatment guidelines. However, uncertainty remains concerning the efficacy of colchicine for stroke prevention, benefits in key subgroups and on serious safety events.
Added value of this study
Pooled study-level data from six trials involving 14,934 participants demonstrate that colchicine in patients with prior stroke or coronary disease lowers the risk of ischaemic stroke and MACE, with consistent efficacy in patients with prior stroke or coronary disease, in key subgroups defined by sex, age, diabetes, and statin use at baseline, and without statistically significant increases of serious safety outcomes (hospitalisation for pneumonia, cancer, and gastro-intestinal events) or death (all-cause death, cardiovascular death, and non-cardiovascular death).
Implications of all the available evidence
The results of our meta-analysis support the routine use of colchicine for secondary prevention of stroke and coronary events in a broad population of patients with prior stroke or coronary artery disease.
Introduction
Stroke and coronary disease are the leading causes of death worldwide. Despite the availability of effective prevention strategies, the burden of cardiovascular disease continues to rise, driven in low- and middle-income countries by growing exposure to cardiovascular risk factors, and, in high income countries, by aging populations.1 Additional effective and affordable therapies are needed to address the growing disease burden. Inflammation is an important risk factor for stroke and cardiovascular outcomes.2, 3, 4, 5 Colchicine is an anti-inflammatory agent with multiple actions on inflammatory pathways, mediated by inhibition of microtubule function.6 Production costs of colchicine are low and its widespread availability make it attractive as an inexpensive agent for secondary prevention in regions of varying economic status.
In the Colchicine Cardiovascular Outcomes Trial (COLCOT) and Low-dose Colchicine for secondary prevention of cardiovascular disease (LoDoCo) randomised clinical trials (RCTs), long-term colchicine reduced recurrence of major adverse cardiovascular events (MACE) in patients with coronary disease.7, 8, 9 Multiple treatment guidelines now recommend low-dose colchicine in coronary disease.10, 11, 12, 13 However, key subgroups of patients, particularly those with prior stroke, were underrepresented in the pivotal trials. The recently completed COlchicine for preveNtion of Vascular Inflammation in Non-CardioEmbolic stroke (CONVINCE) trial showed a numerical, but not statistically significant, reduction of MACE in patients with prior stroke or transient ischaemic attack (TIA).14 Uncertainty thus remains concerning treatment effect of colchicine on stroke outcomes, and in key subgroups including females, older patients, those with diabetes and in statin-untreated patients, and on serious safety events.
The primary aim of this collaborative meta-analysis involving the lead investigators of several trials of colchicine was to evaluate the efficacy of colchicine for the prevention of ischaemic stroke and MACE, as well as to provide comprehensive safety data, and investigate efficacy in key clinical subgroups.
Methods
Search strategy, selection criteria, and data-extraction
This meta-analysis was performed according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) reporting guidelines.15 The protocol was submitted to international prospective register of systematic reviews PROSPERO on April 26, 2024, prior to commencement of the analyses.
PubMed, EMBASE, Cochrane Central Register of Controlled Trials (CENTRAL) and clinicaltrials.gov were searched from inception to May 16, 2024, without restriction on language of publication, to identify RCTs published in peer-reviewed journals that compared colchicine to an active comparator (i.e., placebo or no colchicine control) in patients with cardiovascular disease. The key search terms were ‘atherosclerosis’, ‘myocardial ischemia’, ‘stroke’, ‘transient ischaemic attack’, ‘brain ischemia’, ‘peripheral artery disease’ and ‘colchicine’, including their subheadings and synonyms. Sensitivity-maximising filters were used as recommended by the Cochrane Collaboration to identify RCTs in PubMed and EMBASE.16,17 The search algorithm is presented in Supplemental Table S1.
RCTs were eligible for inclusion if they compared colchicine to an active comparator (placebo or no colchicine) for secondary prevention after stroke or coronary disease, with a least 3 months of treatment duration, reporting data on any of the efficacy or safety outcomes. Unpublished data, observational studies, non-randomised registry studies, narrative reviews, editorials, case series, and duplicate studies were excluded. Two authors (ATLF and MHFP) independently screened titles and abstracts for relevance and duplicates and evaluated full text articles for eligibility with conflicts resolved by consensus discussion with a third reviewer (PJK). For each included trial, summary data were extracted from the principal and relevant subsidiary peer-reviewed publications.
Data analysis
The primary efficacy outcomes were ischaemic stroke and MACE (i.e., the composite of ischaemic stroke, myocardial infarction, coronary revascularisation, or cardiovascular death). We used the definitions of outcomes used in the original trials (Supplemental Table S2). We requested additional data from the principal investigators to create the pre-specified composite outcome for this meta-analysis if needed. Other secondary efficacy outcomes included all stroke (ischaemic and haemorrhagic, including intracerebral haemorrhage and subarachnoid haemorrhage); myocardial infarction; and coronary revascularisation. The main safety outcome was death, which was further classified as all-cause death, cardiovascular death, and non-cardiovascular death. Other serious safety outcomes evaluated included hospitalisation for pneumonia, newly diagnosed cancer, and gastro-intestinal events.
The primary analysis was done in all patients and a sensitivity analysis was done restricting the cohort to patients with prior stroke or TIA. For subgroup analyses, lead investigators of included trials provided outcome data for the primary efficacy outcomes according to prespecified subgroups: sex (female versus male); age (<70 versus ≥70 years); diabetes mellitus (yes versus no); and statin treatment at randomisation (yes versus no).
The relative risks of each outcome from each individual trial were determined and pooled estimates were subsequently calculated by applying inverse-variance weighting using a fixed-effect model. Additional sensitivity analyses encompassed a separate analysis using a random-effect model with a DerSimonian–Laird estimator. The treatment effect was formally tested at a two-sided alpha level of 0.05. Inter-study heterogeneity was assessed with the Higgins and Thompsons’ I2 index. Heterogeneity was considered low if the percentage was approximately 25%, moderate if 50%, and high if 75%.18 Interactions between studies and between subgroups were examined with a test for heterogeneity, with a p value below 0.05 considered significant. Publication bias was assessed if sufficient (i.e., 10 or more) studies were available.
All analyses were performed in STATA (version 15.1) and R (The R Foundation for Statistical Computing, version 3.6.0).
Risk of bias assessment
The methodological quality of the randomised controlled trials was assessed by the Cochrane Collaboration's revised Risk-of-Bias 2 tool.19,20 Two investigators (ATLF and MHFP) independently assessed the five domains for risk of bias: the randomisation process, deviations from intended interventions, missing outcome data, measurement of the outcome, and selection of the reported results.
Role of the funding source
There was no funding source for this study.
Results
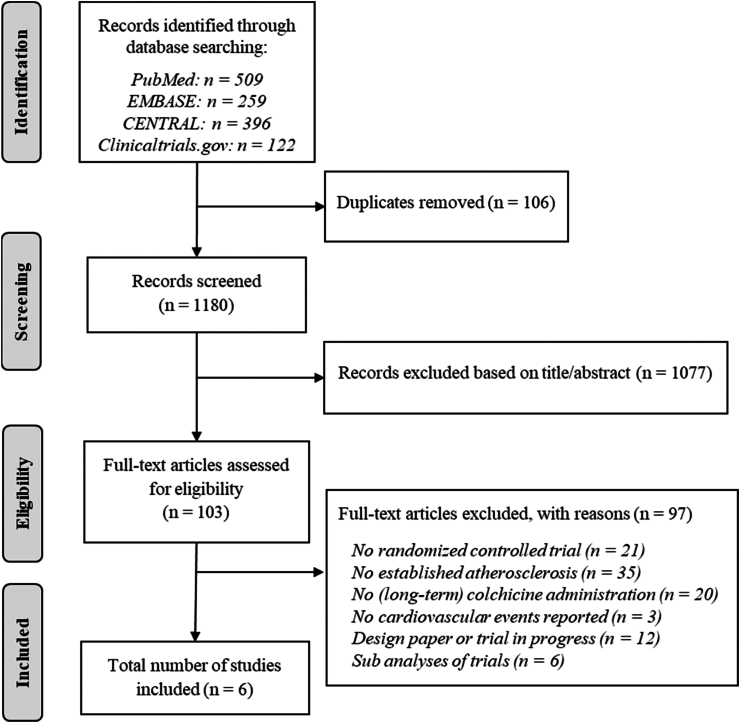
Six eligible trials involving 14,934 participants, of whom 7487 received colchicine and 7473 received placebo or no colchicine, were identified (Fig. 1).7, 8, 9,14,21,22 Table 1 provides a summary of trial designs. One trial was performed in patients with prior non-cardioembolic stroke or TIA (CONVINCE, n = 3144), and five trials evaluated patients with coronary disease (LoDoCo2, n = 5522 participants; Colchicine in Patients With Acute Coronary Syndrome [COPS], n = 795; COLCOT, n = 4745; LoDoCo, n = 532; Deftereos et al., n = 196). LoDoCo2, COLCOT, COPS and the trial by Deftereos et al. were randomised, placebo-controlled, double-blind trials. CONVINCE and LoDoCo were open-label trials with blinded endpoint adjudication.
Fig. 1.
Flowchart of included studies.
Table 1.
Key features of included trials.
| Acronym | Author | Year | Trial size | Key inclusion criteria | Key exclusion criteria | Active treatment | Comparator | Multi-centre | Open label run-in | Follow-up (median, months) |
|---|---|---|---|---|---|---|---|---|---|---|
| CONVINCE | Kelly | 2024 | 3144 | Non-severe ischaemic stroke or high-risk TIA | Stroke/TIA caused by cardio-embolism or other defined cause | Colchicine 0.5 mg once daily | No colchicine | Yes | No | 34 |
| LoDoCo2 | Nidorf | 2020 | 5522 | Chronic coronary disease, clinically stable >6 months | Heart failure (NYHA III/IV); renal failure (eGFR <50 ml/min/1.73 m2); severe valvular heart disease | Colchicine 0.5 mg once daily | Placebo | Yes | Yes | 29 |
| COPS | Tong | 2020 | 795 | Acute coronary syndrome with presence of coronary disease | Requiring bypass surgery; severe liver impairment; severe renal impairment (eGFR <30 ml/min/1.73 m2) | Colchicine 0.5 mg twice daily for one month, followed by 0.5 mg once daily | Placebo | Yes | No | 12 |
| COLCOT | Tardif | 2019 | 4745 | Post myocardial infarction | Heart failure (LVEF <35%); renal impairment (creatinine level >2x upper limit of normal); bypass surgery <3 years or planned | Colchicine 0.5 mg once daily | Placebo | Yes | No | 23 |
| NA | Deftereos | 2013 | 222 | Diabetes and undergoing percutaneous coronary revascularisation | Acute myocardial infarction; renal impairment (eGFR <20 ml/min/1.73 m2); liver failure | Colchicine 0.5 mg twice daily | Placebo | No | No | 6 |
| LoDoCo | Nidorf | 2013 | 532 | Chronic coronary disease, clinically stable >6 months | Bypass surgery <10 years, major competing comorbidities | Colchicine 0.5 mg once daily | No colchicine | No | No | 36 |
eGFR, estimated glomerular filtration rate. LVEF, left ventricular ejection fraction. NA, not available. NYHA, New York Heart Association. TIA, transient ischaemic attack.
Deftereos et al. studied colchicine at a dose of 0.5 mg by mouth twice daily and COPS used this dose for the first month only, followed by 0.5 mg once daily. All other trials used 0.5 mg once daily. The LoDoCo2 trial used an open-label run-in period of 30 days during which all participants received colchicine at a dose of 0.5 mg once daily. Trial medication discontinuation in the colchicine group at the end of the trials ranged from 10.5% to 22.0% (Supplemental Table S4). CONVINCE was finished before the anticipated number of outcomes was accrued due to budget constraints attributable to the Coronavirus disease 2019 (COVID-19) pandemic. Risk of bias was overall low for the primary outcomes the trials. We assigned unclear risk of bias to Deftereos et al., since selection of the reported outcomes could not be verified with a prespecified research protocol (Supplemental Table S3).
Baseline characteristics for each study are shown in Table 2. Percentage of female participants ranged from 11.1% to 34.7%. Median age ranged from 59.9 to 65.5 years. Percentage of current smokers ranged from 4.5% to 37.8% and those of patients with history of hypertension ranged from 48.5% to 65.4%. Percentage of patients with diabetes mellitus ranged from 18.3% to 100%. Prior stroke or TIA ranged from 2% to 100% in the four trials that reported this. Patients were treated according to recommended standards for secondary cardiovascular disease prevention, with 93.5%–99.0% of patients treated with statin therapy and 90.9%–98.8% treated with antiplatelet therapy at the time of randomisation.
Table 2.
Baseline characteristics of included trials.
| Mean age | Females | Current smoking | Hypertension | Diabetes mellitus | eGFR <60 ml/min/1.73m2 | History of stroke or TIA | History of ACS | Antiplatelet therapy | Statin therapy | Beta-blocker therapy | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| CONVINCE | 66.3 ± 10.0 | 30.5% | 22.1% | 65.4% | 22.3% | NA | 100% | 9% | 97.5% | 93.5% | NA |
| LoDoCo2 | 65.8 ± 8.6 | 15.3% | 11.8% | 50.9% | 18.3% | 5.5% | 4.0% | 84.4% | 90.9% | 94.0% | 62.1% |
| COPS | 59.9 ± 10.3 | 20.8% | 34.8% | 50.3% | 19.0% | NA | 2.0% | 100% | 98.6% | 98.9% | 82.6% |
| COLCOT | 60.6 ± 10.7 | 19.2% | 29.9% | 51.0% | 20.2% | NA | 2.6% | 100% | 98.8% | 99.0% | 88.9% |
| Deftereos | 63.3 ± 7.0 | 34.7% | 37.8% | 48.5% | 100% | 33.2% | NA | 31.1% | NA | NA | NA |
| LoDoCo | 67 ± 9.4 | 11.1% | 4.5% | NA | 30.3% | NA | NA | 23.5% | 93.4% | 95.1% | 66.5% |
ACS, acute coronary syndrome. eGFR, estimated glomerular filtration rate. NA, not available. TIA, transient ischaemic attack. History of stroke or TIA was available for 3318 patients, and smoking status was missing for 21 patients in LoDoCo2.
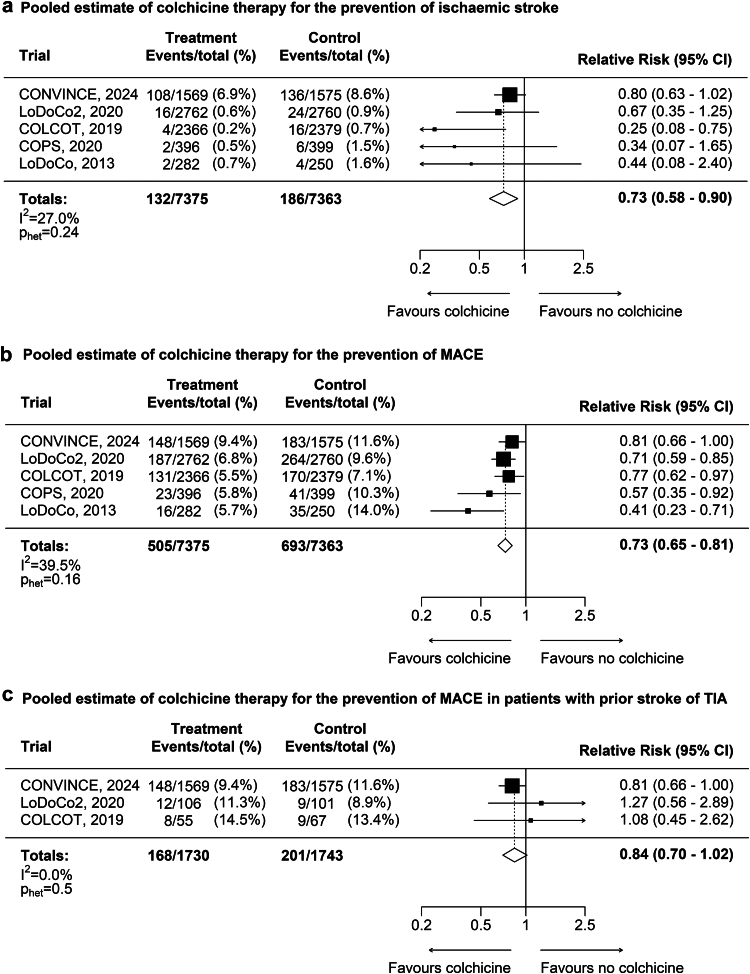
The results for the main outcomes are presented in Fig. 2. Ischaemic stroke occurred in 132 (1.8%) of 7375 patients assigned to colchicine and in 186 (2.5%) of 7363 patients assigned to placebo or no colchicine. Colchicine reduced the risk of ischaemic stroke by 27% (RR 0.73, 95% CI 0.58–0.90; p < 0.004; Fig. 2a), with low heterogeneity among trials. The effect estimates were consistent when comparing CONVINCE to the four trials that included patients with coronary disease (p for heterogeneity = 0.09), and comparing the two acute to the two chronic coronary disease trials (p for heterogeneity = 0.13). The risk of all stroke (ischaemic and haemorrhagic) was reduced by 26% in colchicine-treated patients (RR 0.74, 0.60–0.91; p < 0.004; Supplemental Figure S1). The number of haemorrhagic strokes during follow-up was low and did not differ by treatment group (17 [0.2%] in the colchicine group versus 19 [0.3%] in the no colchicine group).
Fig. 2.
a: Pooled estimate of colchicine treatment for prevention of ischaemic stroke. b: Pooled estimate of colchicine therapy for prevention of ischaemic stroke, myocardial infarction, coronary revascularisation, or cardiovascular death (MACE). c: Pooled estimate of colchicine therapy for prevention of ischaemic stroke, myocardial infarction, coronary revascularisation, or cardiovascular death (MACE) in patients with prior stroke or TIA.
MACE occurred in 505 (6.8%) of 7375 patients assigned to colchicine and 693 (9.4%) of 7363 assigned to no colchicine. Colchicine reduced the risk of MACE by 27% (RR 0.73, 0.65–0.81; p < 0.001; Fig. 2b), with low to moderate heterogeneity among trials. The effect estimates were consistent when comparing CONVINCE to the four trials that included patients with coronary disease (p for heterogeneity = 0.22) and comparing the two acute to the two chronic coronary disease trials (p for heterogeneity = 0.51). Risk reduction was 22% when omitting coronary revascularisation from the composite outcome between the colchicine and no colchicine arms (395 [5.4%] versus 505 [6.9%], RR 0.78, 0.69–0.89; p < 0.001; Supplemental Figure S2). Effects were consistent for the individual components of the composite, with a risk reduction of 20% for myocardial infarction (224 [3.0%] versus 278 [3.8%], RR 0.80, 0.68–0.96, p = 0.01; Supplemental Figure S3), and a risk reduction of 26% for coronary revascularisation (319 [4.3%] versus 415 [5.6%], RR 0.77, 0.67–0.89; p < 0.001; Supplemental Figure S4). Among all trials, 3473 patients had prior stroke or TIA, and 2864 patients had prior stroke at randomisation. Although not meeting the threshold for statistical significance, the direction of effect of colchicine for reduction of MACE was consistent in patients with prior stroke or TIA (RR 0.84, 0.70–1.02; p = 0.09, Fig. 2c) and in prior stroke alone (RR 0.85, 0.69–1.05; p = 0.13, Supplemental Figure S5).
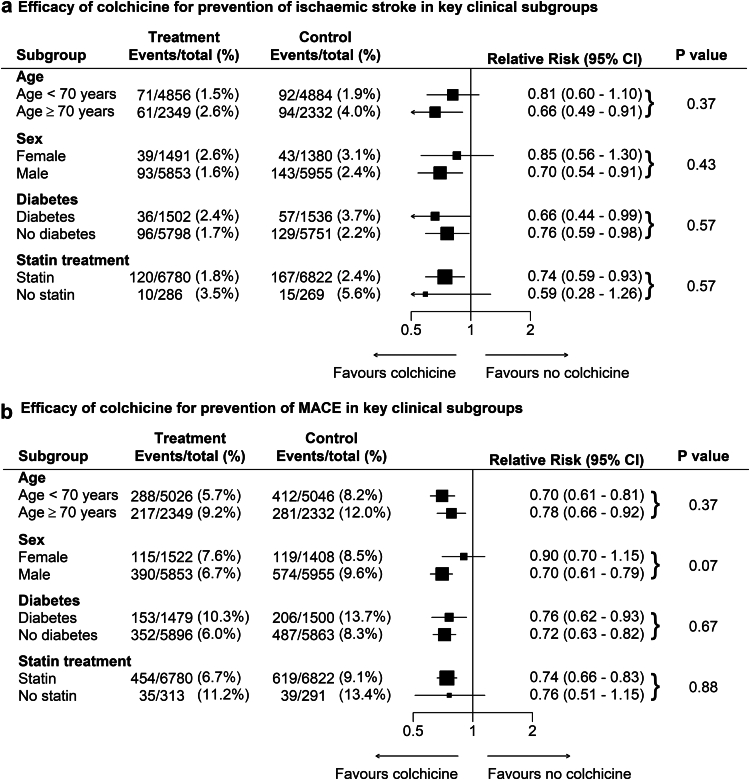
The benefit of low-dose colchicine in reducing the risk of ischaemic stroke was consistent across all subgroups examined, with neither a significant interaction in treatment between those aged below 70 or over 70 years of age (p for heterogeneity = 0.37), nor by sex (p for heterogeneity = 0.43), those with or without diabetes (p for heterogeneity = 0.57), and those using statin therapy at baseline or not (p for heterogeneity = 0.57) (Fig. 3a). The direction of the pooled estimates for the risk of MACE was consistent in all subgroups, without evidence of treatment interaction by subgroup categories (Fig. 3b).
Fig. 3.
a: Efficacy of colchicine for prevention of ischaemic stroke in key clinical subgroups. b: Efficacy of colchicine for prevention of MACE in key clinical subgroups.
Colchicine-treated patients had no excess of hospitalisation for pneumonia (109 [1.5%] versus 106 [1.5%], RR 0.99, 0.76–1.30; p = 0.94), newly diagnosed cancer (247 [3.5%] versus 255 [3.6%], RR 0.97, CI 0.82–1.15; p = 0.72), or gastro-intestinal events (153 [2.2%] versus 135 [1.9%], RR 1.15, CI 0.91–1.44; p = 0.24) (Supplemental Figure S6).
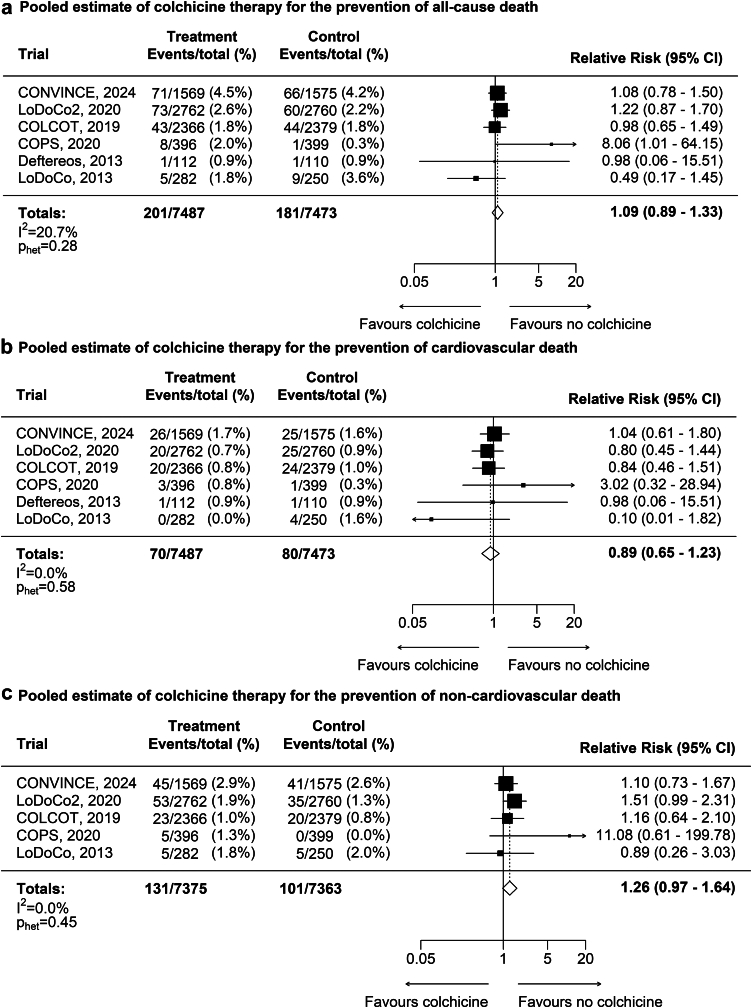
There was no significant difference in all-cause death (201 [2.7%] versus 181 [2.4%], RR 1.09, CI 0.89–1.33; p = 0.39; Fig. 4a); cardiovascular death (70 [0.9%] versus 80 [1.1%], RR 0.89, 0.65–1.23; p = 0.50; Fig. 4b), or non-cardiovascular death (131 [1.8%] versus 101 [1.4%], RR 1.26 CI 0.97–1.64; p = 0.08; Fig. 4c).
Fig. 4.
Mortality (all-cause death, cardiovascular death, and non-cardiovascular death). a: Pooled estimate of colchicine treatment for prevention of all-cause death. b: Pooled estimate of colchicine treatment for prevention of cardiovascular death. c: Pooled estimate of colchicine treatment for prevention of non-cardiovascular death. CONVINCE included 51 cardiovascular deaths, among which are 18 late deaths (10 in the colchicine arm and 8 in the control arm) beyond 30 days of the qualifying event. Deftereos et al., 2013 reported no non-cardiovascular deaths in both treatment and control arm and was therefore omitted in this meta-analysis.
Sensitivity analyses using random-effects models showed similar findings for all outcomes (Supplemental Table S3).
Discussion
Randomised trials of long-term colchicine in patients with coronary disease reported few patients with ischaemic stroke outcomes and a numerical but not statistically significant reduction of recurrent stroke was observed in patients with stroke in the CONVINCE trial, leading to uncertainty about the efficacy of colchicine for secondary prevention of stroke. This study-level meta-analysis of six randomised trials involving 14,934 patients with prior stroke or coronary disease showed consistent benefit for prevention of stroke and MACE, with consistent effects in patients with prior stroke and in key subgroups. The observed risk reductions of 27% for both ischaemic stroke and MACE provide compelling evidence for a benefit of colchicine in high-risk patients with atherosclerosis. The substantial increase in number of stroke outcome events compared with earlier meta-analyses now provides the most robust estimate of treatment effect for stroke prevention by colchicine to date.23, 24, 25 The evidence for benefit in stroke prevention is strengthened by the plausibility of the effect size compared with the earlier reported large effect sizes of colchicine in smaller studies, the consistency of effect when haemorrhagic stroke is included in the stroke outcome definition, the alignment of treatment effect in the pre-specified subgroups, and the reproducibility of our findings in sensitivity analyses using a random-effects model. The magnitude of benefit of these pooled estimates are larger than those seen in contemporary secondary prevention trials of adjunctive lipid-lowering agents such as Ezetimibe or Proprotein Convertase Subtilisin/Kexin Type 9 (PCSK9) inhibition.26 Colchicine can be produced at low-cost and addition of colchicine to contemporary secondary prevention therapies is highly cost-effective at commonly accepted thresholds.27, 28, 29 This favourable combination of a clinically relevant treatment effect and low expense is of importance when considering strategies to address rising cardiovascular disease burden in middle and low-income countries. In the large coronary trials, the curves diverged by 6 months and remain consistently separated after this time. The lack of heterogeneity across trials of differing durations suggests a consistent effect over time, which was also seen in earlier landmark analyses.30 Future individual patient and long-term follow up data will help to confirm consistency of treatment effect over time.
Our study was not designed to investigate the mechanism of stroke prevention by colchicine, but the combined findings correspond to evidence from earlier experimental and clinical mechanistic studies. The causative role of inflammation in large artery and small vessel stroke has been by confirmed by mendelian randomization studies, in which genetically determined lower levels of interleukin-6 are associated with lower risk for stroke, and higher activity of monocyte chemoattractant protein-1 with higher risk for stroke.2,31 In carotid atherosclerosis, inflammation and unstable plaque morphology are associated with higher risk for ischaemic stroke.32,33 Correspondingly, increased carotid plaque inflammation detected with 18F-fluorodeoxyglucose uptake independently predicts early recurrent stroke.34 In addition to these findings in large vessel disease, evidence from experimental studies also have highlighted the causal role of inflammation in the pathogenesis of cardio-embolic stroke, small vessel disease, and cryptogenic stroke.32 Colchicine may have benefit for several stroke subtypes via inhibition of inflammation and platelet activation, such as reduced neutrophil adhesion at sites of inflamed or injured endothelium, decreased expression of platelet activation surface markers, and reduced leukocyte-platelet aggregation.35,36 This leads to changes in plaque morphology and plaque vulnerability.32,37, 38, 39 These mechanisms and the findings of our meta-analysis suggest therapeutic benefit is mainly gained with prolonged treatment, in particular when considering the neutral outcomes of short term treatment in the Colchicine in High-risk Patients with Acute Minor-to-moderate Ischemic Stroke or Transient Ischemic Attack (CHANCE3) trial.40
In our study, the increased number of outcome events and collaborative involvement of individual trialists allowed in-depth analysis of the effect of colchicine in key clinical subgroups. We found consistent treatment effects in patients aged over 70 years as compared to younger patients, which is reassuring since implementation in real-world populations will likely be in older patients than those participating in trials.41 Individual trials of colchicine recruited fewer women than men and were underpowered to detect a treatment differences by sex.42 Our analyses now include the largest-available sample of female participants, with data of 2628 women. The direction of treatment effect was consistent in males and females, albeit with some variation in effect size which was not statistically significant. Although diabetes is associated with a pro-inflammatory state, we observed equal benefit in patients with and without diabetes, consistent with findings from the Canakinumab Anti-inflammatory Thrombosis Outcomes Study (CANTOS) trial.43 Finally, we observed a similar benefit in both statin and non-statin treated patients, supporting an independent and additive effect of anti-inflammatory therapy on top of lipid-lowering therapy and for treatment of statin-intolerant patients. These data support the finding that in patients who receive contemporary statin therapy, inflammation assessed by high-sensitivity C-Reactive protein is a stronger predictor for risk of future cardiovascular events and death than cholesterol assessed by low-density lipoprotein (LDL) cholesterol.44
Randomised trials are not designed to detect uncommon serious safety events, which may be important when new treatments are adopted for widespread clinical use. Consistent with large observational studies of colchicine for various indications, we found no increased risk of pre-specified serious safety outcomes, although rates of nausea and diarrhoea are known to be increased.45, 46, 47 Five of the six trials included in our meta-analysis demonstrated a favourable but non-significant direction of effect of colchicine on cardiovascular death, and four of the five trials which reported non-cardiovascular death reported a higher number of such deaths in the colchicine arm. The numerically higher but statistically non-significant, increase in non-cardiovascular deaths is based on a low absolute number of events, and the surplus almost completely arises from one trial (LoDoCo2). Ancillary analyses on drivers of mortality in this trial and prolonged follow-up of the COPS trial revealed a wide spectrum of causes of death without a clear overarching or consistent signal.48,49 The current meta-analysis showed no increase in specific major causes of death (hospitalisations for new cancer, pneumonia, or gastro-intestinal events). In addition, experience from life-long treatment with colchicine in Familial Mediterranean Fever, in which it is used in children, pregnant and nursing women, has not shown concerns relating to excess non-cardiovascular death.47,50 Since the apparent increase is not consistently seen in studies and lacks a biological explanation at this time this issue requires further study with long term follow-up in future randomised trials and real-world data. The upcoming CLEAR SYNERGY (OASIS 9) trial will provide valuable data for both efficacy and safety data in this regard.51 When introducing colchicine to current regimes of secondary prevention, patients should be counselled about known drug interactions and adverse effects, and renal function should be regularly monitored. Priorities for future trials include further investigation of the effect of colchicine for prevention of vascular events and cognitive decline in patients with stroke, investigating safety in patients with renal impairment, further randomised data of non-cardiovascular death in colchicine-treated patients and controls, and establishing the effect of colchicine therapy on long-term cardiovascular outcomes.
We acknowledge some limitations. Some relevant subgroups, such as ethnicity or race, were not collected at baseline, or were not assessed in this study, such as hypertension or smoking status. Differences in stroke outcome definition between trials may have introduced some variability, although this is unlikely to have materially impacted our overall findings. The possibility of performance bias in the two non-placebo-controlled trials could not be fully excluded, but we believe is unlikely since these trials involved blinded adjudication of outcomes supported by objective biomarker and imaging data. In this meta-analysis, we did not analyse individual patient data which would have allowed more detailed exploration of outcomes, subgroups and interactions based on stroke aetiology.
In conclusion, in patients with prior stroke or coronary disease, low-dose colchicine reduced the risk for ischaemic stroke and MACE, with consistent treatment effects in key clinical subgroups, and without significant increases in other serious safety events or all-cause mortality. Our findings support the use of low-dose colchicine for secondary prevention of stroke and coronary events in clinical practice.
Contributors
ATLF, MHFP, SMN, JE, AM, and PJK wrote the protocol and the statistical analysis plan. ATLF and MHFP performed statistical analyses with oversight from PJK. PIs of individual trials (ATLF (LoDoCo2), JL (COPS), SMN (LoDoCo, LoDoCo2), J-CT (COLCOT), PT (LoDoCo), AM (LoDoCo2), PJK (CONVINCE)) accessed and verified the data. ATLF, MHFP, JE, AM, and PJK wrote the first draft of the manuscript. All authors critically reviewed drafts of this manuscript and approved the final version.
Data sharing statement
Study-level data not published within the article can be shared with researchers who submit a research proposal and request access to the data, for use pending approval of the authors and of the individual trial funders, and after a data access agreement has been signed.
Declaration of interests
Funding: Michiel Poorthuis reports support for travel to Dublin from Dr. Jan Meerwaldt stichting. Pierre Amarenco reports grants from the French Government for Reducing Inflammation in Ischemic Stroke with Colchicine (RIISC); Ticagrelor in High-risk patients-Extended Treatment in Ischemic Stroke (THETIS); Treat Stroke to Target 40; Treat Stroke to Target Cholesterol and Colchicine in Cerebral Small Vessel Disease (TST 3C SVD), from Pfizer for Treat Stroke to Target Trial, consulties fees from Neuraltide for TIDE-IN Trial, honoraria from Novartis, Sanofi, and Viatris, support from Novartis, Participation on a Data Safety Monitoring Board or Advisory Board for Cell Prothera, and from AstraZeneca for Ticagrelor supply (Ticagrelor in High-risk patients-Extended Treatment in Ischemic Stroke (THETIS)). Kevin Boczar reports grants from Novartis paid to his institution. Noel Chan is supported by a Heart and Stroke of Canada New Investigator Award, and received a project grant from the Canadian Institutes of Health Research for a randomized trial investigating the risk-benefit of colchicine in peripheral artery disease. Sanjit Jolly reports honoraria from Pharmascience. Binita Shah reports grants from NIH NHLBI and VA Office of Research and Development paid to her institution, support for attending meetings and/or travel from NovoNordisk as US national leader and co-chair of the global expert panel for the ARTEMIS trial, leadership or fiduciary role for the Society of Cardiovascular Angiography and Interventions (Board of Trustees) and the American Heart Association (Associate Editor for Circulation Cardiovascular Intervention), and from Philips Volcano (advisory board). Jean-Claude Tardif reports grants from Amarin, AstraZeneca, Ceapro, DalCor Pharmaceuticals, Esperion, Ionis, Merck, Novartis, Pfizer paid tot his institution, consulting fees from DalCor Pharmaceuticals paid to him, honoraria for lectures from HLS Pharmaceuticals, Pendopharm, Pfizer paid to him, patent for Pharmacogenomics-guided CETP inhibition (waived his right in this patent), patent for Use of colchicine after myocardial infarction (his institution submitted a patent on this topic and he was an author, but waived his rights in this patent and did not stand to gain financially), and a minor equity interest in DalCor Pharmaceuticals. John Eikelboom reports grants from Anthos, Bayer, BI, BMS, Daiichi-Sankyo, Ionis, Janssen, Merck, Pfizer, USV; consulting fees from Anthos, Bayer, BI, BMS, Daiichi-Sankyo, Ionis, Janssen, Merck, Pfizer, USV.
Acknowledgements
We are grateful to Paul Sherliker (Nuffield Department of Population Health, University of Oxford) for help creating the figures.
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.eclinm.2024.102835.
Appendix A. Supplementary data
References
- 1.Roth G.A., Huffman M.D., Moran A.E., et al. Global and regional patterns in cardiovascular mortality from 1990 to 2013. Circulation. 2015;132:1667–1678. doi: 10.1161/CIRCULATIONAHA.114.008720. [DOI] [PubMed] [Google Scholar]
- 2.Georgakis M.K., Malik R., Gill D., et al. Interleukin-6 signaling effects on ischemic stroke and other cardiovascular outcomes. Circ Genom Precis Med. 2020;13 doi: 10.1161/CIRCGEN.119.002872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kuo H.-K., Yen C.-J., Chang C.-H., Kuo C.-K., Chen J.-H., Sorond F. Relation of C-reactive protein to stroke, cognitive disorders, and depression in the general population: systematic review and meta-analysis. Lancet Neurol. 2005;4:371–380. doi: 10.1016/S1474-4422(05)70099-5. [DOI] [PubMed] [Google Scholar]
- 4.Interleukin-6 Receptor Mendelian Randomisation Analysis (IL6R MR) Consortium The interleukin-6 receptor as a target for prevention of coronary heart disease: a mendelian randomisation analysis. Lancet. 2012;379:1214–1224. doi: 10.1016/S0140-6736(12)60110-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kamtchum-Tatuene J., Saba L., Heldner M.R., et al. Interleukin-6 predicts carotid plaque severity, vulnerability, and progression. Circ Res. 2022;131:e22–e33. doi: 10.1161/CIRCRESAHA.122.320877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Leung Y.Y., Hui L.L.Y., Kraus V.B. Colchicine—update on mechanisms of action and therapeutic uses. Semin Arthritis Rheum. 2015;45:341–350. doi: 10.1016/j.semarthrit.2015.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tardif J.-C.C., Kouz S., Waters D.D., et al. Efficacy and safety of low-dose colchicine after myocardial infarction. N Engl J Med. 2019;381:2497–2505. doi: 10.1056/NEJMoa1912388. [DOI] [PubMed] [Google Scholar]
- 8.Nidorf S.M., Eikelboom J.W., Budgeon C.A., Thompson P.L. Low-dose colchicine for secondary prevention of cardiovascular disease. J Am Coll Cardiol. 2013;61:404–410. doi: 10.1016/j.jacc.2012.10.027. [DOI] [PubMed] [Google Scholar]
- 9.Nidorf S.M., Fiolet A.T.L., Mosterd A., et al. Colchicine in patients with chronic coronary disease. N Engl J Med. 2020;383:1838–1847. doi: 10.1056/NEJMoa2021372. [DOI] [PubMed] [Google Scholar]
- 10.Byrne R.A., Rossello X., Coughlan J.J., et al. 2023 ESC Guidelines for the management of acute coronary syndromes. Eur Heart J. 2023;44:3720–3826. doi: 10.1093/eurheartj/ehad191. [DOI] [PubMed] [Google Scholar]
- 11.Visseren F.L.J., Mach F., Smulders Y.M., et al. 2021 ESC Guidelines on cardiovascular disease prevention in clinical practice: developed by the task force for cardiovascular disease prevention in clinical practice with representatives of the European Society of Cardiology and 12 medical societies. Eur Heart J. 2021;42:3227–3337. [Google Scholar]
- 12.Virani S.S., Newby L.K., Arnold S.V., et al. 2023 AHA/ACC/ACCP/ASPC/NLA/PCNA guideline for the management of patients with chronic coronary disease: a report of the American Heart Association/American College of Cardiology Joint Committee on Clinical Practice Guidelines. J Am Coll Cardiol. 2023;82:833–955. doi: 10.1016/j.jacc.2023.04.003. [DOI] [PubMed] [Google Scholar]
- 13.Ponte-Negretti C.I., Wyss F., Piskorz D., et al. Latin American consensus on management of residual cardiometabolic risk. A consensus paper prepared by the Latin American academy for the study of lipids and cardiometabolic risk (ALALIP) endorsed by the inter-American society of cardiology (IASC), the international atherosclerosis society (IAS), and the Pan-American College of Endothelium (PACE) Arch Cardiol Mex. 2021;92 doi: 10.24875/ACM.21000005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kelly P., Lemmens R., Weimar C., et al. Long-term colchicine for the prevention of vascular recurrent events in non-cardioembolic stroke (CONVINCE): a randomised controlled trial. Lancet. 2024;404:125–133. doi: 10.1016/S0140-6736(24)00968-1. [DOI] [PubMed] [Google Scholar]
- 15.Page M.J., McKenzie J.E., Bossuyt P.M., et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372 doi: 10.1136/bmj.n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lefebvre C., Glanville J., Briscoe S., et al. Cochrane Handbook for Systematic Reviews of Interventions; 2019. Technical supplement to chapter 4: searching for and selecting studies; pp. 1–94. [Google Scholar]
- 17.Glanville J., Foxlee R., Wisniewski S., Noel-Storr A., Edwards M., Dooley G. Translating the Cochrane EMBASE RCT filter from the Ovid interface to Embase.com: a case study. Health Info Libr J. 2019;36:264–277. doi: 10.1111/hir.12269. [DOI] [PubMed] [Google Scholar]
- 18.Higgins J.P.T. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Higgins J.P.T., Altman D.G., Gøtzsche P.C., et al. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ. 2011;343 doi: 10.1136/bmj.d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sterne J.A.C., Savović J., Page M.J., et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. 2019;366 doi: 10.1136/bmj.l4898. [DOI] [PubMed] [Google Scholar]
- 21.Deftereos S., Giannopoulos G., Raisakis K., et al. Colchicine treatment for the prevention of bare-metal stent restenosis in diabetic patients. J Am Coll Cardiol. 2013;61:1679–1685. doi: 10.1016/j.jacc.2013.01.055. [DOI] [PubMed] [Google Scholar]
- 22.Tong D.C., Quinn S., Nasis A., et al. Colchicine in patients with acute coronary syndrome: the Australian COPS randomized clinical trial. Circulation. 2020;142:1890–1900. doi: 10.1161/CIRCULATIONAHA.120.050771. [DOI] [PubMed] [Google Scholar]
- 23.Samuel M., Tardif J.-C., Bouabdallaoui N., et al. Colchicine for secondary prevention of cardiovascular disease: a systematic review and meta-analysis of randomized controlled trials. Can J Cardiol. 2021;37:776–785. doi: 10.1016/j.cjca.2020.10.006. [DOI] [PubMed] [Google Scholar]
- 24.Fiolet A.T.L., Opstal T.S.J., Mosterd A., et al. Efficacy and safety of low-dose colchicine in patients with coronary disease: a systematic review and meta-analysis of randomized trials. Eur Heart J. 2021;42:2765–2775. doi: 10.1093/eurheartj/ehab115. [DOI] [PubMed] [Google Scholar]
- 25.Kofler T., Kurmann R., Lehnick D., et al. Colchicine in patients with coronary artery disease: a systematic review and meta-analysis of randomized trials. J Am Heart Assoc. 2021;10 doi: 10.1161/JAHA.121.021198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nelson K., Fuster V., Ridker P.M. Low-dose colchicine for secondary prevention of coronary artery disease. J Am Coll Cardiol. 2023;82:648–660. doi: 10.1016/j.jacc.2023.05.055. [DOI] [PubMed] [Google Scholar]
- 27.Samuel M., Tardif J.-C., Khairy P., et al. Cost-Effectiveness of low-dose colchicine after myocardial infarction in the colchicine cardiovascular outcomes trial (COLCOT) Eur Heart J Qual Care Clin Outcomes. 2021;7:486–495. doi: 10.1093/ehjqcco/qcaa045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Boczar K.E., Beanlands R., Wells G., Coyle D. Cost-Effectiveness of colchicine for recurrent cardiovascular events. CJC Open. 2023;5:348–356. doi: 10.1016/j.cjco.2023.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fiolet A.T.L., Keusters W., Blokzijl J., et al. Cost-effectiveness of low-dose colchicine in patients with chronic coronary disease in the Netherlands. Eur Heart J Qual Care Clin Outcomes. 2024 doi: 10.1093/ehjqcco/qcae021. [DOI] [PubMed] [Google Scholar]
- 30.Opstal T.S.J., van Broekhoven A., Fiolet A.T.L., et al. Long-term efficacy of colchicine in patients with chronic coronary artery disease. Insights from LoDoCo2. Circulation. 2022;145:626–628. doi: 10.1161/CIRCULATIONAHA.121.058233. [DOI] [PubMed] [Google Scholar]
- 31.Georgakis M.K., Gill D., Rannikmäe K., et al. Genetically determined levels of circulating cytokines and risk of stroke. Circulation. 2019;139:256–268. doi: 10.1161/CIRCULATIONAHA.118.035905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kelly P.J., Lemmens R., Tsivgoulis G. Inflammation and stroke risk: a new target for prevention. Stroke. 2021;52:2697–2706. doi: 10.1161/STROKEAHA.121.034388. [DOI] [PubMed] [Google Scholar]
- 33.Saba L., Saam T., Jäger H.R., et al. Imaging biomarkers of vulnerable carotid plaques for stroke risk prediction and their potential clinical implications. Lancet Neurol. 2019;18:559–572. doi: 10.1016/S1474-4422(19)30035-3. [DOI] [PubMed] [Google Scholar]
- 34.Kelly P.J., Camps-Renom P., Giannotti N., et al. Carotid plaque inflammation imaged by 18-F-fluorodeoxyglucose positron emission tomography and risk of early recurrent stroke. Stroke. 2019;50:1766–1773. doi: 10.1161/STROKEAHA.119.025422. [DOI] [PubMed] [Google Scholar]
- 35.Shah B., Allen N., Harchandani B., et al. Effect of colchicine on platelet-platelet and platelet-leukocyte interactions: a pilot study in healthy subjects. Inflammation. 2016;39:182–189. doi: 10.1007/s10753-015-0237-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cronstein B.N., Molad Y., Reibman J., Balakhane E., Levin R.I., Weissmann G. Colchicine alters the quantitative and qualitative display of selectins on endothelial cells and neutrophils. J Clin Invest. 1995;96:994–1002. doi: 10.1172/JCI118147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Vaidya K., Arnott C., Martínez G.J., et al. Colchicine therapy and plaque stabilization in patients with acute coronary syndrome: a CT coronary angiography study. JACC Cardiovasc Imaging. 2018;11:305–316. doi: 10.1016/j.jcmg.2017.08.013. [DOI] [PubMed] [Google Scholar]
- 38.Cecconi A., Vilchez-Tschischke J.P., Mateo J., et al. Effects of colchicine on atherosclerotic plaque stabilization: a multimodality imaging study in an animal model. J Cardiovasc Transl Res. 2021;14:150–160. doi: 10.1007/s12265-020-09974-7. [DOI] [PubMed] [Google Scholar]
- 39.Buckley L.F., Libby P. Colchicine's role in cardiovascular disease management. Arterioscler Thromb Vasc Biol. 2024;44:1031–1041. doi: 10.1161/ATVBAHA.124.319851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Li J., Meng X., Shi F.-D., et al. Colchicine in patients with acute ischaemic stroke or transient ischaemic attack (CHANCE-3): multicentre, double blind, randomised, placebo controlled trial. BMJ. 2024;385 doi: 10.1136/bmj-2023-079061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dhruva S.S. Variations between clinical trial participants and medicare beneficiaries in evidence used for medicare national coverage decisions. Arch Intern Med. 2008;168:136–140. doi: 10.1001/archinternmed.2007.56. [DOI] [PubMed] [Google Scholar]
- 42.Melloni C., Berger J.S., Wang T.Y., et al. Representation of women in randomized clinical trials of cardiovascular disease prevention. Circ Cardiovasc Qual Outcomes. 2010;3:135–142. doi: 10.1161/CIRCOUTCOMES.110.868307. [DOI] [PubMed] [Google Scholar]
- 43.Everett B.M., Donath M.Y., Pradhan A.D., et al. Anti-inflammatory therapy with Canakinumab for the prevention and management of diabetes. J Am Coll Cardiol. 2018;71:2392–2401. doi: 10.1016/j.jacc.2018.03.002. [DOI] [PubMed] [Google Scholar]
- 44.Ridker P.M., Bhatt D.L., Pradhan A.D., et al. Inflammation and cholesterol as predictors of cardiovascular events among patients receiving statin therapy: a collaborative analysis of three randomised trials. Lancet. 2023;401:1293–1301. doi: 10.1016/S0140-6736(23)00215-5. [DOI] [PubMed] [Google Scholar]
- 45.Robinson P.C., Terkeltaub R., Pillinger M.H., et al. Consensus statement regarding the efficacy and safety of long-term low-dose colchicine in gout and cardiovascular disease. Am J Med. 2022;135:32–38. doi: 10.1016/j.amjmed.2021.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Stewart S., Yang K.C.K., Atkins K., Dalbeth N., Robinson P.C. Adverse events during oral colchicine use: a systematic review and meta-analysis of randomised controlled trials. Arthritis Res Ther. 2020;22:1–15. doi: 10.1186/s13075-020-2120-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nidorf S.M., Ben-Chetrit E., Ridker P.M. Low-dose colchicine for atherosclerosis: long-term safety. Eur Heart J. 2024;45:1596–1601. doi: 10.1093/eurheartj/ehae208. [DOI] [PubMed] [Google Scholar]
- 48.Opstal T.S.J., Nidorf S.M., Fiolet A.T.L., et al. Drivers of mortality in patients with chronic coronary disease in the low-dose colchicine 2 trial. Int J Cardiol. 2023;372:1–5. doi: 10.1016/j.ijcard.2022.12.026. [DOI] [PubMed] [Google Scholar]
- 49.Tong D.C., Bloom J.E., Quinn S., et al. Colchicine in patients with acute coronary syndrome: two-year follow-up of the Australian COPS randomized clinical trial. Circulation. 2021;144:1584–1586. doi: 10.1161/CIRCULATIONAHA.121.054610. [DOI] [PubMed] [Google Scholar]
- 50.Ben-Chetrit E., Levy M. Colchicine prophylaxis in familial Mediterranean fever: reappraisal after 15 years. Semin Arthritis Rheum. 1991;20:241–246. doi: 10.1016/0049-0172(91)90019-v. [DOI] [PubMed] [Google Scholar]
- 51.d'Entremont M.-A., Lee S.F., Mian R., et al. Design and rationale of the CLEAR SYNERGY (OASIS 9) trial: a 2x2 factorial randomized controlled trial of colchicine versus placebo and spironolactone vs placebo in patients with myocardial infarction. Am Heart J. 2024;275:173–182. doi: 10.1016/j.ahj.2024.06.007. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.