Abstract
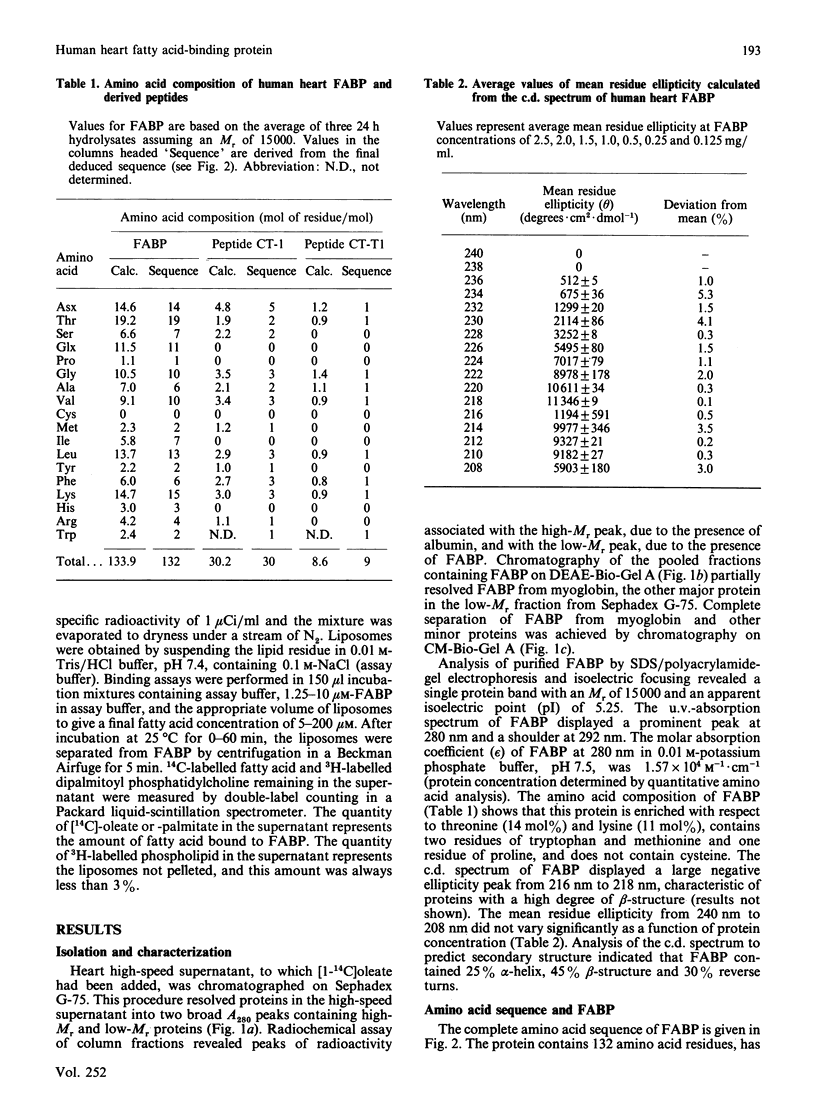
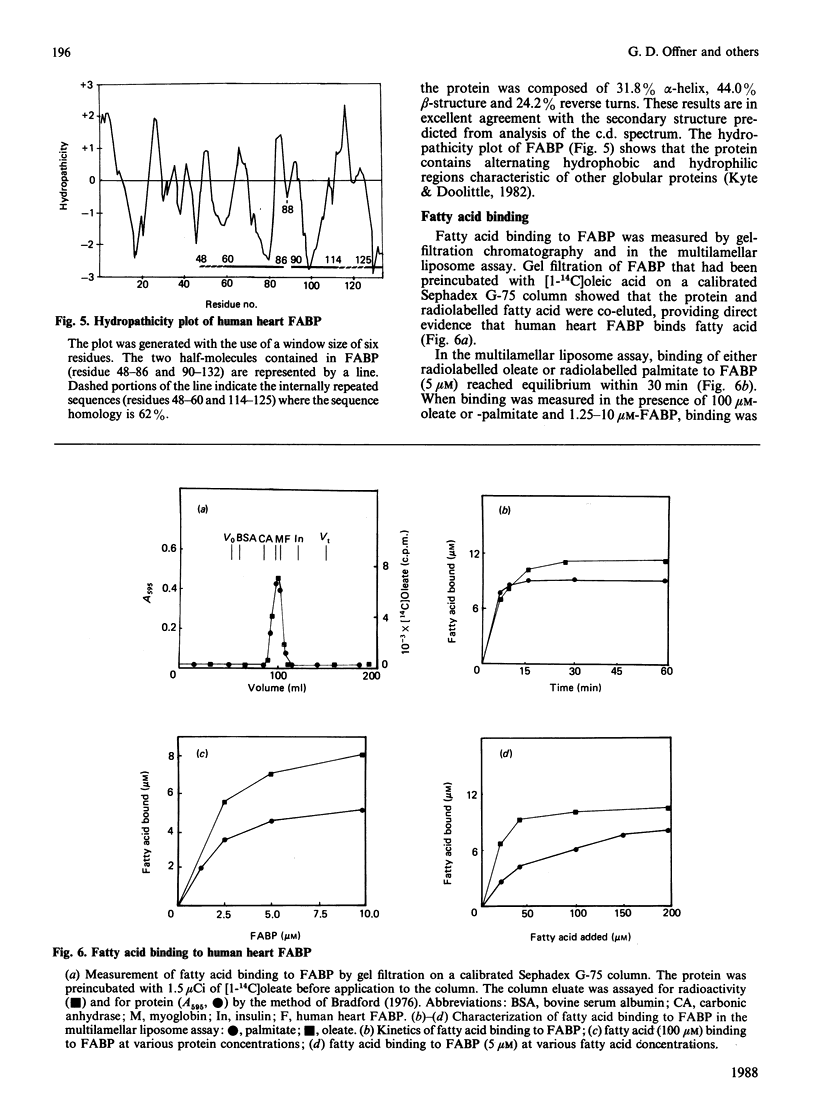
The complete amino acid sequence of a fatty acid-binding protein from human heart was determined by automated Edman degradation of CNBr, BNPS-skatole [3'-bromo-3-methyl-2-(2-nitrobenzenesulphenyl)indolenine], hydroxylamine, Staphylococcus aureus V8 proteinase, tryptic and chymotryptic peptides, and by digestion of the protein with carboxypeptidase A. The sequence of the blocked N-terminal tryptic peptide from citraconylated protein was determined by collisionally induced decomposition mass spectrometry. The protein contains 132 amino acid residues, is enriched with respect to threonine and lysine, lacks cysteine, has an acetylated valine residue at the N-terminus, and has an Mr of 14768 and an isoelectric point of 5.25. This protein contains two short internal repeated sequences from residues 48-54 and from residues 114-119 located within regions of predicted beta-structure and decreasing hydrophobicity. These short repeats are contained within two longer repeated regions from residues 48-60 and residues 114-125, which display 62% sequence similarity. These regions could accommodate the charged and uncharged moieties of long-chain fatty acids and may represent fatty acid-binding domains consistent with the finding that human heart fatty acid-binding protein binds 2 mol of oleate or palmitate/mol of protein. Detailed evidence for the amino acid sequences of the peptides has been deposited as Supplementary Publication SUP 50143 (23 pages) at the British Library Lending Division, Boston Spa, Yorkshire LS23 7BQ, U.K., from whom copies may be obtained as indicated in Biochem. J. (1988) 249, 5.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bass N. M., Manning J. A. Tissue expression of three structurally different fatty acid binding proteins from rat heart muscle, liver, and intestine. Biochem Biophys Res Commun. 1986 Jun 30;137(3):929–935. doi: 10.1016/0006-291x(86)90314-1. [DOI] [PubMed] [Google Scholar]
- Bornstein P., Balian G. Cleavage at Asn-Gly bonds with hydroxylamine. Methods Enzymol. 1977;47:132–145. doi: 10.1016/0076-6879(77)47016-2. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Brecher P., Saouaf R., Sugarman J. M., Eisenberg D., LaRosa K. Fatty acid transfer between multilamellar liposomes and fatty acid-binding proteins. J Biol Chem. 1984 Nov 10;259(21):13395–13401. [PubMed] [Google Scholar]
- Chen Y. H., Yang J. T., Chau K. H. Determination of the helix and beta form of proteins in aqueous solution by circular dichroism. Biochemistry. 1974 Jul 30;13(16):3350–3359. doi: 10.1021/bi00713a027. [DOI] [PubMed] [Google Scholar]
- Chou P. Y., Fasman G. D. Prediction of beta-turns. Biophys J. 1979 Jun;26(3):367–383. doi: 10.1016/S0006-3495(79)85259-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou P. Y., Fasman G. D. Prediction of the secondary structure of proteins from their amino acid sequence. Adv Enzymol Relat Areas Mol Biol. 1978;47:45–148. doi: 10.1002/9780470122921.ch2. [DOI] [PubMed] [Google Scholar]
- Claffey K. P., Herrera V. L., Brecher P., Ruiz-Opazo N. Cloning and tissue distribution of rat heart fatty acid binding protein mRNA: identical forms in heart and skeletal muscle. Biochemistry. 1987 Dec 1;26(24):7900–7904. doi: 10.1021/bi00398a054. [DOI] [PubMed] [Google Scholar]
- Corr P. B., Gross R. W., Sobel B. E. Amphipathic metabolites and membrane dysfunction in ischemic myocardium. Circ Res. 1984 Aug;55(2):135–154. doi: 10.1161/01.res.55.2.135. [DOI] [PubMed] [Google Scholar]
- Crisman T. S., Claffey K. P., Saouaf R., Hanspal J., Brecher P. Measurement of rat heart fatty acid binding protein by ELISA. Tissue distribution, developmental changes and subcellular distribution. J Mol Cell Cardiol. 1987 May;19(5):423–431. doi: 10.1016/s0022-2828(87)80394-2. [DOI] [PubMed] [Google Scholar]
- Fournier N. C., Rahim M. H. Self-aggregation, a new property of cardiac fatty acid-binding protein. Predictable influence on energy production in the heart. J Biol Chem. 1983 Mar 10;258(5):2929–2933. [PubMed] [Google Scholar]
- Fournier N. C., Rahim M. Control of energy production in the heart: a new function for fatty acid binding protein. Biochemistry. 1985 Apr 23;24(9):2387–2396. doi: 10.1021/bi00330a039. [DOI] [PubMed] [Google Scholar]
- Fournier N. C., Zuker M., Williams R. E., Smith I. C. Self-association of the cardiac fatty acid binding protein. Influence on membrane-bound, fatty acid dependent enzymes. Biochemistry. 1983 Apr 12;22(8):1863–1872. doi: 10.1021/bi00277a019. [DOI] [PubMed] [Google Scholar]
- Fournier N., Geoffroy M., Deshusses J. Purification and characterization of a long chain, fatty-acid-binding protein supplying the mitochondrial beta-oxidative system in the heart. Biochim Biophys Acta. 1978 Apr 26;533(2):457–464. doi: 10.1016/0005-2795(78)90391-4. [DOI] [PubMed] [Google Scholar]
- Glatz J. F., Paulussen R. J., Veerkamp J. H. Fatty acid binding proteins from heart. Chem Phys Lipids. 1985 Aug 30;38(1-2):115–129. doi: 10.1016/0009-3084(85)90061-1. [DOI] [PubMed] [Google Scholar]
- Heuckeroth R. O., Birkenmeier E. H., Levin M. S., Gordon J. I. Analysis of the tissue-specific expression, developmental regulation, and linkage relationships of a rodent gene encoding heart fatty acid binding protein. J Biol Chem. 1987 Jul 15;262(20):9709–9717. [PubMed] [Google Scholar]
- Jagschies G., Reers M., Unterberg C., Spener F. Bovine fatty acid binding proteins. Isolation and characterisation of two cardiac fatty acid binding proteins that are distinct from corresponding hepatic proteins. Eur J Biochem. 1985 Nov 4;152(3):537–545. doi: 10.1111/j.1432-1033.1985.tb09229.x. [DOI] [PubMed] [Google Scholar]
- Johnson R. S., Biemann K. The primary structure of thioredoxin from Chromatium vinosum determined by high-performance tandem mass spectrometry. Biochemistry. 1987 Mar 10;26(5):1209–1214. doi: 10.1021/bi00379a001. [DOI] [PubMed] [Google Scholar]
- Kyte J., Doolittle R. F. A simple method for displaying the hydropathic character of a protein. J Mol Biol. 1982 May 5;157(1):105–132. doi: 10.1016/0022-2836(82)90515-0. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Ockner R. K., Manning J. A., Poppenhausen R. B., Ho W. K. A binding protein for fatty acids in cytosol of intestinal mucosa, liver, myocardium, and other tissues. Science. 1972 Jul 7;177(4043):56–58. doi: 10.1126/science.177.4043.56. [DOI] [PubMed] [Google Scholar]
- Offner G. D., Brown-Mason A. S., Ehrhardt M. M., Troxler R. F. Primary structure of phycocyanin from the unicellular rhodophyte Cyanidium caldarium. I. Complete amino acid sequence of the alpha subunit. J Biol Chem. 1981 Dec 10;256(23):12167–12175. [PubMed] [Google Scholar]
- Offner G. D., Troxler R. F., Brecher P. Characterization of a fatty acid-binding protein from rat heart. J Biol Chem. 1986 Apr 25;261(12):5584–5589. [PubMed] [Google Scholar]
- Offner G. D., Troxler R. F. Primary structure of allophycocyanin from the unicellular rhodophyte, Cyanidium caldarium. The complete amino acid sequences of the alpha and beta subunits. J Biol Chem. 1983 Aug 25;258(16):9931–9940. [PubMed] [Google Scholar]
- Persson B., Flinta C., von Heijne G., Jörnvall H. Structures of N-terminally acetylated proteins. Eur J Biochem. 1985 Nov 4;152(3):523–527. doi: 10.1111/j.1432-1033.1985.tb09227.x. [DOI] [PubMed] [Google Scholar]
- Roepstorff P., Fohlman J. Proposal for a common nomenclature for sequence ions in mass spectra of peptides. Biomed Mass Spectrom. 1984 Nov;11(11):601–601. doi: 10.1002/bms.1200111109. [DOI] [PubMed] [Google Scholar]
- Sacchettini J. C., Said B., Schulz H., Gordon J. I. Rat heart fatty acid-binding protein is highly homologous to the murine adipocyte 422 protein and the P2 protein of peripheral nerve myelin. J Biol Chem. 1986 Jun 25;261(18):8218–8223. [PubMed] [Google Scholar]
- Simpson R. J., Neuberger M. R., Liu T. Y. Complete amino acid analysis of proteins from a single hydrolysate. J Biol Chem. 1976 Apr 10;251(7):1936–1940. [PubMed] [Google Scholar]
- Unterberg C., Heidl G., von Bassewitz D. B., Spener F. Isolation and characterization of the fatty acid binding protein from human heart. J Lipid Res. 1986 Dec;27(12):1287–1293. [PubMed] [Google Scholar]


