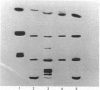
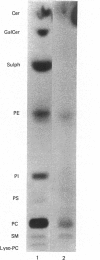
Abstract
Cultured human fibroblasts were fed with two differently labelled sulphatide molecules [one labelled on C-3 of the sphingosine (Sph) moiety [( Sph-3H]sulphatide), the second on C-1 of stearic acid [( stearoyl-14C]sulphatide)], and the intracellular metabolic fate of radioactivity was monitored. Incorporated radioactivity was almost all recovered in the total lipid extract, regardless of the labelling position of the added sulphatide; however, large differences in the level of incorporation occurred among labelled glycosphingolipids. For example, sphingomyelin was present as the major radiolabelled lipid after [Sph-3H]-sulphatide incubation, but was detectable only in trace amounts after [stearoyl-14C]sulphatide administration; in the latter case the radioactivity was located predominantly in glycerophospholipids. From this finding it can be inferred that the free long-chain base (sphingosine) that originates from lysosomal catabolism of sulphatide is mainly, and quite specifically, utilized for sphingomyelin biosynthesis, whereas the ceramide moiety is not; conversely the fatty acid released from ceramide is non-specifically re-utilized for phospholipid biosynthesis.
Full text
PDF

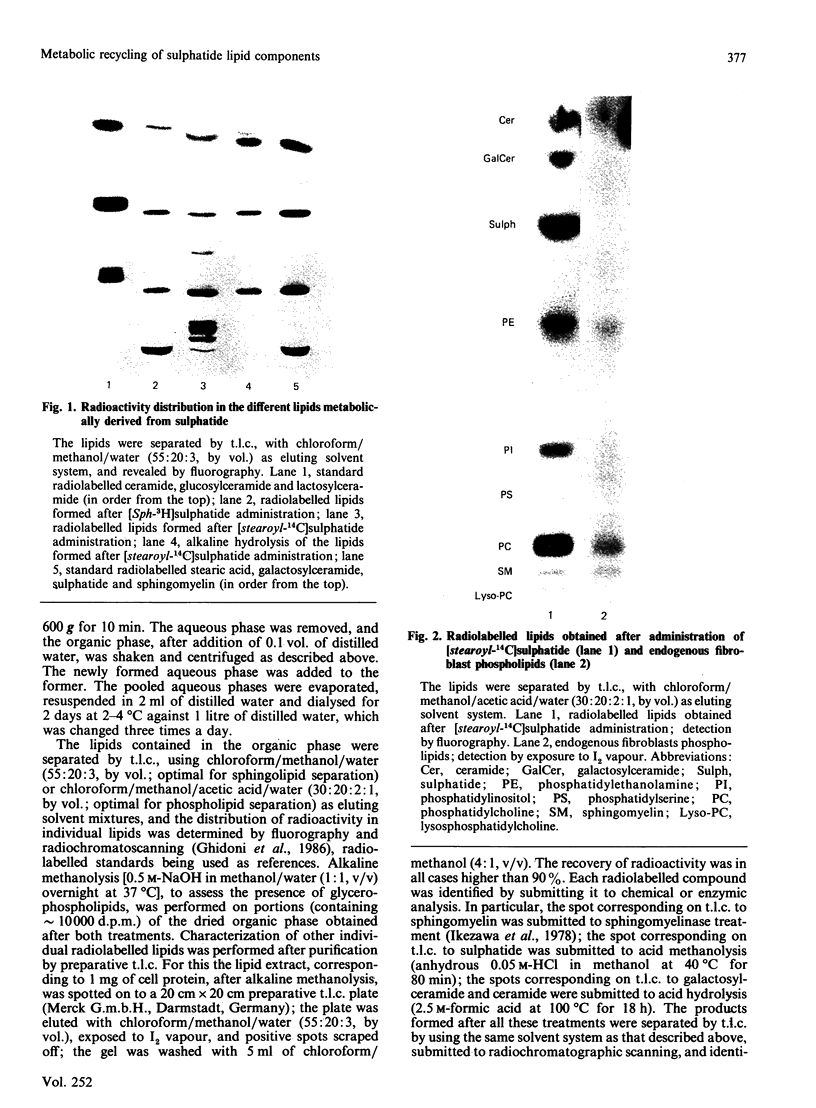


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Dubois G., Zalc B., Le Saux F., Baumann N. Stearoyl[1-14C]sulfogalactosylsphingosine ([14C]sulfatide) as substrate for cerebroside sulfatase assay. Anal Biochem. 1980 Mar 1;102(2):313–317. doi: 10.1016/0003-2697(80)90159-1. [DOI] [PubMed] [Google Scholar]
- Fishman P. H., Bradley R. M., Hom B. E., Moss J. Uptake and metabolism of exogenous gangliosides by cultured cells: effect of choleragen on the turnover of GM1. J Lipid Res. 1983 Aug;24(8):1002–1011. [PubMed] [Google Scholar]
- Ghidoni R., Sonnino S., Chigorno V., Venerando B., Tettamanti G. Occurrence of glycosylation and deglycosylation of exogenously administered ganglioside GM1 in mouse liver. Biochem J. 1983 Aug 1;213(2):321–329. doi: 10.1042/bj2130321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghidoni R., Sonnino S., Masserini M., Orlando P., Tettamanti G. Specific tritium labeling of gangliosides at the 3-position of sphingosines. J Lipid Res. 1981 Nov;22(8):1286–1295. [PubMed] [Google Scholar]
- Ghidoni R., Trinchera M., Venerando B., Fiorilli A., Sonnino S., Tettamanti G. Incorporation and metabolism of exogenous GM1 ganglioside in rat liver. Biochem J. 1986 Jul 1;237(1):147–155. doi: 10.1042/bj2370147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikezawa H., Mori M., Ohyabu T., Taguchi R. Studies on sphingomyelinase of Bacillus cereus. I. Purification and properties. Biochim Biophys Acta. 1978 Feb 27;528(2):247–256. [PubMed] [Google Scholar]
- Kishimoto Y., Mitry M. T. A new procedure for synthesis of 3-keto derivatives of sphingolipids and its application for study of fatty acid composition of brain ceramides and cerebrosides containing dihydrosphingosine of sphingosine. Arch Biochem Biophys. 1974 Apr 2;161(2):426–434. doi: 10.1016/0003-9861(74)90324-5. [DOI] [PubMed] [Google Scholar]
- Leroy J. G., Ho M. W., MacBrinn M. C., Zielke K., Jacob J., O'Brien J. S. I-cell disease: biochemical studies. Pediatr Res. 1972 Oct;6(10):752–757. doi: 10.1203/00006450-197210000-00002. [DOI] [PubMed] [Google Scholar]
- Leskawa K. C., Dasgupta S., Chien J. L., Hogan E. L. A simplified procedure for the preparation of tritiated GM1 ganglioside and other glycosphingolipids. Anal Biochem. 1984 Jul;140(1):172–177. doi: 10.1016/0003-2697(84)90149-0. [DOI] [PubMed] [Google Scholar]
- Porter M. T., Fluharty A. L., Trammell J., Kihara H. A correlation of intracellular cerebroside sulfatase activity in fibroblasts with latency in metachromatic leukodystrophy. Biochem Biophys Res Commun. 1971 Aug 6;44(3):660–666. doi: 10.1016/s0006-291x(71)80134-1. [DOI] [PubMed] [Google Scholar]
- Sonderfeld S., Conzelmann E., Schwarzmann G., Burg J., Hinrichs U., Sandhoff K. Incorporation and metabolism of ganglioside GM2 in skin fibroblasts from normal and GM2 gangliosidosis subjects. Eur J Biochem. 1985 Jun 3;149(2):247–255. doi: 10.1111/j.1432-1033.1985.tb08919.x. [DOI] [PubMed] [Google Scholar]
- Sonnino S., Kirschner G., Ghidoni R., Acquotti D., Tettamanti G. Preparation of GM1 ganglioside molecular species having homogeneous fatty acid and long chain base moieties. J Lipid Res. 1985 Feb;26(2):248–257. [PubMed] [Google Scholar]
- Tettamanti G., Bonali F., Marchesini S., Zambotti V. A new procedure for the extraction, purification and fractionation of brain gangliosides. Biochim Biophys Acta. 1973 Jan 19;296(1):160–170. doi: 10.1016/0005-2760(73)90055-6. [DOI] [PubMed] [Google Scholar]
- Zeigler M., Bach G. Internalization of exogenous gangliosides in cultured skin fibroblasts for the diagnosis of mucolipidosis IV. Clin Chim Acta. 1986 Jun 15;157(2):183–189. doi: 10.1016/0009-8981(86)90224-x. [DOI] [PubMed] [Google Scholar]