Abstract
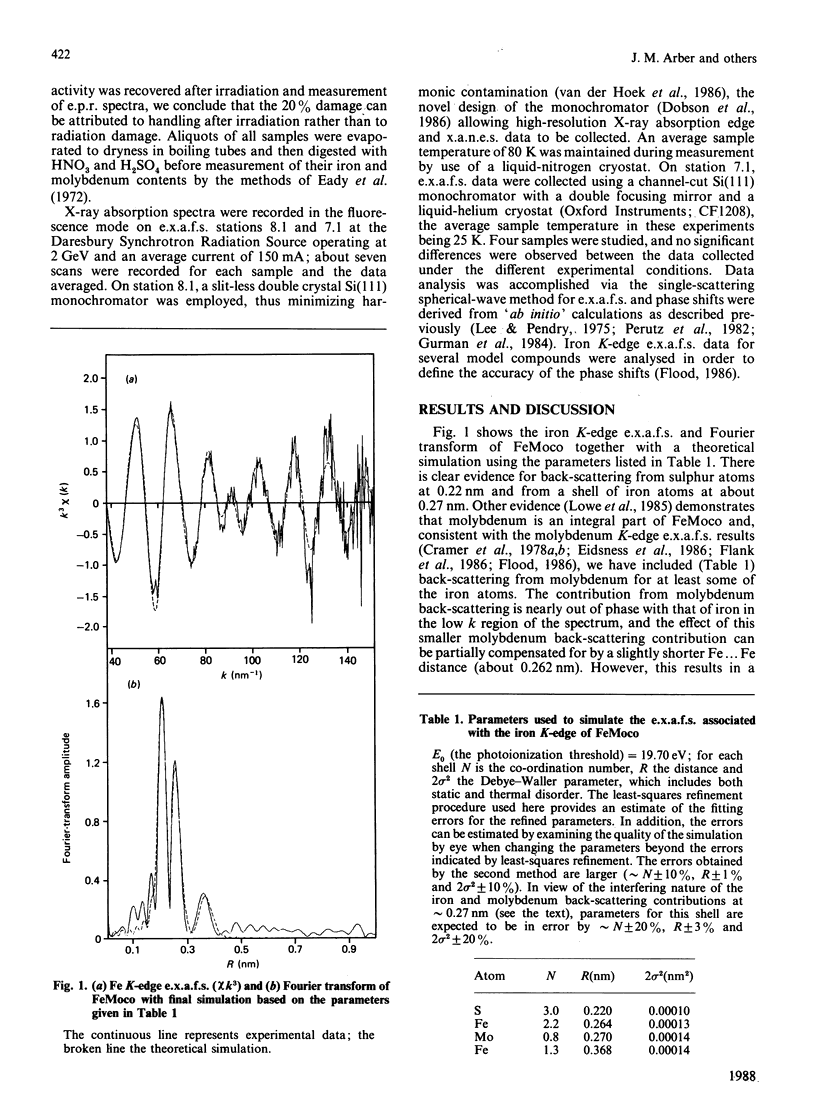
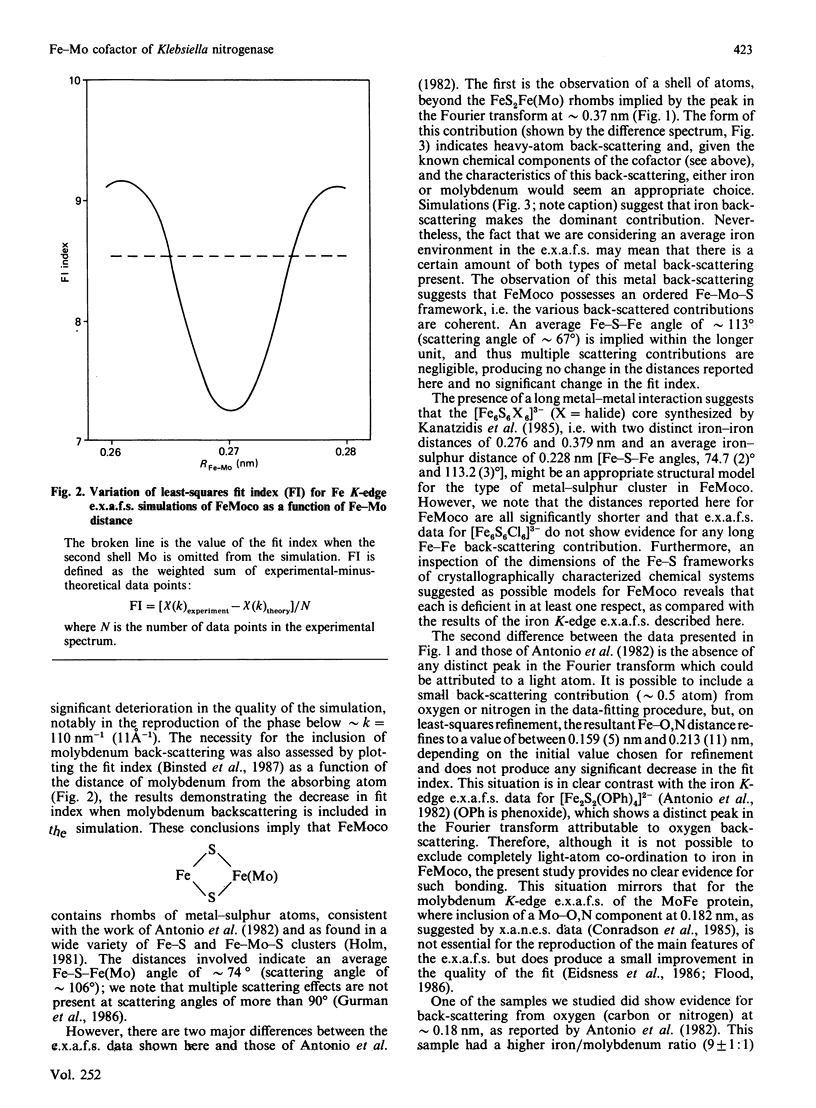
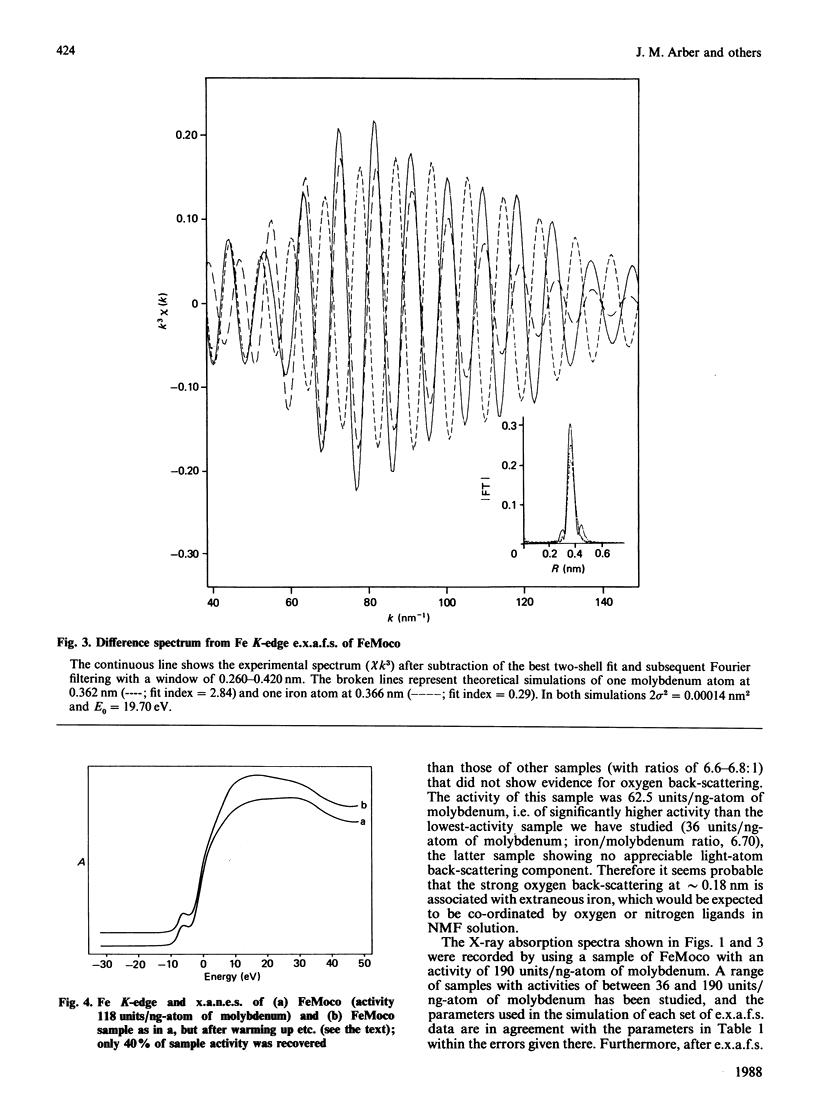
Iron K-edge X-ray absorption data for the iron-molybdenum cofactor ('FeMoco') from Klebsiella pneumoniae reported here provide the first evidence for long-range structural order in the cofactor [Fe...Fe(Mo) = 0.368 nm in addition to Fe...S = 0.22 nm and Fe...Fe(Mo) = 0.27 nm] and, in contrast with previously published data [Antonio, Teo, Orme-Johnson, Nelson, Groh, Lindahl, Kauzlarich & Averill (1982) J. Am. Chem. Soc. 104, 4703-4705], indicate that most of the iron centres are not co-ordinated to light (oxygen, nitrogen) atoms. This demonstrates that presently available chemical models for FeMoco are inadequate.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Eady R. R., Smith B. E., Cook K. A., Postgate J. R. Nitrogenase of Klebsiella pneumoniae. Purification and properties of the component proteins. Biochem J. 1972 Jul;128(3):655–675. doi: 10.1042/bj1280655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawkes T. R., McLean P. A., Smith B. E. Nitrogenase from nifV mutants of Klebsiella pneumoniae contains an altered form of the iron-molybdenum cofactor. Biochem J. 1984 Jan 1;217(1):317–321. doi: 10.1042/bj2170317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawkes T. R., Smith B. E. Purification and characterization of the inactive MoFe protein (NifB-Kp1) of the nitrogenase from nifB mutants of Klebsiella pneumoniae. Biochem J. 1983 Jan 1;209(1):43–50. doi: 10.1042/bj2090043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huynh B. H., Henzl M. T., Christner J. A., Zimmermann R., Orme-Johnson W. H., Münck E. Nitrogenase XII. Mössbauer studies of the MoFe protein from Clostridium pasteurianum W5. Biochim Biophys Acta. 1980 May 29;623(1):124–138. doi: 10.1016/0005-2795(80)90015-x. [DOI] [PubMed] [Google Scholar]
- Huynh B. H., Münck E., Orme-Johnson W. H. Nitrogenase XI: Mössbauer studies on the cofactor centers of the MoFe protein from Azotobacter vinelandii OP. Biochim Biophys Acta. 1979 Jan 25;576(1):192–203. doi: 10.1016/0005-2795(79)90497-5. [DOI] [PubMed] [Google Scholar]
- Perutz M. F., Hasnain S. S., Duke P. J., Sessler J. L., Hahn J. E. Stereochemistry of iron in deoxyhaemoglobin. Nature. 1982 Feb 11;295(5849):535–538. doi: 10.1038/295535a0. [DOI] [PubMed] [Google Scholar]
- Smith B. E., Eady R. R., Lowe D. J., Gormal C. The vanadium-iron protein of vanadium nitrogenase from Azotobacter chroococcum contains an iron-vanadium cofactor. Biochem J. 1988 Feb 15;250(1):299–302. doi: 10.1042/bj2500299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zumft W. G. Isolation of thiomolybdate compounds from the molybdenum-iron protein of clostridial nitrogenase. Eur J Biochem. 1978 Nov 15;91(2):345–350. doi: 10.1111/j.1432-1033.1978.tb12686.x. [DOI] [PubMed] [Google Scholar]


