Abstract
Multiple changes occur across various endocrine systems as an individual ages. The understanding of the factors that cause age-related changes and how they should be managed clinically is evolving. This statement reviews the current state of research in the growth hormone, adrenal, ovarian, testicular, and thyroid axes, as well as in osteoporosis, vitamin D deficiency, type 2 diabetes, and water metabolism, with a specific focus on older individuals. Each section describes the natural history and observational data in older individuals, available therapies, clinical trial data on efficacy and safety in older individuals, key points, and scientific gaps. The goal of this statement is to inform future research that refines prevention and treatment strategies in age-associated endocrine conditions, with the goal of improving the health of older individuals.
Keywords: adrenal, androgen, diabetes, endocrinology, estrogen, growth hormone, water metabolism, osteoporosis, thyroid, vitamin D
Hormones regulate and coordinate multiple physiologic functions. With increasing age, declines in physical and cognitive function occur. The extent to which age-associated changes in hormonal regulation and increases in prevalence of specific endocrine diseases contribute to declines in physical and cognitive function is incompletely understood. This area will only expand in importance as the number of older individuals increases worldwide. Current projections show an increase in those aged 65 years and older from 703 million (1 in 11 people) to 1.5 billion in 2050 (1 in 6 people) (1).
This Scientific Statement was developed to provide a high-level summary of the current state of research across multiple hormonal axes in aging and to identify areas in need of future research. Each section describes the natural history and observational data in older individuals, available therapies, clinical trial data on efficacy and safety in older individuals, key points, and scientific gaps. The extent to which hormonal changes with age are deemed “normal aging” vs “endocrine disease” can be arbitrary and depends in part on whether treatment is currently indicated. Four hypothalamic-pituitary axes are presented: growth hormone, adrenal, gonadal (divided into ovarian and testicular), and thyroid. These are followed by osteoporosis, vitamin D deficiency, diabetes, and water metabolism topics. Geroscience has emerged as a research approach examining biological mechanisms of aging and their interplay with comorbid disease. In the conclusion, cross-cutting themes of research areas in need of further investigation and the need for geroscience approaches are summarized.
Growth Hormone Axis
Natural History/Observational Data in Older Individuals
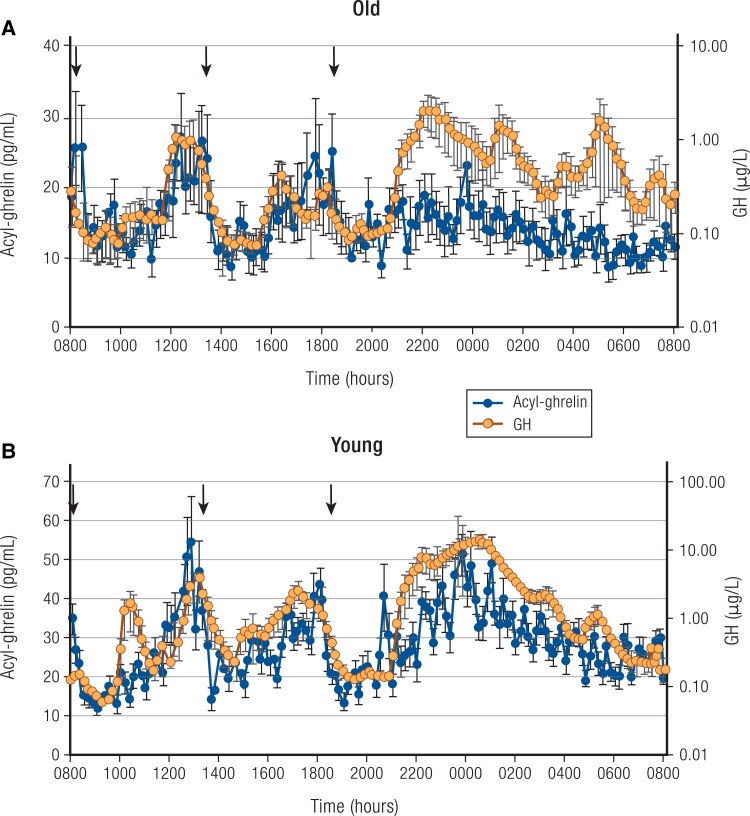
Growth hormone (GH) is secreted in a pulsatile fashion. Peak GH secretion occurs at mid-puberty (2), subsequently declining by 50% every 7 to 10 years. By the time the eighth decade is reached, GH levels are similar to those of GH-deficient young adults (3). Pulse frequency is similar across age, with approximately 18 secretory episodes of GH per 24 hours in children, younger adults, and older individuals (4). The decline in GH with aging is primarily seen in the amplitude of the secretory episodes, although interpulse levels also decline (Fig. 1) (5). A reduction of serum insulin-like growth factor 1 (IGF-1) levels occurs in parallel with the decline in average GH secretion in aging.
Figure 1.
24-hour mean (±SEM) profiles of acyl-ghrelin (left axis) and GH (right axis, note log scale) in 6 healthy older adults (A) and 8 healthy young men (B); young adults are included for comparison. Note different scales for old (upper panel) and young (lower panel) between groups. Arrows indicate standardized meals at 8:00 Am, 1:00 Pm, and 6:00 Pm. Subjects were allowed to sleep after 9:00 Pm. Also, note that in the older adults, GH was assayed in singlicates, which may contribute to some additional measurement variability in this group. Redrawn from Nass R et al (5). © Endocrine Society.
In premenopausal women, GH peak levels are higher than in men (6). This is likely due to reduced GH receptor sensitivity at the liver, and thus higher levels of GH are required to maintain normal serum IGF-1 levels. After menopause, GH levels are similar between women and men of similar age (6). Oral estrogen supplementation inhibits hepatic IGF-1 synthesis and increases GH secretion through reduced feedback inhibition, whereas IGF-1 levels increase and GH secretion is unchanged when estrogen is administered by the transdermal route (7-9).
The decline in GH synthesis and secretion with aging is well-documented in all mammalian species. In humans as well as other species, decreased output by the GH/IGF-1 axis is correlated with increased percentage of total body and visceral fat, decreased muscle mass, decreased physical fitness, decreased immune function, and physiological declines in estrogen and androgen concentrations. Whether this decline in GH secretion is causative or only correlative is controversial. In children and adults with GH deficiency, GH replacement has demonstrated benefits on body composition, serum lipids, fitness, and bone density; it also increases growth velocity in children. However, potential adverse effects of GH stimulation on malignancy, senescence, and telomere shortening are concerns of GH therapy in older individuals.
Controversy of whether GH deficiency extends life span
Caloric restriction and genetic alterations that reduce function in the GH/IGF-1/insulin pathways have been shown in experimental invertebrate and vertebrate animal models to extend life span. Mouse models of mutants that lack GH release (growth hormone-releasing hormone [GHRH], GHRH receptor, Prop1, and Pouf1) and that are GH insensitive (GHR) live significantly longer, and overexpression of GH reduces life span (bovine GH transgenic) (10). Whether this translates to humans is unclear. However, these are lifelong experiments and are likely not applicable to aging in humans in the Western world. This has also been recently reviewed in the context of humans with isolated GH deficiency (IGHD) type 1B, owing to a mutation of the GHRH receptor gene, in Itabaianinha County, Brazil. Individuals with IGHD are characterized by proportional short stature, doll facies, high-pitched voices, and central obesity. They have delayed puberty but are fertile and generally healthy. Moreover, these IGHD individuals are partially protected from cancer and some of the common effects of aging and can attain extreme longevity (10). In contrast, dwarfism associated with GH deficiency in patients with GH1 mutations is reported to significantly shorten median life span (11). There are studies that suggest that individuals with lower serum IGF-I levels have longer lives, potentially due to GH receptor exon 3 deletions (12), and that individuals with other GHR variants have major reductions in cancer and diabetes incidence without effects on life span (13). IGF-I receptor mutations have also been associated with longevity. In the Leiden Longevity Study, evidence has been presented that GH secretion is more tightly controlled in the offspring of long-lived families than in their partners, who served as age-matched controls (14).
Age, gender, percentage body fat, body fat mass, sleep disruption, aerobic fitness, and IGF-1 and gonadal steroid concentrations are all related to 24-hour GH release in adults. A major question is whether the decline of GH is due only to age or whether other factors are at play. It is well established that obesity, particularly increased visceral fat, is associated with reduced GH levels (15). In a study of highly and homogeneously active older male (n = 84) and female (n = 41) cyclists aged 55 to 79 years, it was shown that serum IGF-1 declined with age, while testosterone in men did not. The authors suggest that the hormonal changes of aging involve not only the aging process but also inactivity (16).
Available Therapies
There are no approved therapies for reversing the age-associated decline of GH secretion. Recombinant human GH (rhGH) is approved in pediatric patients with disorders of growth failure or short stature and in adults with growth hormone deficiency or with HIV/AIDS wasting and cachexia. Both GHRH and GH secretagogues exist but are not approved for use as anti-aging agents.
Clinical Trial Data on Efficacy and Safety in Older Individuals
In 2007, Liu et al published a systematic review of clinical trials of rhGH vs placebo, with or without lifestyle interventions (17). A total of 220 healthy older participants were enrolled and followed for a combined 107 patient years. Mean treatment was 27 weeks at a mean dose of 14 ug/kg day. Small changes in body composition (reduction in fat mass [−2.1 kg (95% CI, −2.8 to −1.35 kg)] and increase in lean body mass [2.1 kg (CI, 1.3 to 2.9 kg)], greater in men than in women) were found at the expense of an increased rate of adverse events. These included soft tissue edema, arthralgias, carpal tunnel syndrome, and gynecomastia, as well as a higher onset of diabetes mellitus and impaired fasting glucose. The conclusion of this review was that rhGH cannot be recommended as an anti-aging therapy.
Two randomized, placebo-controlled studies of the GH secretagogues MK-677 and capromorelin in older individuals demonstrated that these oral agents increase GH levels by enhancing the amplitude of GH pulses to levels reported in young individuals (4, 18). These compounds also have the advantage that they cannot be overdosed, due to IGF-1 feedback. The major difference between the 2 studies was in the selection of participants. In the MK-0677 study, healthy individuals were studied, whereas in the capromorelin study, participants had mild functional impairment.
Sixty-five healthy adults ranging from 60 to 81 years of age were randomized to the GH secretagogue receptor agonist MK-677 to determine whether MK-677, an oral ghrelin mimetic, could increase growth hormone secretion into the young adult range without serious adverse effects, prevent the decline of fat-free mass, and decrease abdominal visceral fat compared with placebo (4). Over 12 months, MK-677 enhanced pulsatile growth hormone secretion and significantly increased fat-free mass vs placebo (1.1 kg [CI, 0.7 to 1.5 kg] vs −0.5 kg [95% CI, −1.1 to 0.2 kg]), but did not affect abdominal visceral fat, total fat mass, strength, or physical function. Body weight increased with an increase in appetite, mild lower-extremity edema, and muscle pain, along with small increases in fasting glucose and cortisol and a decrease in insulin sensitivity. Further development of this compound was not pursued.
A randomized trial with the GH secretagogue agonist capromorelin was conducted in 395 adults aged 65 to 84 years of age with mild functional limitation to investigate the hormonal, body composition, and physical performance effects as well as the safety of 4 capromorelin dosing groups vs placebo (18). Although the study was terminated early due to failure to meet predetermined treatment effect criteria, a sustained, dose-related rise in IGF-I concentrations occurred in all active treatment groups. At 6 months, body weight increased 1.4 kg in participants receiving capromorelin and decreased 0.2 kg in those receiving placebo (P = .006). Lean body mass increased 1.4 vs 0.3 kg (P = .001), and tandem walk improved by 0.9 seconds (P = .02) in the pooled treatment vs placebo groups. By 12 months, stair climb also improved (P = .04). Adverse events included fatigue, insomnia, and small increases in fasting glucose, glycated hemoglobin, and indices of insulin resistance. No additional studies are planned for this compound.
Key Points
At present, no therapy to increase GH secretion or action is approved as an anti-aging intervention.
Studies with rhGH and GH secretagogues failed to demonstrate benefits that outweigh risks. However, it is possible that benefit could be maximized with the use of lower doses, in study populations with worse physical function, and in combination with exercise and adequate nutrition, without the adverse effects seen in previous studies.
Gaps in the Research
Studies in invertebrate and vertebrate models are important but may not be translatable to humans. Most animal studies have investigated lifelong interventions of over- or underactive somatotroph function. Intervening at different stages of the life cycle may help explain the conflicting data on whether too little or too much somatotroph function may be beneficial to extending life span.
The changes in GH secretion across the life cycle make the interpretation of animal studies and their translation to humans problematic. The objective should be to improve health span rather than life span. Thus, further studies of increasing or decreasing somatotrope function at different stages of the life cycle will be important, particularly to evaluate whether restoring pulsatile GH secretion as seen in 20- to 30-year-old individuals would help prevent development of frailty and sarcopenia without increasing risks. It is clear that hormonal treatment alone will not be sufficient, so future trials will require evaluation of lower doses of GH/GH secretagogues with consideration of combination with exercise, nutrition interventions, and/or co-supplementation of other hormones (eg, testosterone). Targeting the right populations, such as those who have developed, or are at high risk for, frailty and sarcopenia, will also be vital. Further studies will need to be carried out for several years or longer.
Adrenal Axis
Natural History/Observational Data in Older Individuals
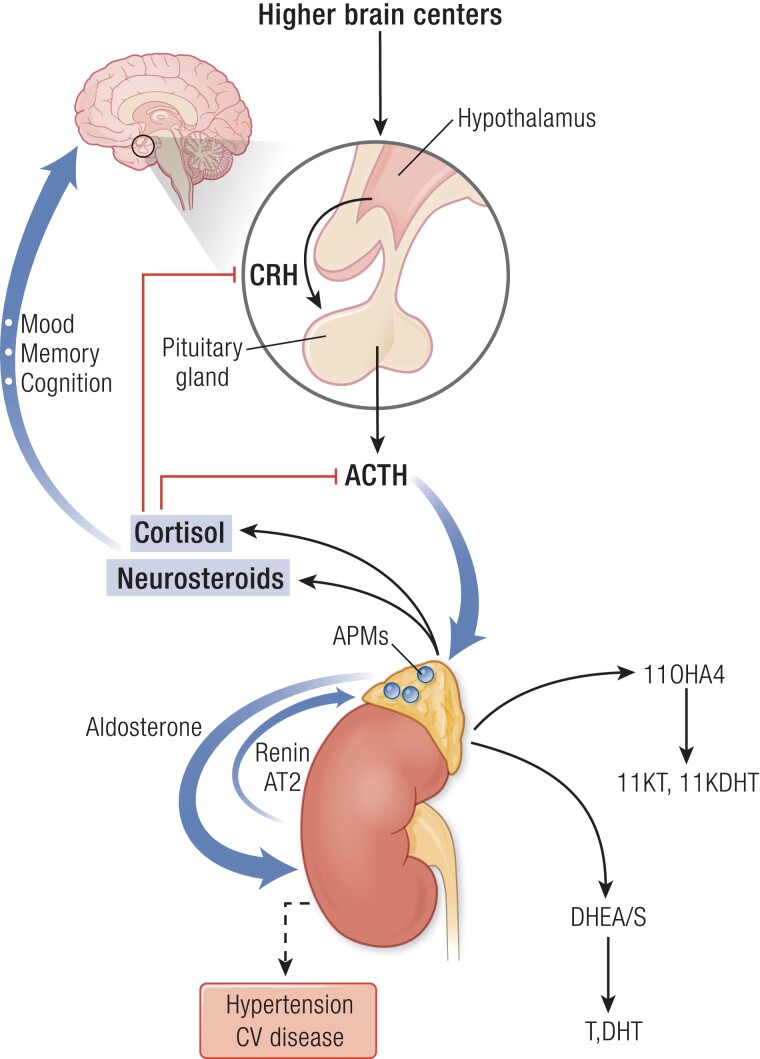
The adrenal glands produce several classes of hormones from different cell types or zones. The adrenal cortex synthesizes steroid hormones and hormone precursors, primarily the mineralocorticoid aldosterone from the zona glomerulosa, the glucocorticoid cortisol from the zona fasciculata, and the androgen precursors dehydroepiandrosterone (DHEA) and its sulfate (DHEAS) from the zona reticularis (19) (Fig. 2). DHEAS is largely a storage form and excreted product, with conversion to DHEA in a few tissues. The adrenal medulla is an extension of the sympathetic nervous system, which secretes epinephrine.
Figure 2.
The hypothalamus integrates signals from the environment and higher brain centers to release corticotropin-releasing hormone (CRH), which stimulates pituitary production of adrenocorticotropin (ACTH). ACTH drives production of cortisol, as well as neurosteroids and their precursors, 11β-hydroxyandrostenedione (11OHA4), and dehydroepiandrosterone and its sulfate (DHEA[S]). Cortisol provides negative feedback (red lines) to the hypothalamus and pituitary, not just to cortisol but also to all other ACTH-stimulated steroids. DHEA and DHEAS can be metabolized to the androgens testosterone and dihydrotestosterone (T, DHT), whereas 11OHA4 is metabolized to the androgens 11-ketotestosterone (11KT) and 11-ketodihydrotestosterone (11KDHT). Cortisol and neurosteroids exert important actions on the brain that control mood, memory, and cognition. Independently, aldosterone is produced under the renin-angiotensin 2 (AT2) system, or autonomously such as from aldosterone-producing micronodules (APMs). Aldosterone regulates sodium balance, and aldosterone excess causes hypertension and cardiovascular (CV) disease. In aging, cortisol negative feedback is attenuated, and while DHEAS production falls, cortisol and 11OHA4 synthesis is preserved. APMs increase with age, and the potential deleterious effects of cortisol and aldosterone excess are magnified with aging.
Infants produce large amounts of aldosterone to compensate for the resistance of the neonatal kidney to mineralocorticoids and the low sodium content of human milk. Over time, the sodium content of the diet increases, and the need for aldosterone decreases; most American adults consume over 150 meq of sodium daily. Rather than having a uniform, continuous zona glomerulosa as seen in children and young adults, adrenal glands of adults in Western countries become increasingly discontinuous after age 40. Immunohistochemistry studies reveal pockets of cells that express the aldosterone synthase enzyme (CYP11B2) beneath the adrenal capsule (20), initially termed aldosterone-producing cell clusters and now called aldosterone-producing micronodules (APMs). APM cells commonly harbor somatic mutations in genes encoding subunits of ion channels that regulate aldosterone production (21). As the number of adrenal glands with a continuous zona glomerulosa declines with age, the number of these APMs and their total area increases in parallel (22). A theoretical, but plausible, interpretation of these findings is that, with a chronic high-salt diet and renin suppression, the normal zona glomerulosa atrophies. At the same time, adrenal precursor cells undergo selection for clones with somatic mutations in ion channel genes that allow survival and aldosterone production in the absence of angiotensin II stimulation (23). This process could give rise to the cells that proliferate into APMs. The accumulation of APMs translates to various degrees of autonomous aldosterone production, and if the burden becomes high enough, could result in unilateral or bilateral primary aldosteronism. Other subclones might undergo further genetic changes that drive formation of aldosterone-producing adenomas. This model could explain the development of various forms of primary aldosteronism and why the prevalence of this disease and of salt-sensitive hypertension increases with age.
Like that of other axes, the dynamic behavior of the hypothalamic-pituitary-adrenal (HPA) axis undergoes changes with age, including a flattening of the diurnal rhythm and earlier morning peak (24, 25). This results in higher 24-hour cortisol production rates and free cortisol levels, but no difference in cortisol binding globulin levels, with increasing age (26). In addition, the HPA axis appears to be more responsive to stress, with some differences between men and women (27), in part due to reduced negative feedback inhibition from cortisol (28). Similarly, the cortisol response to exogenous adrenocorticotropin (ACTH) is prolonged at older ages (29). Given the potential contributions of cortisol to a multitude of age-dependent diseases and decline in physical function, these changes and individual variations in magnitude could have broad consequences (30).
Regulation of local glucocorticoid activity, independent of the HPA axis, may occur with cortisol regeneration from cortisone via the enzyme 11β-hydroxysteroid dehydrogenase type 1 (11βHSD1). The expression of 11βHSD1 in skin increases with age (31), which could potentiate the catabolic action of cortisol on skin without affecting adrenal cortisol production. 11βHSD1 expression in muscle is inversely correlated with strength in older individuals (32), suggesting that, in aging, enhanced catabolic action of cortisol could occur through this mechanism in several tissues.
The prevalence of overt Cushing syndrome does not rise with age, but the development of mild ACTH-independent hypercortisolemia due to adrenal adenomas and hyperplasia does increase over time (33). Several studies have provided evidence that even mild cortisol excess is not benign and is associated with hypertension, glucose intolerance, cardiovascular events, and vertebral fractures (34, 35). Consequently, occult and smoldering hypercortisolemia could predispose to common disorders in older persons.
Furthermore, although cortisol does not directly cause major age-related diseases, such as cancer and dementia, preclinical and human studies suggest that modulation of cortisol action could evolve into treatment strategies for these diseases. In castration-resistant prostate cancer, sustained treatment with the potent androgen-receptor antagonist enzalutamide results in upregulation of glucocorticoid-receptor expression, which drives the expression of previously androgen-regulated oncogenes (36). In parallel, rapid degradation of 11β-hydroxysteroid dehydrogenase type 2, the enzyme that converts cortisol to inactive cortisone, potentiates cortisol action (37). Consequently, the glucocorticoid signaling pathway could be a target for the treatment of advanced prostate cancer (38). In patients with early Alzheimer disease, higher morning plasma cortisol predicts more rapid progression of dementia symptoms and deterioration of temporal lobe function (39). In a rat model, glucocorticoid-receptor antagonists attenuated the augmented rise in morning corticosterone and hippocampal amyloid deposition, and some agents delayed the progression of cognitive dysfunction (40). These studies demonstrate that manipulation of cortisol bioactivity, particularly in a tissue-selective manner, could have benefits in certain maladies common in older individuals.
Circulating concentrations of DHEAS peak at about age 25 and then decline gradually with age, falling to childhood concentrations by age 80 in most adults (41), reflecting a gradual reduction in the size of the zona reticularis (42). The reason for this change is not known, and rodents secrete small amounts of DHEA and therefore cannot serve as a research model for this hormone. The peak concentrations and trajectory of decline, however, vary significantly among individuals, and in population studies, DHEAS concentrations are higher in men than women. The developmental changes and age-related decline in DHEAS have attracted considerable attention as a potential mediator of the aging process (43), reflecting the anabolic actions of androgens.
In women, half or more of circulating testosterone derives from 19-carbon androgen precursors from the adrenal cortex, including DHEA, DHEAS, and androstenedione (44). In contrast, the vast majority of testosterone in men derives from the testes throughout adult life. Consequently, an age-related decline in steroid production from the zona reticularis could have greater impact in women and in men with primary or secondary testicular dysfunction than in normal men. While the decline in DHEAS with age is well substantiated, many of the data about the consequences of this phenomenon derive from epidemiologic and cross-sectional studies (45, 46), rather than large randomized controlled trials (RCTs) of DHEA supplementation.
Another product of the adrenal cortex that has been understudied until recently is the robust synthesis of 11-oxygenated androgens, primarily 11β-hydroxyandrostenedione (11OHA4), which is converted through metabolism in other tissues to the androgen 11-ketotestosterone (11KT) (47). While the biosynthetic pathways of 11-oxygenated pro-androgen production via the human adrenal cortex have been described, the location(s) of their synthesis, the zona fasciculata and/or zona reticularis, is not known. In women, DHEA, DHEAS, androstenedione, and testosterone all decline from age 30 onward; however, 11OHA4 and 11KT increase slightly into the ninth decade and decline only slightly during this age window in men (48). For nearly all women (48) and prepubertal children (49), 11KT is the most abundant bioactive androgen in the circulation, and this adrenal androgen component is preserved throughout life. Because 11KT could theoretically provide negative feedback on the gonadal axes, this contribution could become important in older men, although direct evidence to this effect is lacking.
Available Therapies
Spironolactone and eplerenone, and more recently, finerenone, are available as aldosterone (mineralocorticoid) antagonists for the treatment of primary aldosteronism and hypertension. Although the US Food and Drug Administration (FDA) has approved several treatments for Cushing syndrome (mifepristone, pasireotide, osilodrostat, and levoketoconazole) in recent years, these drugs are not well-studied for subtle ACTH-independent hypercortisolemia or the cortisol-mediated contributions to other diseases. DHEA (prasterone) administered as a 6.5 mg intravaginal insert improves symptoms of vulvovaginal atrophy in postmenopausal women and is FDA-approved for this purpose (50). DHEA is available over the counter as a dietary supplement and is not regulated by the FDA.
Clinical Trial Data on Efficacy and Safety in Older Individuals
Small studies have found conflicting results from DHEA replacement in older women (51-53). A few moderately large studies of DHEA supplementation at 25 to 50 mg/d for 1 or 2 years in older men and women have consistently shown restoration of DHEAS concentrations to the young adult range, as well as increased circulating concentrations of testosterone in women and of estradiol in postmenopausal women (54, 55). In these trials, postmenopausal women experienced small improvements in bone density at some sites, and these changes could be ascribed to the rise in estradiol. In one of these studies, no improvement of muscle cross-sectional area or strength was observed (56), and improvements in quality of life could not be demonstrated (55). These studies do not support the widespread use of DHEA supplementation as an anti-aging agent, despite claims otherwise to be found on the internet. Some studies of DHEA supplementation in women with adrenal insufficiency, in whom production of DHEA, DHEAS, testosterone, and all adrenal-derived androgens is low, have reported improvements in sexual satisfaction and interest (57), but similar results have not been obtained in trials with older women.
Key Points
APMs that autonomously produce aldosterone begin to develop in adulthood and accumulate with age.
The HPA axis shows less sensitivity to negative feedback, blunted diurnal changes, and alterations in cortisol/cortisone interconversion with aging.
Although circulating concentrations of DHEA and DHEAS decline with age, cortisol and 11-keto androgens do not decline or rise slightly.
Modulation of cortisol signaling could be beneficial in a host of diseases that become more common in older men and women.
Systemic DHEA supplementation has not shown major benefits in older individuals.
Gaps in the Research
Because rodent adrenals make neither cortisol nor androgens due to lack of the gene Cyp17, engineered or humanized strains that include Cyp17 and recapitulate the zonation and steroidogenic repertoire of the human adrenal would be valuable animal models to study human aging and targeted interventions.
Additional research is needed to chart the development of APMs in aging adrenals and to define the role of autonomous aldosterone production in the age-associated increase of salt-sensitive hypertension. Incorporation of cortisol modulation, including tissue-selective agonists and antagonists, into treatment regimens for diseases from cancer to Alzheimer disease is only beginning to emerge. Previous conclusions regarding adrenal androgens during aging, including 11-oxygenated androgens, need to be reassessed using modern mass spectrometry–based steroid profiling. Studies designed to dissect the contributions of adrenal steroids to the aging process using longitudinal cohorts would add to the understanding of whether these changes are detrimental, compensatory, or clinically insignificant.
Ovarian Axis
Natural History/Observational Data in Older Individuals
Biology of menopause/ovarian aging
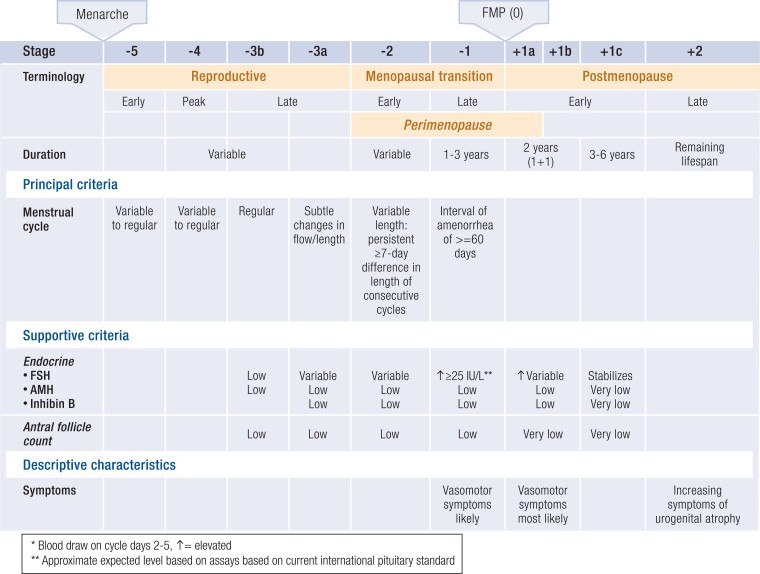
In contrast to other endocrine axes, aging of the human ovary is programmed—before birth—for midlife senescence. A full complement of ovarian follicles develops in utero, peaking at approximately 7 months of gestation with 6 to 7 million follicles, and then, via atresia, is gradually reduced to 1 to 2 million follicles by birth. The progressive decline in ovarian follicle number follows a curvilinear pattern, with accelerated loss with increasing age (58). Menopause, the final menstrual period, is diagnosed retrospectively after 12 months of amenorrhea, at an average age of 51 years, when total follicles number approximately 1000 (Fig. 3) (59).
Figure 3.
The Staging of Reproductive Aging Workshop (STRAW)+10 staging system for reproductive aging in women. Abbreviations: AMH, anti-Mullerian hormone; FMP, final menstrual period; FSH, follicle-stimulating hormone. Redrawn from Harlow SD et al (59). © Endocrine Society.
The average human reproductive life span, ranging from menarche to menopause, is currently estimated at 37 years in duration (60). Genetic, autoimmune, metabolic, environmental, and iatrogenic factors can accelerate follicular atresia resulting in early (40 to 45 years) or premature (<40 years) menopause (61). The progression of ovarian aging can be monitored by measurement of anti-Mullerian hormone (AMH) and ultrasound determination of antral follicle count (AFC) (62, 63). These parameters are useful for determining ovarian reserve and timing of menopause, but paradoxically, do not necessarily correlate with fertility, likely due to the multiple other factors influencing female fertility. By the time follicle-stimulating hormone (FSH) increases during the late menopausal transition, AMH levels are low to undetectable.
Genetic contributions to age of menopause
Population-based genome-wide association studies have identified 290 genomic loci associated with age of natural menopause (64). The loci identified harbor a broad range of DNA damage-response processes, highlighting the importance of these pathways in determining ovarian reserve (64). Additional factors include cohesion deterioration and chromosome mis-segregation, meiotic recombination errors, spindle assembly checkpoint, genetic mutations, telomere length and telomerase activity, reactive oxygen species, mitochondrial dysfunction, and ovarian fibrosis and inflammation (65, 66). The inability to repair DNA damage in both somatic and germ cells could explain the link between reproductive and overall aging (67).
The “epigenetic clock,” based on DNA methylation levels, provides more evidence that menopause accelerates at least some components of biological aging (68). Conversely, increased epigenetic age acceleration in blood is significantly associated with earlier menopause, bilateral oophorectomy, and a longer time since menopause (68). Furthermore, the age at menopause and epigenetic age acceleration share common genetic origins (68). The telomerase reverse transcriptase gene provides critical regulation of the epigenetic clock (69).
Hypothalamic-pituitary contributions to ovarian aging
In spite of the primary focus on the ovary as the key determinant of reproductive senescence, the central nervous system has been explored as a critical pacemaker of reproductive aging with evidence that central changes (70-72), regulated by DNA methylation (73), contribute to the timing of menopause. Manifestations of aging on gonadotropin secretion include diminution of the preovulatory luteinizing hormone (LH) surge (74) and marked elevation of pituitary LH and FSH during the late reproductive phase and the menopause transition. Diminished pituitary responsiveness to gonadotrophin-releasing hormone (GnRH) after menopause (75) is accompanied by alterations in the forms of secreted LH and FSH, resulting in slower clearance and prolonged half-life (76). Pituitary-ovarian axis hormones—particularly FSH and estradiol—are also hypothesized to play a role in regulating ovarian mitochondrial activity (77, 78). Elucidation of hypothalamic kisspeptin, neurokinin B, and dynorphin neuronal morphology and physiology in postmenopausal women provides insights regarding postmenopausal gonadotropin control and a new mechanism to reduce vasomotor symptoms (VMS) with NK3R (neurokinin3 receptor) antagonists (79-81).
Challenges to traditional thinking about the postmenopausal effects of elevated FSH have emerged. Mouse studies utilizing a blocking antibody to the FSH receptor revealed preservation of bone density (82), subsequent browning of white fat cells, decrease in subcutaneous and visceral fat accumulation, and improved muscle mass (83, 84), although contrary evidence of bone anabolic effects of FSH, mediated through the ovary, has also been reported (85). Possible links of FSH with cardiovascular disease (CVD) risk have been proposed. However, in the Study of Women Across the Nation (SWAN), a multiethnic cohort of US women, higher FSH also predicted lower systolic blood pressure (86).
Ovarian steroid hormone status with aging
Estradiol secretion is maintained in older, reproductive aged women by increased ovarian aromatase function (87, 88). Granulosa cell production of estradiol, AMH, and inhibin eventually declines with age, possibly reflecting progressive mitochondrial aging (89). In the postmenopausal state, estrogen synthesis continues, but at much lower levels, via aromatase conversion of ovarian androstenedione to estrone, the predominant postmenopausal estrogen, and of testosterone to estradiol. Obesity, with an attendant increase in aromatase activity, is associated with higher serum concentrations of estrogens and testosterone (90, 91).
Circulating testosterone concentration within the low female range declines with reproductive aging (92-94). Ovarian testosterone production falls in a linear pattern with age; in longitudinal studies, testosterone levels were not directly affected by menopause. The theca cells of the postmenopausal ovary continue to produce testosterone in response to elevated gonadotropins. With advancing age, to 70 (93, 94) to 80 years (93-95), higher testosterone concentrations are associated with detrimental metabolic and cardiovascular effects (96) yet increased bone mineral density (BMD) and lean body mass (91).
Clinical aspects of ovarian aging
Regardless of the etiology of ovarian insufficiency, 2 key clinical sequelae arise: a progressive decline in fertility, reflecting the reduction in ovarian follicle number and quality, and the cessation of monthly menstrual cycles, reflecting the parallel decline of ovarian steroid hormones. Consequently, symptoms (VMS, genitourinary syndrome of menopause [GSM] (97), disordered mood, sleep disruption, sexual disorders) and systemic effects (amenorrhea, bone loss, metabolic syndrome, increased cardiovascular risk, cognitive decline) can result (98).
The menopause transition
The updated Stages of Reproductive Aging Workshop (STRAW+10) report provides standardized criteria for identifying the transition from the reproductive years to the postmenopausal, with the goal of enhancing the design and reporting of research studies of ovarian aging while establishing accepted nomenclature to be applied to patient care (59) (Fig. 3). Prospective, longitudinal observational studies (99-104) (Table 1), such as SWAN (104), continue to clarify the timing of perimenopausal symptom onset, duration during and beyond the menopause transition, relationship with pituitary and ovarian hormone concentrations, clinical correlations with race and ethnicity, linkage of multiple perimenopausal symptoms, and association of symptoms with chronic diseases previously solely attributed to aging.
Table 1.
Prospective longitudinal studies of the menopausal transition
| Study name | N | Age at baseline (y) | Dates | Duration (y) |
|---|---|---|---|---|
| The Massachusetts Women's Health Study (99) | 2570 | 45-55 | 1981-1986 | 5 |
| The Melbourne Women's Midlife Health Project (100) | 438 | 45-55 | 1996-2005 | 9 |
| Penn Ovarian Aging Study (101) | 436 | 35-47 | 1996-2014 | 18 |
| The Seattle Midlife Women's Health Study (102) | 508 | 35-55 | 1990-2013 | 23 |
| University of Pittsburgh Healthy Women Study (103) | 532 | 42-50 | 1983-2008 | 25 |
| Study of Women's Health Across the Nation (104) | 3302 | 40-55 | 1994-ongoing | 28 |
Clinical sequelae of ovarian aging
Ovarian aging is associated with deteriorating lipid profiles; accelerated cardiovascular risk; adverse changes in body composition including distribution of adipose tissue; accelerated lumbar spine BMD loss; and negative effects on sleep, cognition, and mood (105, 106). Early (< age 45 years) and premature (< age 40 years) menopause (natural or surgical) appear to accelerate chronic diseases of aging, including type 2 diabetes, illustrated by studies of women experiencing bilateral oophorectomy before age 46 (107, 108). A truncated “reproductive life span” is associated with higher risk of CVD events and mortality (109). Alternatively, cardiovascular health has been hypothesized by some to contribute to the timing of menopause, so a bidirectional association could be considered (105, 110).
Vasomotor symptoms and cardiovascular risk
Reports from longitudinal, prospective studies provide compelling evidence that for approximately a quarter of women, VMS start more than a decade prior to menopause and last more than a dozen years after (111-113). Long-term SWAN follow-up showed an association between frequency of VMS and increased CVD risk factors, subclinical CVD, and CVD events (113, 114). Ongoing studies will examine whether this association reflects causation and if treating VMS modifies CVD risk.
Observations of VMS with increasing age
Observational studies and clinical trials with participants of advanced age suggest that approximately 7% of older women continue to experience VMS (115). Whether VMS persist from the time of menopause, recur after a period of quiescence, or arise de novo decades later has not been ascertained. The complex interplay between VMS and a 5- to 9-fold increase of CVD events following menopausal hormone therapy (MHT) initiation in older women participating in the Heart and Estrogen/progestin Replacement Study (HERS) (116) and the Women's Health Initiative (WHI) (117) underscores the need for more research into the etiology, characteristics, and consequences of VMS with aging.
Available Therapies
The spectrum of evidence-based therapies for relief of VMS ranges from MHT to prescription nonhormonal drugs to mind-brain-behavioral approaches, including cognitive behavioral therapy and hypnosis (118, 119). Decisions regarding the optimal choice for an individual woman incorporate her degree of symptom bother, personal preferences, CVD and breast cancer risk assessments, and uterine status (118, 120, 121). Treatment of GSM includes over-the-counter moisturizers and lubricants, vaginal estrogens, DHEA, and oral ospemifene (97, 118). As no testosterone preparation is approved by the FDA for women, titration of approved therapies dosed for men has been recommended for treatment of hypoactive sexual desire disorders in women (122, 123).
Clinical Trial Data on Efficacy and Safety in Older Individuals
For this discussion, “older” encompasses women after menopause (usually > age 50), bearing in mind that hormone replacement therapy is indicated for younger women who experience hypogonadism or primary ovarian insufficiency and is recommended until the anticipated age of natural menopause (61, 118, 120). Although preparations, routes of administration, and dosages of MHT have markedly expanded since the first use of conjugated equine estrogens (CEE) in the 1940s, the primary indication for MHT in women experiencing natural menopause remains treatment of symptoms (VMS and GSM) (118, 120). Prevention of osteoporosis is another approved indication of MHT, for postmenopausal women at significant risk of osteoporosis for whom other approved therapies are neither tolerated nor appropriate. Additional preventive indications have been considered and are currently under review (124).
The results of secondary coronary heart disease (CHD) prevention trials have been disappointing (125). In contrast to anticipated CHD benefit based upon myriad observational studies, trials revealed an increase in myocardial infarction within the first year of therapy, and failure to reduce CHD events or coronary atherosclerosis progression (125).
The Women’s Health Initiative clinical trials were initiated in 1992 to determine whether MHT (CEE ± medroxyprogesterone acetate ([MPA]), depending upon uterine status), when started in healthy women ages 50 to 79 at enrollment, reduced the incidence of chronic diseases of aging (myocardial infarction and CHD death, osteoporosis, colon cancer) while evaluating safety outcomes (stroke, venous thromboembolic disease, breast and endometrial cancer) (126). The combined therapy arm was halted after 5.6 years, and the estrogen-only arm after 7.2 years, because overall risks (increased stroke in both trials and heart attack, pulmonary emboli, and breast cancer in the combined arm) exceeded preventive benefits (reduced fractures, colon cancer, diabetes) (117). Subsequent analyses showed a more favorable benefit/risk profile in younger women (ages 50 to 59) or those closer (<10 years) to menopause, whereas stroke risk increased when MHT was initiated > age 60 (127), dementia risk increased > age 65 (126), and CHD events increased > age 70 (127). The 13-year cumulative follow-up provided additional supportive evidence (117). At 18 years, overall mortality was not increased for any group. Moreover, all-cause mortality decreased by 21% in those ages 50 to 59 at enrollment in the CEE-alone arm (128), with maximal mortality benefit—a 40% decrease—for those with bilateral oophorectomy < age 45 (108).
Breast cancer outcomes at 13 years of cumulative follow-up showed persistence of the significant 28% increase in breast cancer risk with combined therapy initially reported at trial termination (117). In contrast, a 21% decrease with CEE alone became statistically significant (117). At 20 years of cumulative follow-up, these findings persisted, with the added caveat that breast cancer mortality—without effect in the combined therapy arm—was significantly reduced in the CEE-alone arm (129). These findings reflect the complexities of these specific hormone preparations on breast cancer incidence and mortality and should not be extrapolated to other MHT preparations. Although adequately powered RCTs are lacking, observational studies do not suggest that estradiol administration inhibits breast cancer, whereas progesterone may have less breast cancer–stimulating effects than MPA (118). The paucity of RCT safety evidence means that MHT is usually not prescribed for women with a history of breast cancer; symptom relief with nonhormonal options is recommended (118, 130).
In summary, the Women’s Health Initiative established the safety of MHT for younger postmenopausal women (< age 60 or <10 years since menopause), highlighted the divergence of CVD and breast cancer outcomes for CEE alone vs combined therapy with MPA, and confirmed observational studies suggesting mortality benefit for women with early menopause who used CEE alone following oophorectomy.
The timing hypothesis
The timing hypothesis suggests that MHT reduces atherosclerosis when initiated close to menopause, but not if started at a later point, possibly due to changes in estrogen receptor signaling with time since menopause and altered estrogen milieu (131, 132). The timing hypothesis could also explain findings from a trial evaluating effects of transdermal estradiol on insulin sensitivity (133). Several RCTs designed specifically to examine the CHD effects of the timing hypothesis yielded inconsistent results (117, 134-136) (Table 2). Current guidelines recommend against prescribing MHT solely for CHD prevention in naturally postmenopausal women (118, 120, 124, 137).
Table 2.
Randomized primary prevention trials evaluating effects of menopausal hormone therapy on clinical and surrogate cardiovascular outcomes in healthy, recently postmenopausal women
| Trial | MHT preparation and dose | N | Age (y) | Duration (y) | Outcomes |
|---|---|---|---|---|---|
| Clinical outcomes | |||||
| WHI E-alone (117) | CEE 0.625 mg/d po | 3313 | 50-59 | 7.2 | Reduced MI, CAC, and revascularization |
| WHI E + P (117) | CEE 0.625 mg/d and MPA 2.5 mg/d po | 5520 | 50-59 | 5.6 | No benefit |
| DOPS (134) | 17-B E2 2 mg/day and norethisterone acetate 1 mg 10 days/mo po | 1006 | 45-58 | 10 | Reduced composite serious adverse events: death, hospitalized MI, or CHF |
| Surrogate outcomes | |||||
| KEEPS (135) | CEE 0.45 mg/d po or TD E2 50 mcg and progesterone 200 mg 12 days/mo po | 727 | 42-58 | 4 | No benefit cIMT or CAC |
| ELITE (136) | 17-B E2 1 mg/d po and progesterone 45 mg vaginal gel 10 days/mo | 596 | 55-64 | 5 | Reduced cIMT early group No benefit CAC |
Early <6 years since menopause vs late ≥10 years since menopause.
Abbreviations: CAC, coronary artery calcium; CEE, conjugated equine estrogens; CHF, congestive heart failure; cIMT, carotid intima-medial thickness; DOPS, Danish Osteoporosis Prevention Study (randomized, not blinded); E2, estradiol; E-alone, estrogen alone trial; E + P, estrogen plus progestogen; ELITE, Early vs Late Postmenopausal Treatment with Estradiol; KEEPS, Kronos Early Estrogen Prevention Study; MHT, menopausal hormone therapy; MI, myocardial infarction; MPA, medroxyprogesterone acetate; po, oral; TD, transdermal; WHI, Women's Health Initiative; y, years.
Dose/type of MHT and duration of therapy
In the absence of adequately powered clinical trials, observational studies and meta-analyses provide some evidence that safety outcomes—particularly for venous thromboembolic disease and possibly stroke risks—are improved with lower doses and transdermal estradiol preparations (105, 118, 138).
Following the initial reports of the Women’s Health Initiative, limiting MHT to 3 to 5 years was recommended to minimize breast cancer risk. Both the North American Menopause Society (NAMS) and the American College of Obstetricians and Gynecologists (ACOG) subsequently issued statements allowing for longer duration of MHT in healthy women ≥ age 65 without contraindications, following an annual discussion of anticipated risks and benefits, and reevaluation of individual health status (120, 139). The recommendation for shared decision making reflects the absence of long-term evidence to inform decisions regarding risks and benefits for women who initiate MHT for symptom relief at menopause and continue for an extended time. Common sense measures include progressively reducing the dose and switching to transdermal from oral preparations (115, 118, 120).
Key Points
Menopause and the postmenopausal state are natural, preprogrammed manifestations of ovarian aging characterized by fertility loss and profound reduction in ovarian hormone production.
Menopausal symptoms are common, vary in degree of bother, and can be effectively treated with a variety of agents proven effective in RCTs.
Initiation of MHT is safest when reserved for women in close proximity (<10 years) to the menopause transition or less than age 60, without contraindications, and with acceptable CVD and breast cancer risks.
Continuation of MHT can be considered individually depending on personal desires, health status, and documented shared decision making.
Although oral MHT has been studied most extensively, depending upon health status and age, based upon prospective observational studies, lower doses and transdermal therapies may be safer with fewer venous thromboembolic events, fewer undesirable metabolic effects, and possibly fewer CVD events.
Delineation of the physiological role of the kisspeptin, neurokinin Y, and dynorphin neurons in control of VMS and gonadotropin and sex steroid secretion allows for potential new treatment options as demonstrated in completed and ongoing RCTs of neurokinin3 receptor (NK3R) antagonists.
Gaps in the Research
Factors that affect the timing and consequences of menopause across diverse races, ethnicities, lifestyles, genetics, environmental influences, metabolic factors, and polycystic ovary syndrome (PCOS) require additional study. The SWAN study provides some insight into differences in reproductive aging and midlife health between Black and White women, but additional work is needed (140).
The natural history and physiologic characteristics of VMS, including the prevalence of ongoing or recurrent VMS in older women, CVD impact of VMS, and safe and effective treatment options in this age group, require more study, optimally utilizing investigative techniques measuring both subjective and objective VMS.
Steroid hormone and gonadotropin concentrations with advanced age have not been well delineated. Additional follow-up of ongoing studies such as SWAN and new population studies is needed.
Adequately powered RCTs with clinical outcomes of MHT would ideally be completed in symptomatic, recently postmenopausal women. Head-to-head randomized trials in this population could confirm risks and benefits of transdermal estradiol and micronized progesterone vs oral estrogen therapies and synthetic progestins.
Further study of selective estrogen receptor modulator (SERM) therapies alone or in combination (eg, CEE with bazedoxifene) could expand therapeutic and preventive strategies for aging women for whom available estrogen and progestogen therapies may no longer be tolerated or appropriate. The impact of FSH-blocking agents on bone health and other outcomes should be examined.
Novel investigational techniques proposed to preserve or revitalize ovarian function—derivation of oocytes from stem cells (141); ovarian transplantation of mesenchymal stem cells from amniotic membrane, umbilical cord, placenta, human menstrual blood, adipose tissue, and bone marrow; intra-ovarian injection of autologous platelet-rich plasma; and in vitro activation of dormant primordial follicles (142)—merit additional study. Investigational approaches to maintain “ovarian fitness” and promote reproductive longevity include dietary restriction, rapamycin, metformin, resveratrol, and melatonin administration (143, 144).
Testicular Axis
Natural History/Observational Data in Older Individuals
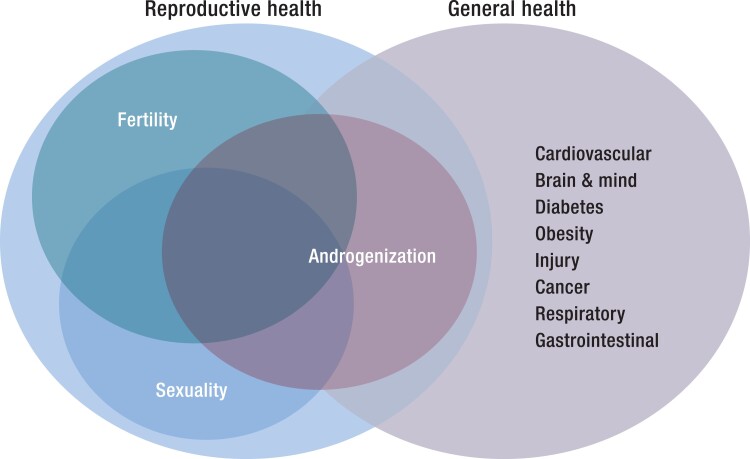
The 3 key dimensions of male reproductive health—fertility, sexuality, and androgenization—all interact with male general health, with the largest overlap with androgenization (Fig. 4).
Figure 4.
Overlap of the 3 dimensions of men's reproductive health—fertility, sexuality and androgenization—with general health. There is overlap of all dimensions but greatest for androgenization.
Biology of testicular aging
The twin functions of the testis—spermatogenesis to produce spermatozoa that can fertilize an oocyte and steroidogenesis to produce the bioactive androgens testosterone and dihydrotestosterone—are both impacted by aging, with effects mediated mainly by accumulation of aging comorbidities rather than aging itself. Hence, reproductive function of the healthiest of men remains largely undiminished throughout life, unless disrupted by intercurrent disease, a natural history differing starkly from female reproductive aging where an intrinsic, abrupt loss of ovarian function occurs at the midpoint of life for modern women.
Testosterone is necessary for reproduction (to make and deliver sperm) but not for life itself (as complete androgen insensitivity resulting from a genetic defect in XY individuals allows for a healthy but infertile life as a phenotypic woman). Uniquely among major human hormones, there is no naturally occurring excess testosterone syndrome in men, possibly reflecting the evolutionary role of the dramatic surge in androgens during male puberty required for species propagation. Testosterone is produced by all steroidogenic organs (testis, ovary, adrenal, placenta) and, while present in the circulation of all humans, blood testosterone displays a marked sexual dichotomy, with testicular secretion of 20 times more testosterone after puberty than is produced from non-testicular sources in children and women.
Male fertility
Paternity requires producing mature, fertile spermatozoa that are delivered by male sexual function to the female reproductive tract. After spermatogenesis is initiated at puberty, it is minimally affected by aging unless impacted by gonadotoxic chemicals or ionizing radiation (to which it is exquisitely sensitive) or severe withdrawal of gonadotropin drive essential to maintain the intratesticular androgen milieu required for completion of meiosis. Hence, on average the fertility of older men, either naturally or via in vitro fertilization, is only modestly diminished by reduced sperm output and motility (145, 146) so that paternity at advanced age is well known (147). However, unexplained impairment of sperm production in otherwise healthy men, the most frequent cause of male infertility, remains an important research challenge for both younger and older men (148). Modern genetics has still more to reveal about the heritable origins of spermatogenic failure and sperm (dys)function through genetic (149) and epigenetic (150, 151) mechanisms. Insight into acquired (nongenetic) causes of reproductive failure has, however, advanced only minimally. Data have been inconclusive about whether there is a secular trend for diminished human sperm production (152), due to potential bias from low participation of healthy, non-infertile men (153), whereas excellent animal studies are clearly negative (154). Many possibly damaging environmental impacts on spermatogenesis, from prenatal to adult life, are proposed but remain speculative (155).
Genetic risk of older fathers
Male aging has modest but significant effects of increasing the very low absolute risk of some rare autosomal dominant genetic disorders (eg, achondroplasia, Apert syndrome, Noonan syndrome, and Costello syndrome), genetic mutations, chromosomal defects, and epigenetic changes (147), as well as neuropsychiatric disorders (156). These paternal age effects, arising from cumulative de novo DNA copying errors during hundreds of rounds of mitotic and meiotic replication during spermatogenesis over a man's lifetime, can become entrenched in the genome through selection of mutations that enhance proliferation of their own spermatagonial clone over others (157); however, their low prevalence makes them difficult to fully disentangle from more potent overlaid teratogenic effects of female aging and pregnancy. Further insight into the testicular origins of paternal age effects on reproductive outcomes (158) is highly desirable given the increasing rates of older men fathering children both naturally and via in vitro fertilization after remarriage to younger women.
Sexual function in male aging
Male sexual function operates as a hydraulic neurovascular mechanism subserving erection and culminating in an autonomic neural reflex for ejaculation. Although initiation of adult male sexual function at puberty requires adult male blood testosterone exposure, maintenance of men's sexual function requires only a low blood testosterone threshold. Hence erectile dysfunction (ED), the most prevalent male sexual dysfunction, which is steeply age dependent, is both associated with age-related comorbidities and predicts future cardiovascular events (159). However, ED is rarely due to androgen deficiency when it is part of a pathologic form of hypogonadism. Furthermore, in a longitudinal cohort study, reduced sexual activity from any cause (drugs, depression, organic ED) was associated with decreases in blood testosterone concentrations (160), whereas concentrations increased with increased sexual activity (161). This overlooked observation often leads to confusing mildly reduced blood testosterone as the cause rather than the effect of reduced sexual activity, a major contributor to the excess of unjustified testosterone prescribing over recent decades (162). As a sound alternative, the safety and efficacy of phosphodiesterase type 5 inhibitors for ED in older men is now well established for many underlying medical causes of ED, subject to avoidance of adverse drug interactions such as with nitrates (163).
Testosterone measurement
Analytical research into the impact of male aging on reproductive and general health depends crucially on accurate measurement of testosterone and its bioactive metabolites dihydrotestosterone and estradiol (as well as ideally precursors and other metabolites). For this purpose, steroid liquid chromatography–mass spectrometry (LC-MS) can provide accurate, multi-analyte profiles, allowing for a dynamic picture of net androgen action. However, although steroid LC-MS is now dominant in clinical research as the steroid immunoassay era draws to a close, affordability and general availability of steroid LC-MS methods in clinical practice remains challenging. This is due to commercial lock-in of pathology laboratories to multiplex immunoassay platforms in which steroid analytes remain a minor component but provide quick, inexpensive, albeit often inaccurate results. Laboratory measurements of testosterone fractions (“free,” “bioavailable”) are technically demanding, laborious manual methods which remain unstandardized and lack reference standards, quality control, or reference ranges (164). Consequently, lab measurements of derived fractions of blood testosterone are rarely available and are replaced by inaccurate calculational formulas. These formulas are inevitably a deterministic (inverse) function of age (165) but empirically add no significant prognostic information to accurate LC-MS testosterone measurements (166). The circadian and ultradian pattern of testosterone release should also be considered in interpretation of testosterone measurements.
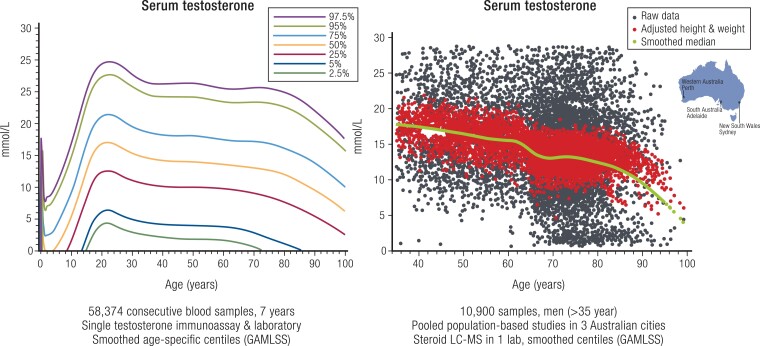
LC-MS measurement of testosterone and related steroids in population-based studies is supplanting immunoassay use in determining the natural history of blood testosterone levels in male aging (167-171). Whereas immunoassay studies reported a gradual, modest, but inconsistent decline in testosterone levels with age among Western men (Fig. 5), recent evidence shows no age-related changes in Japanese (172) or Chinese (173) men, nor in LC-MS data from pooled Western studies (174). These studies highlight lifestyle confounders of the age-related reduction in blood testosterone, notably overweight/obesity, insulin resistance or diabetes, smoking, cardiovascular disease, and depression (175-177), which explain most or all apparent age-related reductions in serum testosterone. There is inadequate research on whether testosterone improves these comorbidities of aging. In addition, there are interesting speculations based on limited interventional (178), observational (179), and mechanistic (180) studies suggesting androgen effects on telomerase as a potential hormonal influence on an underlying mechanism of aging.
Figure 5.
Age-specific profile of serum testosterone in men. Left panel comprises 58 374 consecutive serum samples over 7 years measured in a single immunoassay and pathology laboratory with population centiles deduced by smoothed GAMLSS methodology. Right panel comprises 10 900 serum samples pooled from 3 population-based Australian studies showing the raw scatter (black dots), height and weight-adjusted scatter (red dots), and the smoothed median (green solid line) deduced by GAMLSS methodology. Redrawn and adapted from Handelsman DJ et al Ann Clin Biochem, 2015; 53(3):377-385, © SAGE Publications (left), and redrawn from Handelsman DJ et al (168), © 2015 European Society of Endocrinology (right).
Although the sole unequivocal indication for testosterone treatment is for replacement therapy in men with pathological reproductive disorders, there is strong public interest in extending the use of testosterone outside endocrine disorders, notably for rejuvenation, an application with a deep aspirational history throughout human civilization long preceding modern endocrinology. The modern embodiment of this prescientific belief in testosterone as the pivot of male sexual, reproductive, and general rejuvenation was the re-emergence as “andropause” over the turn of the 21st century (181). That wishful thinking underlies the 100-fold increases in global pharmaceutical testosterone sales over 3 decades (182), including 10-fold increases in the United States and 40-fold in Canada over the first decade of the 21st century (162), in the absence of any new approved indications for testosterone treatment. An important public health challenge is to evaluate the impact of this decades-long epidemic of testosterone prescribing, possibly abating recently (183, 184), on underlying rates of cardiovascular and prostate diseases. Both of these diseases have displayed significant temporal changes over recent decades, which makes discerning an overlaid impact of changes in testosterone administration challenging.
Available Therapies
While numerous testosterone products are approved for oral, transdermal, injectable, or implantable (and in some countries, buccal and intranasal) administration to men with pathologic hypogonadism (185), none are approved for use in male aging. In men of any age without contraindications (nitrate vasodilators) or CYP3A drug interaction, phosphodiesterase type 5 inhibitors (sildenafil, tadalafil, and congeners) are highly effective and well tolerated for improving erectile function (186). Urinary human chorionic gonadotropin (hCG) is approved for treatment of gonadotropin-deficient male infertility but has little applicability to male aging where the predominant testicular defect is intrinsic Leydig cell failure, and hCG does not achieve sustained benefits. Likewise, clomiphene and aromatase inhibitors should not be used to increase endogenous testosterone due to their adverse effects on estrogen-dependent male sexual function and bone density.
Clinical Trial Data on Efficacy and Safety in Older Individuals
Based on testosterone's prominent effects on muscle structure and function, placebo-controlled interventional studies investigating potential effects of testosterone aiming to reverse age-related muscle loss (sarcopenia) or weakness (frailty) have been conducted. However, these studies have produced inconsistent and/or inconclusive findings, largely due to relatively small sample sizes (vs small magnitude of benefits) and heterogeneity of study cohorts and endpoints. Salutary findings were produced by the Testosterone in Older Men with Mobility Limitations (TOM) trial in which 209 men aged 65 years or over (average 74 years) with a high prevalence of obesity, hypertension, diabetes, and hyperlipidemia were treated with daily transdermal testosterone or placebo gel for 6 months; however, the study was terminated prematurely for an excess of cardiovascular adverse effects (187). Analogous studies of testosterone treatment in frail and/or sarcopenic older men also had minor benefits but without these adverse cardiovascular effects (188-190).
The 1994 Institute of Medicine (IOM, now National Academy of Medicine) review of male aging concluded there was insufficient efficacy evidence to justify a large, placebo-controlled RCT of testosterone for an age-related reduction in blood testosterone in men without reproductive pathology. They recommended short-term efficacy studies to justify a costly, large-scale trial. Subsequently, the National Institutes of Health (NIH)-funded Testosterone Trials, a series of 7 well-integrated, overlapping RCTs involving daily transdermal testosterone or placebo gel for 12 months were conducted. These studies recruited 790 men aged 65 years and older who had consistently low morning serum testosterone (<9.5 nmol/L) and a high prevalence of obesity (63%), hypertension (72%), diabetes (37%), and current or former smoking (66%) (191). The key findings were a modest but transient benefit for sexual function, small and expected increases in hemoglobin and bone density, but no benefits for vitality or physical or cognitive function (192). Findings also included adverse effects of testosterone on erythrocytosis and an increase of noncalcified coronary plaque size (192-194). Although the T Trials were not powered to detect cardiovascular endpoints, this latter safety signal needs evaluation, given the widescale usage of off-label testosterone in older men.
An adequately powered long-term safety study is needed to determine whether testosterone treatment of older men without reproductive pathology causes adverse cardiovascular or prostate events. Although the Testosterone Trials failed to meet the IOM mandate for a public sector placebo-controlled efficacy study, a large-scale, long-term industry-funded FDA-mandated safety study (TRAVERSE) is underway aiming to define the cardiovascular safety of testosterone treatment of men with age-related low blood testosterone in the absence of reproductive pathology (195). In the interim, numerous meta-analyses aggregating smaller, shorter-term RCTs report inconsistent and inconclusive evidence for cardiovascular effects (196-198), largely due to underpowering (especially exposure duration), failure to recognize transient adverse effects (196, 199), and industry source funding bias (200). In the T4DM study, 1007 men with impaired glucose tolerance were randomized to injectable testosterone undecanoate (1000 mg) or placebo every 3 months for 2 years, with a reduction in the incidence of diabetes along with an unacceptably high rate of erythrocytosis (22%) (201), together with a slow recovery of testicular endocrine function of at least 12 months (202).
Furthermore, the consequences of testosterone treatment on late-life prostate diseases, including cancer and hyperplasia, require elucidation. While strong evidence exists against any predictive relationship between endogenous testosterone and its metabolites with future diagnosis of prostate cancer over the following decade (203, 204), and there is no evidence of increased prostate disease in meta-analysis of short-term trials of testosterone treatment (205), more powerful RCT evidence is required before the risk of exogenous testosterone administration accelerating late-life prostate diseases can be considered dispelled.
Key Points
Spermatogenesis and steroidogenesis are both negatively impacted by comorbidities associated with aging rather than aging itself.
ED is rarely due to androgen deficiency. Phosphodiesterase type 5 inhibitors are an effective treatment for older men with ED.
Use of steroid immunoassays for measurement of testosterone rather than the preferred LC-MS assays may result in inappropriate diagnosis of low testosterone levels.
The Testosterone Trials showed modest but transient benefits in testosterone treatment for sexual function, small and expected increases in hemoglobin and bone density, but no benefits for vitality or physical or cognitive function and an adverse effect of testosterone to increase noncalcified coronary plaque size. These data do not support the use of testosterone to treat these comorbidities of older men.
A large safety study (TRAVERSE) is underway to evaluate the cardiovascular events during 5 years of daily testosterone vs placebo gel treatment.
Gaps in the Research
Given the lack of convincing efficacy and uncertain safety of testosterone administration to aging men without reproductive pathology, future clinical research on testosterone treatment should focus primarily on whether testosterone administration improves the comorbidities of aging and/or has direct effects on putative underlying mechanisms of aging, and at what threshold of testosterone level. The potential adverse effects of long-term testosterone administration on cardiovascular and prostate diseases in such men also require additional research. Additionally, in the absence of any natural disorders of excessive testosterone secretion in men, possibly reflecting the evolutionary tolerance for sharp increases in testosterone secretion during male puberty, careful exploration of the efficacy and safety of short-term, higher doses of testosterone or other natural nonaromatizable androgens (eg, DHT, nonsteroidal androgens) for specific aging comorbidities may be warranted.
While clinical therapeutics will always require adequately powered, placebo-controlled study of natural or synthetic androgens, analytical research into cellular and molecular mechanisms of androgen action in key target tissues (muscle, liver, erythroid cell lineages, bone, prostate, skin, brain) are needed to identify targeted paracrine or intermediary modulators of androgen action, which could point the way to gaining the benefits of target-specific androgen action while avoiding detrimental off-target effects. Further analytical research is also needed to understand the testicular origins of paternal age effects on reproductive outcomes and on the preservation of testicular function.
Thyroid Axis
Natural History/Observational Data in Older Individuals
Clearance of circulating thyroxine (T4) and triiodothyronine (T3) declines with age, resulting in an increase in half-life from 7 days in younger individuals to 9 days in those aged 80 years and older (206). There is a compensatory reduction in the production of T4 and T3. Production of T4 declines from 80 μg to 60 μg daily and production of T3 declines from 30 μg to 20 μg daily (207). In euthyroid individuals with thyrotropin (thyroid-stimulating hormone [TSH]) and free T4 concentrations within the reference range, T3 concentrations are lower in community-dwelling older individuals without acute illness than in younger individuals, suggesting an age-related decline in 5′-deiodinase activity (208, 209).
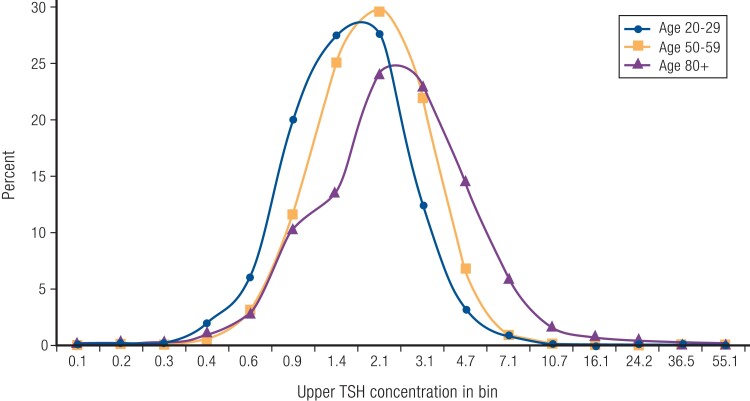
Both cross-sectional and longitudinal studies have shown an increase in TSH concentrations with age, even when limiting to a reference population of individuals without thyroid disease or anti-thyroid antibodies, without any changes in free T4 concentrations (208, 210, 211). The shape of the TSH distribution suggests a population shift to higher levels rather than increased incidence of hypothyroidism at older ages (Fig. 6) (210). Accordingly, a TSH value above the reference range is found in 14.5% of those aged 80 years and older, compared with 2.5% of those aged 20 to 29 years (210). The prevalence of anti-thyroid antibodies also increases with age, particularly in women, consistent with an age-related increase in autoimmune thyroid disease (210). However, anti-thyroid antibody levels are lower in the oldest old (209).
Figure 6.
Distribution of TSH concentrations in a reference population from the National Health and Nutrition Examination Survey. Redrawn from Surks MI & Hollowell JG (210). © Endocrine Society.
The majority of older individuals with elevated TSH concentrations have normal free T4 concentrations, a combination of thyroid test results known as subclinical hypothyroidism. It should be noted that subclinical hypothyroidism persists on repeat testing in only 38% of older individuals, with reversion to euthyroidism in the remaining 62% (212). Subclinical hypothyroidism is not associated with an increase in risk of CHD, stroke, heart failure, dementia, disability, or mortality, overall or in the subgroup of individuals with TSH concentrations of <7 mIU/L (213-217). Furthermore, older individuals with subclinical hypothyroidism may have better mobility and functional status than their euthyroid peers (218, 219). Observational data have shown an increased risk of cardiovascular mortality and stroke in subgroups of patients with subclinical hypothyroidism with TSH levels of 7 to 9.9 mIU/L and of CHD, cardiovascular mortality, and heart failure for TSH ≥10 mIU/L (213, 214, 217). Clinical data do not suggest that levothyroxine treatment reduces the risk of cardiovascular events in older patients with subclinical hypothyroidism (220, 221). Furthermore, overtreatment with levothyroxine to TSH concentrations below the reference range is common in older individuals (222).
Subclinical hyperthyroidism—low TSH concentrations with normal concentrations of free T4—is more common in older than in younger individuals due to an increase in autonomous thyroid hormone secretion from thyroid nodules. Subclinical hyperthyroidism is associated with an increased risk of atrial fibrillation, hip fracture, and dementia if left untreated (223-225). Even patients with low, but not suppressed, TSH levels (TSH 0.1-0.44 mIU/L) are at increased risk of atrial fibrillation, CHD, and hip fracture (223, 224). Because older patients have a high baseline risk of these outcomes, subclinical hyperthyroidism is more likely to have clinically meaningful effects in these patients. Furthermore, in euthyroid older patients, free T4 concentrations within the reference range are associated with increased risk of atrial fibrillation, CHD, heart failure, dementia, and mortality (226, 227). These data support a potential role of free T4 concentrations in identifying increased risk of adverse events, independent of TSH concentrations.
Overt hypothyroidism and hyperthyroidism each are more common in older individuals, as are comorbid conditions or medications that affect thyroid function (228, 229). Recognition of overt thyroid dysfunction can be challenging; the classic symptoms of hypothyroidism and hyperthyroidism are reported less frequently in older patients than in younger patients with a similar degree of thyroid dysfunction (230-232). Clinicians may fail to identify common age-related symptoms and syndromes, such as fatigue, depression, cognitive decline, constipation, and falls as related to thyroid dysfunction. In addition, older patients with hyperthyroidism are more likely to have atypical symptoms, such as apathy and anorexia, and less commonly have hyperadrenergic symptoms (231).
Available Therapies
Treatment of both hyperthyroidism and hypothyroidism should take into account the underlying health status of the patient, particularly underlying cardiovascular comorbidities. Levothyroxine is the primary treatment for thyroid insufficiency. Levothyroxine doses in older individuals correlate with total lean body mass and renal function, leading to lower requirements at the time of diagnosis and increased risk of overtreatment (202). Patients with longstanding levothyroxine use may require a dose reduction over time (201). In addition, multiple over-the-counter and prescription medications affect absorption, protein binding, or metabolism of levothyroxine (233). Three options are available for management of an overactive thyroid: antithyroid medication, radioactive iodine, and thyroidectomy.
Clinical Trial Data on Efficacy and Safety in Older Individuals
There have been 2 RCTs of treatment of subclinical hypothyroidism in older individuals, one with 737 adults aged 65 years and older and the second with 105 adults aged 80 years and older (212, 234). Data from individuals aged 80 years and older from the first trial (n = 146) were merged with data from the second trial for analysis. Both RCTs were conducted in participants with persistent subclinical hypothyroidism who were randomized to levothyroxine or placebo and followed for 12 months. The primary outcome was improvement in hypothyroid symptoms or tiredness, with additional secondary outcomes of quality of life, hand-grip strength, cognitive function, blood pressure, weight, waist circumference, and activities of daily living. No benefit was found in either trial of a low dose of levothyroxine (mean dose 50 mcg daily) compared with placebo, as well as no increase in risk. These trials were not adequately powered to examine cardiovascular or other events, nor were they powered to examine subgroups of TSH at 7 to 9.9 mIU/L or 10 to 19.9 mIU/L that observational data suggested were at higher risk of adverse events. Participants enrolled in both trials showed a low thyroid symptom burden, leaving residual questions about management of patients with symptoms of hypothyroidism.
There have been no trials of similar size in older patients with subclinical hyperthyroidism, and the management is based on thresholds established from observational data.
Key Points
A TSH concentration above the reference range in conjunction with a normal free T4 concentration is common in older individuals. Isolated T3 concentrations below the reference range are also common in this age group.
Older individuals with persistent subclinical hypothyroidism with TSH concentrations of <7 mIU/L should not be treated with levothyroxine. This recommendation is based on RCT data.
Whether or not subgroups of older individuals with persistent subclinical hypothyroidism who have TSH concentration of ≥7 mIU/L or significant symptoms should be treated with levothyroxine is debated.
TSH thresholds for treatment of subclinical hyperthyroidism have been established from observational data, but these treatment thresholds and optimal management have not been tested in RCTs.
Gaps in the Research
The etiology of age-associated changes in thyroid function testing is not known. The Centers for Disease Control and Prevention Clinical Standardization program has created a standardization program for free T4 based on the International Federation of Clinical Chemistry and Laboratory Medicine reference system and is standardizing free T4 and harmonizing TSH testing globally. These efforts represent an important step toward establishing whether age-based reference ranges are needed for diagnosis and management of thyroid dysfunction. Potential causes of TSH elevation such as a decrease in the bioactivity of TSH or diminished response of the thyroid to TSH are untested. Whether the age-associated effect on T4 to T3 conversion is persistent and is due to declines in deiodinase activity in older individuals requires further study. In addition, methods to distinguish between age-associated adaptive changes in thyroid function and early hypothyroidism are needed.
RCT data are needed to assess the risks and benefits of treatment of older individuals with subclinical hypothyroidism with symptoms or higher TSH levels and with subclinical hyperthyroidism. RCT data are also needed in patients with subclinical thyroid dysfunction and pre-existing cardiovascular disease or cognitive impairment. Whether the target TSH range for treated thyroid dysfunction should be the same as the range used to define thyroid dysfunction in an older individual also requires evaluation. Additional study of the clinical importance of free T4 measurement in euthyroid older individuals is needed.
Osteoporosis
Natural History/Observational Data in Older Individuals
Osteoporosis is a chronic skeletal disorder resulting from progressive bone loss after menopause in women and with advancing age in both men and women (235). This bone loss gradually disrupts bone microarchitecture, impairing bone strength and predisposing to fracture. Patients at high risk of fracture can be readily identified, effective strategies for reducing fracture risk are available, and evidence-based guidelines for managing osteoporosis have been published (235-237).
The prevalence of osteoporosis, defined as bone mineral density (BMD) T-score of ≤ −2.5 at the lumbar spine or femoral neck, increases from 6.8% in women aged 50 to 59 years, to 25.7% for those aged 70 to 79 years, and to 34.9% in women aged 80 years and older (238). Osteoporosis is present in 5% of men aged 70 to 79 years and 10.9% of men ≥80 years. In addition, about half of adults aged 70 years and older have low bone density, which, in combination with other risk factors, conveys high fracture risk.
Rates and severity of fractures increase exponentially with age; vertebral (spine) and hip fractures account for 24% of all fractures in women aged 60 to 69 years, but account for 67% in the larger number of women aged 80 years and older with fractures (239) (Fig. 7). About half of women and 20% of men will experience a fracture related to osteoporosis in their lifetime; two-thirds of these fractures occur after age 75 (240). More than 2 million osteoporotic fractures occur each year in the United States, including 700 000 vertebral (spine) fractures and 300 000 hip fractures, resulting in more than 500 000 hospital admissions (241).
Figure 7.
Prevalence of hip, spine, and all fractures in women by decade of age in the DUBBO study. The combination of hip and spine fractures comprised 24% of all fractures between ages 60 and 69, 44% between ages 70 and 79 years, and 67% in those 80 years and older. Redrawn and adapted Center JR et al (239). © Elsevier Ltd.
Both hip and vertebral fractures are associated with substantial morbidity and mortality (242-245). Hip fractures, occurring on average at age 82, are associated with higher health care cost and disability than all other fracture types combined (246). Despite this knowledge and availability of effective treatments, most older patients with fractures do not receive osteoporosis therapy. Fewer than 15% of Medicare patients (average age 80.9 years) began osteoporosis therapy in the year following a fracture, > 60% of which were hip or spine fractures (247). In the United States, age-adjusted rates of hip fracture began decreasing after 1997, but recent data suggest that those rates are increasing again due to widening of a treatment gap (248).
Most fractures occur after a fall. Osteoporosis and sarcopenia, a risk factor for falls, frequently occur together in older adults (249). At least a third of women aged 65 or older experience a fall each year, with the risk of falls increasing with advancing age (250).
Important interplays exist among the strongest risk factors for fracture: advanced age, low BMD and a history of prior fracture or fall. Older women are at higher risk than are younger women with the same T-score and can be at high fracture risk without low BMD (251) (Fig. 8). A history of previous fracture results in a doubling of future fracture risk, and this risk is especially high in the first 2 years after an incident fracture (252). Additionally, the subsequent fracture in older adults is more likely to be a serious fracture (253).
Figure 8.
Relationship between age and hip fracture risk in women with femoral neck T-score values of < −1 and < −2.5. Redrawn and adapted from Kanis JA et al (251). © International Osteoporosis Foundation and National Osteoporosis Foundation.
Societal guidelines and the US Preventive Services Task Force (USPSTF) recommend BMD testing for all women aged 65 and older (254-256). BMD testing in men has been suggested to begin at age 70 (257). Evaluation for secondary causes of osteoporosis is warranted, including endogenous and exogenous Cushing syndrome, male hypogonadism, clinical hyperthyroidism, and severe vitamin D deficiency. The BMD result can be combined with other risk factors in FRAX™, a validated fracture risk algorithm, to estimate fracture probability in individual patients (258). FRAX underestimates fracture risk in patients with recent fractures or falls.
Available Therapies
Therapy to reduce fracture risk begins by minimizing risk factors and with general measures including good nutrition, avoidance of smoking, and regular physical activity. Multidisciplinary approaches to fall risk prevention—including exercises to promote strength and balance, correcting visual deficits, avoiding or minimizing medications such as thiazide diuretics, sedatives, and alpha blockers that are associated with fall risk, removing risks in the home, and appropriate use of assistive devices—can reduce fall risk, but none of these approaches have been evaluated in large enough or long enough studies to demonstrate reduction in fracture risk. These general measures and fall prevention strategies are recommended for all older adults to promote bone health as well as general health, with pharmacological therapy reserved for patients at high risk of fracture (235).
Multiple drugs with varying mechanisms of action are government-approved for treating osteoporosis (237) (Table 3). Each approved drug reduces vertebral fracture risk in postmenopausal women with osteoporosis, and all drugs except raloxifene and ibandronate reduce nonvertebral fracture risk. Hip fracture risk reduction has been demonstrated with alendronate, risedronate, zoledronate, denosumab, and romosozumab. Anti-remodeling agents reduce bone turnover and increase BMD and strength but do not repair the microarchitectural damage of osteoporosis. Osteoanabolic or bone-building agents increase bone formation and improve trabecular architecture. Osteoanabolic agents are more effective than oral bisphosphonates at improving BMD and reducing fracture risk in older adults (259). Bone-forming drugs are recommended for patients at very high fracture risk (T-score of ≤ −3.0 in the absence of fragility fracture, T-score of ≤ −2.5 plus a fragility fracture, severe or multiple vertebral fractures) (236, 256, 260). Details about the efficacy, safety and use of individual drugs are provided in an Endocrine Society Clinical Practice Guideline and its Guideline Update (235, 236).
Table 3.
Drugs approved in United States for treating osteoporosis
| Drug | Drug class | Dose, route of administration and dosing interval | Approved for treating men with osteoporosis | Fracture risk reduction (in primary analyses of registration trials) | Subgroup analysis of older study participants | ||
|---|---|---|---|---|---|---|---|
| Vertebral fracture | Nonvertebral fracture | Hip fracture | |||||
| Raloxifene | EAA | 60 mg po daily | ✓ | ||||
| Alendronate | bisphosphonate | 70 mg po once weekly | ✓ | ✓ | ✓ | ✓ | |
| Risedronate | bisphosphonate | 35 mg po once weekly or 150 mg po once monthly | ✓ | ✓ | ✓ | ✓ | ✓ |
| Ibandronate | bisphosphonate | 150 mg po once monthly or 3 mg IV every 3 months | ✓ | ||||
| Zoledronate | bisphosphonate | 5 mg IV every year | ✓ | ✓ | ✓ | ✓ | ✓ |
| Denosumab | RANK ligand inhibitor | 60 mg SQ once every 6 months | ✓ | ✓ | ✓ | ✓ | ✓ |
| Teriparatide | PTH receptor agonist | 20 mcg SQ daily | ✓ | ✓ | ✓ | ✓ | |
| Abaloparatide | PTH receptor agonist | 80 mcg SQ daily | ✓ | ✓ | ✓ | ||
| Romosozumab | sclerostin inhibitor | 210 mg SQ once monthly | ✓ | ✓ | ✓ | ||
| Calcitonin-salmon | calcitonin | 200 USP units by nasal spray daily | ✓ | ||||
Abbreviations: EAA, estrogen agonist/antagonist; IV, intravenous; PTH, parathyroid hormone; SQ, subcutaneous.
Recent data demonstrate a strong relationship between treatment-associated changes in BMD and fracture risk reduction (261). This has led to an emerging concept of goal-directed therapy using total hip BMD as a “target” informing the choice of initial therapy and decisions about subsequent therapies (262).
Raloxifene, an estrogen agonist/antagonist, is a weak anti-remodeling agent that reduces the risk of vertebral but not other fractures.
Calcitonin-salmon is a weak inhibitor of bone resorption that may reduce vertebral fracture risk. Because of a possible cancer risk associated with calcitonin-salmon therapy, this drug is no longer approved in Europe. Short-term therapy may be considered for pain relief following an acute vertebral fracture (263).
Bisphosphonates are the most commonly used drugs for osteoporosis treatment. Except for ibandronate, the approved bisphosphonates reduce risks of vertebral, nonvertebral, and hip fracture. While osteonecrosis of the jaw and femoral shaft fractures with atypical features have been described with long-term bisphosphonate therapy, the benefit/risk profile remains favorable for up to 10 years in patients at high fracture risk. However, bisphosphonate use beyond 5 years does not result in additional BMD increase or fracture risk reduction. Guidelines recommend re-evaluating fracture risk after 3 to 5 years of bisphosphonate therapy. For patients who are no longer at high risk of fracture and who no longer meet criteria for treatment, interruption of therapy can be considered until the patient again meets treatment criteria (235). For patients remaining at high risk of fracture after 5 years of bisphosphonate treatment, switching to denosumab or one of the bone-building agents could be considered.
Denosumab is a human monoclonal antibody administered subcutaneously every 6 months that reduces risks of vertebral, nonvertebral, and hip fracture. Progressive increases in BMD, maintenance of or improved fracture risk reduction, and no major safety issues were seen over 10 years of therapy. While there is no limit on the duration of denosumab therapy, discontinuation of therapy results in a rebound in bone turnover markers, rapid loss of BMD and vertebral fracture protection, and increased risk of multiple fractures. Alendronate or zoledronate should be given whenever denosumab is discontinued to mitigate these effects (264).
Teriparatide and abaloparatide are parathyroid hormone receptor agonists that activate bone formation and, to a lesser extent, bone resorption. Both drugs have been demonstrated to reduce vertebral and nonvertebral fractures, but neither was shown to reduce hip fracture risk in the pivotal clinical trials that were not designed to evaluate that outcome. These drugs are administered by daily subcutaneous injection, usually for 18 to 24 months because their anabolic effects diminish with longer use. Potent anti-remodeling agents given after a course of these agents are recommended to maintain the skeletal benefits.
Romosozumab, an anti-sclerostin antibody that activates bone formation while inhibiting bone resorption, is administered by subcutaneous injection once monthly for 12 months, followed by either a bisphosphonate or denosumab. These regimens induce larger increases in BMD and greater reduction in fracture risk within 12 months when compared to placebo and to alendronate. Increased cardiovascular risk was observed compared to alendronate but not to placebo (265).
Clinical Trial Data on Efficacy and Safety in Older Individuals
The IOM, now the National Academy of Medicine, recommends a total daily intake of calcium of 1200 mg for older adults, based on inconsistent data (266). Higher daily calcium intakes are not beneficial and may be harmful. The role of vitamin D supplementation in older adults is even less certain and is discussed in detail in the following section. Based on available evidence, 1 to 1.2 g protein/kg body weight per day is recommended for older adults (267). High protein intake may slow muscle loss and reduce fall frequency (268).
Weight-bearing exercises do not generally increase BMD in older adults, whereas a regular walking program may attenuate bone loss in sedentary older adults (269). Multicomponent exercise programs targeting balance, gait, and muscle strength reduce the frequency of falls and possibly fractures in older people (250). Correcting cataracts and limiting the use of neuroactive sedative drugs reduces fall risk. Hip protectors may be considered in patients at high risk for falling, especially for patients in supervised settings (270). The Centers for Disease Control and Prevention has provided useful tools for fall risk assessment and management, based on published guidelines (271). For older patients who have experienced fractures, individualized rehabilitation programs are helpful (272, 273). Back strengthening exercises improve symptoms in patients with vertebral fractures and reduce subsequent fracture risk (274).
The average ages of participants in the pivotal fracture trials with drugs have been between 65 and 75 years; some have enrolled participants up to 100 years old. Subgroup analyses of responses to 3 bisphosphonates (alendronate, risedronate, and zoledronate), denosumab, teriparatide, and abaloparatide in subsets of older participants enrolled in the pivotal trials have been published (275-280). These analyses demonstrate that effectiveness, safety, and tolerability of therapies in the oldest subgroups are generally similar to responses in the entire study cohorts. Importantly for older patients, fracture risk reduction is evident as early as 6 months after beginning therapy. Specific issues relevant to the use of these drugs in older patients with osteoporosis are presented here.
Because neither raloxifene nor calcitonin-salmon reduce the risk of nonvertebral or hip fracture, they are not recommended for treating older patients with osteoporosis. Bisphosphonates should be used with caution in patients with significantly impaired renal function. When oral bisphosphonate use is difficult because of dosing rules and/or too many other medications, annual or biannual zoledronate infusion is an alternative (281). In a RCT in patients treated within 3 months of a hip fracture, average age 74, zoledronate reduced both fracture risk (35%) and mortality (28%) compared with placebo (282). The twice-yearly parenteral dosing of denosumab is an appealing option for older patients taking many oral medications. Denosumab can be used in patients with impaired renal function, but the risk of hypocalcemia is higher in those patients. Compared with placebo, denosumab reduced hip fracture risk by 62% in patients aged 75 and older (277). Teriparatide and abaloparatide may be associated with palpitations and postural hypotension and are not recommended in patients at increased risk for osteosarcoma, including those with a history of skeletal radiation. Patients at very high cardiovascular risk are not good candidates for romosozumab.
Key Points
Fractures related to osteoporosis are common and often serious problems in older individuals.
Older patients at high risk of fracture can be readily identified, especially those with a recent fracture.
Ensuring good nutrition and encouraging regular physical activity promotes bone health.
Drugs to reduce fracture risk are effective and well tolerated in older patients and should be considered in all older patients with osteoporosis, especially those with prior fracture.
Gaps in the Research
Fractures are often not recognized as being related to osteoporosis. As a result, most older patients with fracture are not treated for osteoporosis. Studies evaluating strategies to educate patients and clinicians about the importance of osteoporosis and the benefits of therapy would be helpful.
Studies are needed comparing the efficacy and safety of osteoporosis drugs, especially in older patients. None of the studies evaluating approaches to reducing the risk of falls have been designed to evaluate effects on fracture risk. Evaluation of the role of senolytic therapies to forestall effects of aging through selective induction of death of senescent cells should include skeletal outcomes.
Vitamin D
Natural History/Observational Data in Older Individuals
The activated form of vitamin D is a steroid hormone that controls several hundred genes (283, 284). It modulates a wide range of molecular and cellular functions, including immune functions, inflammation, cellular senescence, and telomere biology (284, 285). Vitamin D may play a dual role in aging, as a risk factor or marker of ill health, and as a possible therapeutic drug (286, 287).
Vitamin D physiology
Vitamin D is available in 2 major forms, ergocalciferol (vitamin D2) originating from plant sources or supplements, and cholecalciferol (vitamin D3), the animal form that represents its major (>90%) source. Cholecalciferol is synthesized in the epidermis, from 7-hydrocholesterol after exposure to short UV-B sunlight radiation (290-315 nm, Fig. 9). Both vitamin D3 and vitamin D2 are readily hydroxylated in the liver, in an unregulated, substrate-dependent pathway, leading to the most abundant circulating, but biologically inactive form, 25-hydroxyvitamin D (25(OH)D, calcifediol). Serum 25(OH)D circulates in serum bound to a specific, high affinity, transport protein, vitamin D–binding protein (VDBP), with relatively low free levels. Biological activity is conferred by 1 α-hydroxylation by the renal CY27B1 enzyme into 1,25-dihydroxy vitamin D [1,25(OH)2D3]. This step is tightly regulated by parathyroid hormone (PTH), and under negative feedback by calcium, phosphate, fibroblast growth factor 23 (FGF23), 1,25(OH)2 D3 itself, and to a lesser extent, calcitonin, GH/IGF-1, and leptin. Calcitriol is the ligand for the nuclear vitamin D receptor (VDR), and its high affinity for its receptor and much lower affinity for vitamin D–binding protein favors its selective nuclear uptake, whereas its precursor(s) remain in the bloodstream (288). 25(OH)D has the longest half-life, approximately 2 to 3 weeks, and is the best nutritional index of vitamin D. This prohormone can be inactivated (CYP24A1) or activated (CYP27B1) systemically or locally for autocrine/paracrine actions across organ systems (288).
Figure 9.
Vitamin D metabolism. Reprinted with permission from Fuleihan GEH et al (283). © American Society for Bone and Mineral Research.
The biological effects of 1,25(OH)2D are mediated through genomic effects via its nuclear receptor VDR, by forming a dimer with retinoid X receptor (RXR) and activating vitamin D response elements; and nongenomic effects via intracellular signaling pathways through putative plasma membrane receptors (288, 289). 1,25(OH)2D increases calcium absorption from the intestine through the genomic actions of 1,25(OH)2D3, an active, energy dependent, transcellular pathway, mostly in the duodenum and jejunum (290), and by a passive paracellular pathway. Other classical target organs for calcitriol are the skeleton and parathyroid glands. Calcitriol also modulates several other organ systems through autocrine and paracrine pathways.
Altered vitamin D metabolism with aging
Aging affects vitamin D metabolism at the level of several key organ systems (291-293). The large capacity of the skin to produce vitamin D3 decreases with aging, by an estimated 13% each decade (294, 295). Older individuals are, however, still able to increase their serum vitamin D3 in response to exposure to UVB (294). The age-related decrease in calcium absorption is multifactorial. It includes reductions in serum 25(OH)D levels, impaired 1α-hydroxylation to calcitriol from declining renal function, gut resistance to the effect of vitamin D, and postmenopausal reductions in estrogen levels (291, 292). Renal resistance to the parathyroid hormone stimulating effect on 1α-hydroxylase (CYP27B1), and fibroblast growth factor 23 suppression of this hydroxylase, are other possible factors. Animal studies have also shown enhanced degradation and decreased production of calcitriol and age-related decrements in VDR and in the renal calcium transporter TRPV5 (292). The contribution of an age-related decrease in VDR to impaired organ function (muscle, intestine) is, however, debatable (292).
Because of these age-related alterations in vitamin D metabolism, and lifestyle changes, vitamin D deficiency is highly prevalent in high-risk populations, namely older individuals. A plethora of systematic reviews and meta-analyses have scrutinized the impact of vitamin D on health and disease over the last 5 decades (286, 296). A few of the most recent and rigorous systematic reviews and trials that enroll more than 2000 participants are highlighted herein.
Associations of vitamin D and major health outcomes in older individuals
Significant and consistent inverse associations have been reported by many systematic review/meta-analyses between vitamin D and many major health outcomes.
Musculoskeletal
Vitamin D deficiency results in calcium malabsorption, secondary hyperparathyroidism, increased bone resorption, bone loss, and fractures (297). The evidence regarding vitamin D levels and muscle performance in older individuals, based on large cohort studies from the United States and Europe, is contradictory (298). A dose-response meta-analysis of individuals aged 62 to 79 years indicated that serum 25(OH)D levels are directly associated with the risk of frailty (299).
Cardiovascular and cerebrovascular
The Copenhagen City Heart Study revealed a stepwise increase in the risk of ischemic heart disease, myocardial infarction, and early death, with decreasing 25(OH)D levels, in individuals with a mean age of 57 years (56% women), after a 29-year follow-up period (300). A similar increase in the risk of ischemic stroke in the same cohort after 21 years was reported (301). Both findings were substantiated in meta-analyses inclusive of several major cohorts from Europe and the United States (300, 301).
Cancer
The most consistent relationship between serum 25(OH)D levels and cancers was for colorectal cancer, while no association could be detected for breast and prostate cancer (302, 303). The mean age in individual studies ranged between 30 and 76 years (302, 303).
Cognition
A meta-analysis of 26 observational studies of participants who were mostly older than 65 years revealed that low vitamin D was associated with worse cognitive performance and cognitive decline. Cross-sectional studies revealed a stronger effect compared with longitudinal studies (304).
Mortality
In an individual patient meta-analysis of 26 916 study participants from 8 independent prospective European cohort studies, median age 61.6 years, 58% females, with a 25(OH)D concentration of 21 ng/mL, and follow-up time of 10.5 years, 6802 of whom died, serum 25(OH)D was associated with overall mortality and cardiovascular mortality, but not cancer mortality (305).
Available Therapies
Treatment of vitamin D deficiency could be with cholecalciferol (Vitamin D3), the most widely used form, ergocalciferol (Vitamin D2), or calcifediol (25-hydroxycholecalciferol, that is 25(OH)D) (Fig. 9). Calcifediol may be faster and more potent than cholecalciferol, and D3 superior to D2, in terms of increasing serum 25(OH)D levels (306-308). These findings may be explained by differences in absorption, as well as assay differences in detecting D2, by many platform assays (309, 310). Serum 25(OH)D levels reached with equivalent doses of vitamin D, given as daily, weekly, or monthly to patients post hip fractures, were comparable (311). High doses given periodically may increase the risk of falls or fractures (312, 313). Therapy with an active oral vitamin D sterol such as calcitriol is required in patients with stage 3 or 4 chronic kidney disease (314).
Clinical Trial Data on Efficacy and Safety in Older Individuals
The negative associations between vitamin D and major disease outcomes from 290 prospective cohort studies contrast with null findings from 172 randomized trials, therefore suggesting that vitamin D may be a marker of ill health (286). Interestingly, centenarians have a high frequency of severe vitamin D deficiency, and yet live beyond their expected country longevity (315). We summarize results of most recent and rigorous meta-analyses and of large RCTs (Table 4).
Table 4.
Major placebo-controlled megatrialsa of vitamin D therapy and impact on major outcomes
| Trial | N | Baseline 25OHD ng/mL | Age years/gender | Doses & frequency | Median duration | Primary outcomes |
|---|---|---|---|---|---|---|
| Trivedi, 2003 (312) | 2686 | NA | 65-85; both | Monthly 100 000 IU oral vitamin D3 | 5 years | Vitamin D reduced any first fracture (RR = 0.78 [0.61-0.99]) and first hip, wrist or forearm, or vertebral fracture (RR = 0.67 [0.48-0.93]) and did not significantly reduce mortality (RR = 0.88 [0.74-1.06]). |
| RECORD Grant, 2005 (316) |
5292 | 15.2 ± 6.5 [n = 60] |
>70; both | Daily 800 IU oral vitamin D3 | 45 months | Vitamin D did not significantly reduce the incidence of new, low-trauma fractures (HR = 1.02 [0.88-1.19]). |
| WHI Jackson, 2006 (317) |
36 282 | NAb | 50-79; women | Daily 400 IU oral vitamin D3 | 7 years | Vitamin D with calcium did not significantly reduce hip fracture (HR = 0.88 [0.72-1.08]), clinical spine fracture (HR = 0.90 [0.74-1.10]), and total fractures (HR = 0.96 [0.91-1.02]). |
| CAPS Lappe, 2017 (318) |
2303 | 32.8 ± 10.5 | ≥55 women | Daily 2000 IU oral vitamin D3 | 4 years | Vitamin D did not reduce cancer incidence (difference of 1.69% [−0.06-3.46%]). |
| ViDa Study Scragg, 2017 (319) |
5110 | 26.5 ± 9 | 50-84; both | Monthly 100 000 IU oral vitamin D3 | 3.3 years | Vitamin D did not significantly reduce the primary endpoint of incident cardiovascular disease (HR = 1.02 [0.87-1.20]). |
| D2d Pittas, 2019 (320) |
2423 | 28.0 ± 10.2 | >30; both | Daily 4000 IU oral vitamin D3 | 2.5 years | Vitamin D did not significantly reduce the risk of diabetes among persons at high risk for type 2 diabetes (HR = 0.88 [0.75-1.04]). |
| VITAL Manson, 2019 (321) |
25 871 | 30.8 ± 10.0 [n = 15 787] |
Men ≥50 Women ≥55 |
Daily 2000 IU oral vitamin D3 | 5.3 years | Vitamin D did not significantly reduce the co-primary endpoints of any invasive cancer incidence (HR = 0.96 [0.88-1.06]) or major cardiovascular events (HR = 0.97 [0.85-1.12]). |
| DO-HEALTH Bischoff-Ferrari, 2020 (322) |
2157 | 22.4 ± 8.4 | ≥70; both | Daily 2000 IU oral vitamin D3 | 3 years | Vitamin D did not significantly reduce incident nonvertebral fractures, cognitive decline, or rate of infections, or improve physical performance or systolic and diastolic blood pressure. |
| D-Health Trial Neale, 2022 (323) |
21 315 | NAc | ≥60; both | Monthly 60 000 IU oral vitamin D3 | 5.7 years | Vitamin D did not significantly reduce mortality (HR = 1.04 [0.93-1.18]). |
| FIND Virtanen, 2022 (324) |
2495 | 29.9 ± 7.3 [n = 551] |
Men ≥60 Women ≥65 |
Daily 1600 IU or 3200 IU oral vitamin D3 | 5 years | Vitamin D did not significantly reduce the incidence of major cardiovascular events (HR = 0.90 [0.62-1.32]) or invasive cancer (HR = 1.04 [0.72-1.51]). |
Abbreviations: 25(OH)D, 25-hydroxyvitamin D (calcifediol); HR, hazard ratio; NA, not available; RR, relative risk.
Megatrials are trials that included ≥2000 study subjects.
Mean 25(OH)D in a nested case-control assessment was 18.42 ± 9.1 ng/mL for participants who had hip fracture and 19.39 ± 9.41 ng/mL among their controls (P = .17).
Predicted de-seasonalized serum 25(OH)D concentration [N (%)]: <50 [2562 (24.0)] Vitamin D group; [2638 (24.8)] placebo group ≥50 [8099 (76.0)] Vitamin D group; [8011 (75.2)] placebo group.
Fractures
In a recent umbrella review of meta-analyses of vitamin D RCTs, the only consistent significant findings were for calcium and vitamin D (Ca/D), not vitamin D alone, in reducing the risk of hip fractures, by 16% to 39%, in 8/13 meta-analyses, and of any fracture, by 5% to 26%, in 8/14 meta-analyses (325). Subgroup analyses by residential status suggested a reduction in hip fractures in 2 meta-analyses, and any fractures in 4 meta-analyses, but only with Ca/D, and in institutionalized but not community-dwelling adults. These findings were driven by 2 trials in older institutionalized vitamin D–deficient individuals (297, 326). These findings are also consistent with results of earlier systematic reviews demonstrating that older age and 25(OH)D levels <20 ng/mL may indeed be predictors of fracture reduction in response to vitamin D (327-329). Vitamin D, without calcium, did not have a beneficial effect on risk of fractures (296, 325, 326). Table 4 highlights 4 large vitamin D trials, including the Women’s Health Initiative, that did not show any beneficial effect of vitamin D on fracture reduction (312, 316, 317, 322). Only 2 trials were conducted exclusively in older subjects, and serum 25OHD levels were essentially not measured (3 trials) or had a mean above 20 ng/mL (1 trial) (Table 4). Recent analyses from the VITAL (Vitamin D and Omega-3 Trial) study did not show any beneficial effect of vitamin D3 on fracture reduction compared to placebo, in generally healthy midlife and older adults, who were not selected for vitamin D deficiency (330).
Falls
The US Preventive Services Task Force (USPSTF) systematic review assessed the impact of various interventions to prevent falls in 7500 older subjects recruited to 7 heterogeneous trials of vitamin D formulations (with or without calcium), with overall null findings (331). A Cochrane systematic review evaluated the effectiveness of various interventions in 159 RCTs inclusive of 79 193 predominantly older, community-dwelling women, and concluded that vitamin D supplements did not reduce falls in this population (332).
Cardiovascular diseases
Two meta-analyses, one of 11 trials inclusive of 50 252 individuals, and another of 10 trials mostly inclusive of 79 111 older women, did not reveal any effect of calcium or vitamin D supplementation on major cardiovascular events, myocardial infarction or stroke when compared to placebo (333, 334). These findings were corroborated by individual vitamin D trials, VITAL (Vitamin D and Omega-3 Trial) and VIDa (Vitamin D Assessment Study), that were not included in these meta-analyses (Table 4) (319, 321, 324).
Cognition
A Cochrane meta-analysis examined the effect of nutritional interventions on cognitive function, including vitamin D3 (400 IU/day) and calcium compared to placebo, and demonstrated no effect of vitamin D3 and calcium supplements, on overall cognitive function, at a follow-up interval of up to 10 years (335).
Cancer
A Cochrane systematic review/meta-analysis of 18 RCTs of more than 50 000 community-dwelling women aged 47 to 97 years revealed that vitamin D, administered for a weighted mean of 6 years, did not have any significant effect on cancer incidence (336). This is consistent with results from the 3 larger RCTs with cancer as the primary outcome (Table 4) (318, 321, 324).
Mortality
A systematic review inclusive of 172 randomized trials, consisting mostly of women living in institutions, concluded that supplementation in older people (mainly women) with 20 μg vitamin D per day seemed to slightly reduce all-cause mortality (286). A Cochrane meta-analysis of 56 randomized trials, with 95 286 participants, mostly women older than 70 years, revealed that vitamin D, administered over 4 years decreased mortality with RR 0.97 (95% CI, 0.94 to 0.99) (337). This effect was seen in 38 trials of vitamin D3, RR 0.94 (95% CI, 0.91 to 0.98), but not with other forms of vitamin D (337). These findings were not validated in 2 trials of vitamin D3 supplements in adults older than 60 (Table 4) (312, 323). However, neither of these trials reported serum 25(OH)D levels at study entry.
Desirable 25(OH)D level, recommended daily allowance, and safety
The IOM defined the sufficient 25(OH)D level based on observational BMD data, as ≥20 ng/mL (266, 283). It defined the recommended daily allowance (RDA), the dose covering the requirements of 97.5% of the population to the desirable level, at 600 IU/day in adults, and 800 IU/day if above 70 years, and for calcium to range between 1000 and 1200 mg/day (266). The Endocrine Society defined a sufficient 25(OH)D level as ≥30 ng/mL (338). These numbers were derived from and for White individuals. Worthy of note, all pivotal phase 3 osteoporosis trials that led to drug approval by the FDA co-administered Ca/D in their treatment arms. Age, BMI, ethnicity, season, baseline 25(OH)D level, type of vitamin D, and treatment duration and dose predict achieved level (339). In older individuals, the increment was 1.3 ng/mL per 100 IU/day with a weighted mean dose of 606 IU, whereas it was 0.68 ng/mL per 100 IU/day with higher doses of 3900 IU/day (339). Obese, dark-skinned individuals have lower serum 25(OH)D levels and may need higher doses to reach desirable levels established for light-skinned individuals (340, 341). However, the optimal concentration in these populations remains unknown. Most trials used vitamin D3. Ca/D3 increased the risk of nephrolithiasis in 4 trials with 42 876 participants, findings reported in individual trials (339). Another meta-analysis inclusive of 3 trials (710 subjects) showed that alfacalcidol and calcitriol increased the risk of hypercalcemia (337).
Key Points
There is consistent evidence for a beneficial effect of Ca/D (mostly as D3), but not vitamin D alone, in reducing the risk of hip fractures and any fractures. This evidence may be driven by findings in older, institutionalized participants, mostly women. There is no benefit of such supplementation in vitamin D–replete individuals.
There are data to support the efficacy of vitamin D in reducing mortality.
Data for falls, cardiovascular diseases, cognition, and cancer are mostly null, and consistent with individual results from the latest large RCTs.
Gaps in the Research
The RCTs and meta-analyses published to date do not have adequate power to evaluate important subgroups, specifically those at high risk of adverse outcomes. This includes subjects with low 25(OH)D levels, men, the oldest old, ethnic groups other than White individuals, and those from low-income countries. In addition, the mean 25(OH)D in these RCTs was ≥20 ng/mL, many lacked measurement of vitamin D levels during treatment, used nonstandardized assays, and used adverse events data to identify fractures.
Implementation of individual patient data meta-analyses and meta-regressions combining data from the latest mega-trials, to investigate the efficacy of vitamin D on prespecified primary outcomes in these modern trials, with subgroup analyses by gender, ethnicity, baseline 25(OH)D level, and dose are needed. Major organizations and scientific journals should require that vitamin D assays be standardized, with results traceable to universal standards, as a condition for publication. This is necessary to enable meaningful guidance on desirable 25(OH)D levels.
Type 2 Diabetes
Natural History/Observational Data in Older Individuals
Diabetes in older adults is a growing public health concern with one-quarter of US adults aged 65 years or older having diabetes and an additional half of older adults having prediabetes (342). Of all age categories, the prevalence of diabetes is highest in the older US adult population. More than 130 million people worldwide aged 65 to 79 years and older have diabetes; the global prevalence of diabetes increases with age, with the highest proportion (24.0%) observed in those 75 to 79 years of age (343).
Impaired glucose tolerance is associated with aging (344). Data from the Baltimore Longitudinal Study of Aging demonstrate an age-related increase in progression rate from normal glucose status to impaired glucose tolerance that is markedly greater than the progression rate from normal to impaired fasting glucose after 20 years of follow-up (Fig. 10) (345). These findings suggest that oral glucose tolerance testing, in particular, is important to consider when characterizing abnormal glucose status in older individuals. Using the hyperinsulinemic-euglycemic clamp, whole body insulin sensitivity is demonstrably reduced in older relative to younger adults (346). This is largely due to age-associated increases in insulin resistance and, to some extent, due to decreased beta cell function with aging. Body composition changes that occur during aging, including increased central adiposity and progressive declines in skeletal muscle mass, may increase insulin resistance (347). In addition, decreased physical activity, mitochondrial dysfunction, inflammatory pathways, and hormonal changes with aging (ie, lower testosterone levels in men) contribute to insulin resistance (344). Insulin secretory defects have also been described, which may impair the compensatory beta-cell response to increases in insulin resistance with aging and further increase the risk for development of prediabetes and diabetes (348).
Figure 10.
Natural history of progression from normal glucose tolerance to type 2 diabetes with aging, Baltimore Longitudinal Study of Aging. Redrawn from Meigs JB et al (345). © American Diabetes Association.
While rates of diabetes-related microvascular and macrovascular complications have declined over time in the US population overall, the absolute rates of end-stage renal disease, acute myocardial infarction, stroke, and cardiovascular disease remain higher in older relative to younger adults (349). However, diabetes in the older adult population is heterogeneous and includes individuals with both middle-age and older-onset diabetes (350), with the latter group accounting for up to a third of older adults with newly diagnosed diabetes. Older adults with middle-age onset diabetes had a greater burden of retinopathy but a similar burden of macrovascular complications compared with older-onset diabetes (350). Thus, the age of diabetes onset may impact the burden of disease and presence of diabetic complications in the older patient with diabetes.
While the aging process can be associated with alterations in glucose metabolism, including both progressive insulin resistance and relative beta cell dysfunction, abnormal glucose metabolism is not present in all older adults. Descriptions of otherwise healthy Italian centenarians without impaired glucose uptake suggest that insulin resistance is not a necessary component of the aging process (351). Instead, insulin resistance may accelerate the aging process. Older adults with diabetes represent a vulnerable population at higher risk for geriatric syndromes such as depression, cognitive dysfunction, chronic pain, injurious falls, urinary incontinence, and polypharmacy (352). Other adverse geriatric conditions that have been described to occur more frequently in persons with diabetes include functional and mobility limitations, disability, and frailty (353, 354)—all of which can significantly impact quality of life in the older patient. Importantly, frail older women have dysregulated glucose and insulin dynamics with higher postchallenge glucose and insulin levels during a 75-gram oral glucose tolerance test compared with non-frail women (355). Studies of older adults with diabetes have demonstrated decreased muscle strength and mass, especially in the lower extremities, compared to those without diabetes (356). Further, greater levels of hyperglycemia are related to steeper declines in muscle strength with aging (357). Even among persons without diabetes, the presence of greater degrees of insulin resistance and/or impaired glucose tolerance is associated with decreased muscle mass and strength in older adults (354, 358).
Available Therapies
As with younger persons, there are many treatment options available for the older person with prediabetes or diabetes, though with unique management considerations for the older population (359). Lifestyle recommendations for older adults may be more appropriate for obese older individuals than those who are underweight. Importantly, the oldest age (>60 years at of age at baseline) group in the Diabetes Prevention Program had the largest reduction in the incidence of diabetes with the lifestyle intervention compared to placebo (71% reduction) and better adherence to lifestyle programs compared to younger age groups, whereas metformin was less effective in the older group (360). The Medicare Diabetes Prevention Program was officially launched in 2018 and is a structured behavior change intervention that aims to prevent development of type 2 diabetes among Medicare beneficiaries who have prediabetes. Such evidence-based, structured programs in the community can effectively facilitate lifestyle changes among older adults with prediabetes.
All antihyperglycemic therapies currently available can be prescribed in the older patient with diabetes, but the choice of pharmacologic therapy may be affected by changes in renal and hepatic functions with aging, susceptibility to hypoglycemia, and the physical and neurocognitive abilities of the individual, in addition to the presence of other comorbidities and potential side effects of medications. Further, newer classes of agents (ie, dipeptidyl peptidase 4 [DPP4] inhibitors, glucagon-like peptide 1 [GLP-1] receptor agonists, and sodium-glucose cotransporter 2 [SGLT2] inhibitors) have generally demonstrated similar safety and cardiovascular outcomes in older and younger individuals in their respective cardiovascular outcome trials, as mandated by the FDA since 2008 for all newly approved antihyperglycemic therapies to date (361).
Optimal glycemic control is often the focus for health care providers when caring for patients with diabetes. However, data have emerged challenging the benefits of tight glycemic control in older adults due to concerns of potentially increased mortality with aggressive glucose lowering (362). Overtreatment is unfortunately common in older adults with diabetes and may be associated with significant hypoglycemia (363). On the other hand, observational studies have linked high blood glucose levels with an increased risk of cognitive impairment—an important comorbidity in older adults (364, 365). Preferential utilization of medications with lower risk of hypoglycemia, as well as liberalization, deintensification, or simplification of diabetes regimens may also be considered where appropriate (366).
Other clinical considerations include evaluation of the older patient's living situation and presence of social support networks that may contribute to diabetes management. Self-monitoring of blood glucose may be implemented, depending on the patient's cognitive ability, functional status, and risk of hypoglycemia. Methods for monitoring of blood glucose in older persons with diabetes are similar to those for younger adults, although some glucose meters may have features that are preferred for older individuals with visual impairments (ie, easy to read screens for low vision or “talking” glucose meters). Use of the continuous glucose monitor's vibratory function, instead of sound alerts for glucose levels that are out of range may be beneficial for older adults with hearing impairments. Insulin pens may also provide advantages over use of syringes in older adults with vision and/or fine motor impairment. Regular exercise as tolerated, including a combination of both aerobic and muscle strengthening exercises, and weight loss can improve insulin sensitivity in older adults with diabetes. Cardiovascular risk factor control (ie, lowering blood pressure, treating dyslipidemia, smoking cessation) is recommended for most older adults with diabetes based on health status. Of note, older adults living in long-term skilled nursing facilities or nursing homes or with substantial cognitive impairment may not be able to self-administer medications and often have additional considerations for goals of care.
Glycemic targets in older adults with diabetes
The United Kingdom Prospective Diabetes Study (UKPDS) demonstrated that randomization of adults with newly diagnosed type 2 diabetes to the intensive vs standard glycemic control arms (mean attained hemoglobin A1c 7% vs 7.9%, respectively) reduced the risk of microvascular complications over 10 years of study follow-up (367). However, most participants were middle-aged; individuals aged over 65 years were excluded from trial enrollment. After study termination, a continued reduction in microvascular complications and emergent risk reductions for myocardial infarction and death from any cause in long-term observation were found; the average age of participants who had data available in the final year of post-trial monitoring was 62 years (368). Further, randomized trials that included older adults at study enrollment (average age 60 years or older) such as the Action in Diabetes and Vascular Disease: Preterax and Diamicron MR Controlled Evaluation (ADVANCE), Action to Control Cardiovascular Risk in Diabetes (ACCORD), and Veterans Affairs Diabetes Trial (VADT) did not demonstrate significant cardiovascular or mortality benefits with more vs less aggressive glucose targets in older adults (362, 369, 370). Older adults are also at a higher risk of hypoglycemia compared to younger adults; thus, glycemic targets in older adults need to be individualized based on cognitive and functional status, life expectancy, and the presence of comorbidities (371, 372).
Key Points
Diabetes and altered glucose metabolism commonly occur with aging but are not universal in aging.
Oral glucose tolerance testing may reveal abnormal glucose status in the older population not detected through fasting glucose or hemoglobin A1c measurement.
Diabetes in this population is heterogeneous, with middle-age onset vs older-onset individuals possibly representing groups at different risk for the development of complications.
Both hyperglycemia and hypoglycemia are related to an increased risk of geriatric syndromes such as cognitive impairment, depression, falls, fractures, and functional disability in most observational studies.
Other geriatric conditions such as muscle loss, mobility disability, and frailty are more prevalent in older patients with diabetes.
Treatment of diabetes in older individuals includes lifestyle recommendations when appropriate and the use of pharmacologic therapies which account for the presence of comorbidities, especially renal and hepatic impairment, as well as the physical and cognitive abilities of the patient, while seeking to minimize hypoglycemia.
There have been few studies investigating glycemic targets in older adults; in general, more vs less aggressive targets have not been found to reduce cardiovascular events or mortality in this population.
Clinical care needs to be individualized for the older adult with diabetes, with simultaneous goals of management of hyperglycemia, prevention and treatment of both macrovascular and microvascular complications of diabetes, avoidance of hypoglycemia, and preservation of quality of life.
Gaps in the Research
Well-designed RCTs are needed to study the effects of more vs less aggressive glycemic goals in an older adult population with diabetes, beyond traditional microvascular and macrovascular complications, particularly for patient-reported outcomes such as quality of life and functional status. More studies are needed to better understand the bidirectional relationship between age-related insulin resistance and geriatric conditions such as skeletal muscle loss, mobility disability, and frailty in older persons with diabetes or at high risk for diabetes. Clinical research focused on management strategies that can slow or prevent functional decline in older persons with diabetes can advance our knowledge in this population. Potential ethical considerations for deintensification of therapy in older adults require continued investigation.
Effective strategies for the prevention of type 2 diabetes in older adults need to be better understood. Tools that may be embedded in electronic health records to help clinicians estimate life expectancy and inform glycemic targets will be helpful in the future for clinical care. Ongoing disparities in the treatment of cardiovascular risk factors by race or ethnicity need to be addressed, and effective population-level approaches to reduce these disparities in older adults should be investigated. Optimal methods of delivering diabetes education to older adults with diabetes, and in particular the role of technology, need to be better understood. The ideal frequency and cost-effectiveness of self-monitored blood glucose testing in older adults with diabetes, many of whom have multimorbidity and may be limited in their functional status, requires further investigation.
Laboratory-based studies investigating the pathophysiology of insulin resistance and beta cell dysfunction with aging are needed. While mitochondrial dysfunction has been linked to both insulin resistance and aging, and studies have reported cellular senescence in persons with diabetes, the underlying mechanisms need to be better understood to facilitate the development of novel targeted therapies.
The Hypothalamic-Neurohypophyseal-Renal Axis
Natural History/Observational Data in Older Individuals
Aging causes distinct changes that impact normal water homeostasis at multiple locations responsible for maintaining normal water balance. The net result of these changes is that older individuals experience a loss of homeostatic reserve, with subsequent increased susceptibility to pathologic and iatrogenic causes of disturbed water homeostasis (373).
A clear age-related deficit in the thirst response appears to arise from decreased sensitivity to osmotic stimulation. The sensation of thirst and the appropriate drinking response to thirst in response to increases in plasma osmolality is compromised in older individuals (Fig. 11) (374). It is likely that this defect occurs, at least in part, through decreased activity of neural pathways that convey osmotic sensory input to the higher cortical centers where thirst is perceived, and from which the thirst-activated drinking responses emanate (375). Studies have suggested that this defect may be due to a higher osmotic set point, leading to a blunted thirst response in older individuals (376). Other studies have demonstrated that there is also a change in baroreceptor-mediated control of thirst in older individuals; plasma volume expansion in older individuals does not generate the normal suppression of thirst found in the young (377). Importantly, the loss of appropriate thirst responses to both osmotic and volume stimuli compromises the critical compensatory mechanisms responsible for the drive to replace lost body fluid, the major physiologic means of correcting a hyperosmolar state.
Figure 11.
Plasma sodium concentration (Na+]) and total water intake in healthy older and younger subjects following 24 hours of dehydration. Baseline sodium concentrations before dehydration (Pre) and after dehydration (Post) are shown. Free access to water was allowed for 60 minutes following dehydration starting at time = 0 minutes. Cumulative water intake during the free drinking period by young and old subjects is depicted in the bar graph. Despite a greater initial increase in serum [Na+], older participants drank significantly less water, resulting in lesser correction of the elevated serum [Na+]. Redrawn with permission from Phillips PA, Johnston CI, & Gray L. Thirst and fluid intake in the elderly. In Ramsay DJ, Booth DA eds., Thirst: Physiological and Psychological Aspects. London; Springer-Verlag, 1991. © Springer-Verlag London Limited.
Impaired glomerular filtration rate and resultant loss of maximal urinary concentrating ability appear a common, if not certain, consequence of aging (378, 379). The importance of such defects is clear: inability to maximally conserve free water favors development of body water deficits. This can contribute to the development of hyperosmolality and hypovolemia. In combination with decreased thirst, this represents a likely cause of the observed increase in the frequency of hypernatremia in older individuals.
Somewhat paradoxically, a decrement in maximal water excretion also occurs in older individuals (380, 381). In addition, older individuals are at a higher risk of developing diseases such as heart failure and cirrhosis that are associated with volume overload. So too, they are at risk for inadvertent iatrogenic overhydration from intravenous and enteral hydration therapy. The inability to appropriately excrete fluid loads therefore predisposes to the development of hypo-osmolar hyponatremia in older individuals.
The secretion and end-organ effects of arginine vasopressin (AVP) account for 2 of the most interesting, and perhaps least well understood aspects of water homeostasis in older individuals. Although a few exceptions exist, most agree that basal AVP secretion is at least maintained, and more likely increased, with normal aging (382). Furthermore, the AVP secretory response, ie, the osmoreceptor sensitivity to osmolar stimuli, is also increased in normal aging (383). Thus, AVP secretion represents one of the few endocrine stimulatory responses that appears to increase rather than diminish with age. It is likely that enhanced secretion of AVP in older individuals and inability to maximally suppress AVP secretion during fluid intake (375), combined with an intrinsic inability to maximally excrete free water (380, 381), increase the likelihood that hypo-osmolar hyponatremia will occur with increased frequency in older individuals.
Hyperosmolality and hypernatremia with aging
Hypernatremia necessarily reflects an increase in plasma osmolality. Cross-sectional studies of both hospitalized older patients and older residents of long-term care facilities show incidences of hypernatremia that vary between 0.3% and 8.9% (384, 385). While hypernatremia is a common presenting diagnosis in older individuals, 60% to 80% of hypernatremia in older populations occurs after hospital admission (384). Similarly, up to 30% of older nursing home patients experience hypernatremia following hospital admission (386).
As hypernatremia develops, normal physiologic responses preserve water homeostasis through osmotically stimulated secretion of AVP to promote renal water conservation along with accompanying potent stimulation of thirst to restore body water deficits (382). Although renal water conservation can forestall the development of severe hyperosmolality, only appropriate stimulation of thirst with subsequent increase in water ingestion can replace body fluid deficits thereby reversing hyperosmolality (387). This entire physiologic response is impaired with aging: older patients have a decreased thirst perception (374), and blunted ability to maximally concentrate their urine in response to AVP (386). An additional factor that can cause and/or exacerbate hypernatremia in hospitalized older patients is osmotic diuresis from a variety of causes: mobilization of urea following hydration for pre-renal azotemia, increased protein load from parenteral or enteral nutrition, and increased tissue catabolism (388). Thus, older individuals have a greatly increased susceptibility to a variety of situations that can induce hypernatremia and hyperosmolality, with the attendant increases in morbidity and mortality that accompany this disorder (389-391).
The clinical implications of hypernatremia in hospitalized older individuals are significant. In a retrospective study, outcomes in 162 hypernatremic older patients, representing 1.1% of all older patients admitted for acute hospital care to a community teaching hospital, were reviewed (389). All patients were at least 60 years of age with a serum [Na+] >148 mmol/L. All-cause mortality in the hypernatremic patients was 42%, which was 7 times greater than age-matched normonatremic patients. Furthermore, 38% of the hypernatremic patients who survived to discharge had a significantly decreased ability to provide self-care (389). More recent analyses of large registry databases have confirmed the relation between hypernatremia and increased all-cause mortality, as well as mortality from coronary events and infections (391).
Although hypernatremia is associated with worse outcomes in all patients, it is particularly associated with increased mortality in patients in intensive care units, with adjusted odd ratios for mortality ranging from 2.03 with serum [Na+] 146-150 mmol/L to 2.67 with serum [Na+] >150 mmol/L.
Hypo-osmolality and hyponatremia with aging
Hyponatremia is the most common electrolyte disorder encountered in clinical practice (392). Hyponatremia becomes clinically significant when accompanied by plasma hypo-osmolality. When hyponatremia is defined as a serum [Na+] of <135 mmol/L, the inpatient incidence is reported to be between 15% and 22%. Studies that define hyponatremia as a serum [Na+] <130 mmol/L demonstrate a lower, but still significant, incidence of 1% to 4% (393). The incidence of hyponatremia in older populations has been reported to vary widely between 0.2% and 29.8%, depending on the criteria used (385). While the true incidence of hyponatremia in older individuals is difficult to define given differing diagnostic criteria across studies, it is clear that the problem is common.
The most common causes of hyponatremia in older individuals are the syndrome of inappropriate antidiuresis (SIAD), drug therapy, and low solute intake. SIAD is the most common cause of hyponatremia in older populations. SIAD can be caused by many types of diseases and injuries common in older individuals, including central nervous system injury and degeneration, pulmonary diseases, paraneoplastic malignancy, nausea, and pain. An idiopathic form of SIAD associated with aging is also quite common. Several studies have demonstrated that SIAD accounts for approximately half (50%-59%) of the hyponatremia observed in some older populations (394-396), and 26% to 60% of older patients with SIAD appear to have the idiopathic form of this disorder (394-396).
Many drugs can cause or exacerbate hyponatremia in older individuals. Some have been associated with SIAD, including many antipsychotic, antidepressant, and antiepileptic drugs (397). Risk factors for the development of hyponatremia with selective serotonin reuptake inhibitor (SSRI) antidepressants include older age, female gender, concomitant use of diuretics, low body weight, and lower baseline serum sodium concentration (398). However, the drug class most commonly implicated with causing hyponatremia in older patients is thiazide diuretics, which does not cause SIAD but rather secondary AVP secretion due to solute depletion and baroreceptor stimulation (399). The incidence of hyponatremia in patients treated with a thiazide diuretic in a primary care database was 13.7%, even higher than hypokalemia (8.5%), and the odds ratio for hyponatremia in patients older than 70 years was 3.87 compared with those younger than 70 (400). Although thiazide diuretics cause hyponatremia in part by solute depletion, this can also occur in the absence of diuretic therapy in individuals eating a low sodium and low protein diet, called the “tea and toast” syndrome (401).
Hyponatremia in older individuals is associated with multiple clinically significant outcomes including neurocognitive effects and falls (402, 403), hospital readmission and need for long-term care (404), incidence of bone fractures (405), and osteoporosis (406). Hyponatremia is a strong independent predictor of mortality, reported to be as high as 60% in some series (384, 407), in outpatient as well as inpatient studies (408). In a study of the association between asymptomatic hyponatremia and gait instability and attention deficits, a subset of 12 patients with hyponatremia secondary to SIAD with [Na+] in the range of 124 to 130 mmol/L demonstrated significant gait instability that normalized with correction of hyponatremia (409). The patients were asked to walk a tandem gait on a computerized platform that measured the center of gravity on the ball of their foot. Deviation from the straight line was measured as “Total Traveled Way.” The hyponatremic patients wandered markedly off the tandem gait line in terms of their center of balance, but corrected significantly once their hyponatremia was corrected (Fig. 12). When performing a series of attention tests, patients in the hyponatremic subset (mean [Na+] = 128 mmol/L) had prolonged response latencies compared with a group of patients after acute alcohol intake (blood alcohol concentration 0.6 g/L). These impairments suggested a global decrease of attentional capabilities that is more pronounced in hyponatremic patients (409), which may contribute to gait instability and falls in older individuals.
Figure 12.
Total traveled way measured by the center of pressure during a dynamic walking test consisting of 3 stereotyped steps “in tandem,” eyes open, in 3 patients (A–C) with mild asymptomatic hyponatremia before (left) and after (right) correction. Patients are walking from right to left. Markedly irregular paths of the center of pressure were observed in the hyponatremia condition (arrows). Redrawn from Renneboog B et al (409). © Elsevier Inc.
Verbalis et al explored the effect of hyponatremia and bone quality and demonstrated a link between chronic hyponatremia and metabolic bone loss (406). This study demonstrated that chronic hyponatremia causes a significant reduction of bone mass at the cellular level. Subsequent epidemiological analysis of 2.9 million patient records showed that chronic hyponatremia was associated with odds ratios of 3.99 for osteoporosis and 3.05 for fractures, thus confirming the translational significance of the animal studies (410). Hyponatremia-induced bone resorption and osteoporosis are unique in that they represent attempts of the body to preserve sodium homeostasis at the expense of bone structural integrity (411).
Available Therapies
Hyperosmolality and hypernatremia
Adequate hydration is the cornerstone of preventing hyperosmolality and hypernatremia in older patients. Aggressive hydration with hypotonic fluids (D5W or D5/0.5 NSS) is indicated to lower the serum [Na+] to normal levels in the first 48 hours of hospital admission. A recent retrospective study of 449 patients hospitalized with a serum [Na+] >155 mmol/L showed that there was no evidence that rapid correction of hypernatremia (>0.5 mmol/L/h) was associated with a higher risk for mortality, seizure, alteration of consciousness, and/or cerebral edema in critically ill adult patients with either admission or hospital-acquired hypernatremia (412).
Older patients with an established diagnosis of AVP deficiency (cranial diabetes insipidus) should be treated with desmopressin as other adult patients (413). However, because desmopressin is largely metabolized through renal excretion, older individuals are more prone to hyponatremia with desmopressin therapy because of age-associated decreases in glomerular filtration rate.
Hypo-osmolality and hyponatremia
Treatment of hypo-osmolality and hyponatremia in older individuals should follow the same guidelines as in younger individuals, particularly with regard to limits of daily correction of serum [Na+] to avoid the osmotic demyelination syndrome. Fluid restriction is usually the first therapy employed, but it has limited efficacy with mean increases in serum [Na+] in the range of 3 to 5 mmol/L in RCTs (414). If pharmacologic treatment is necessary, the choices include urea, furosemide in combination with NaCl tablets, demeclocycline, and the vasopressin receptor antagonists (393, 415). Although each of these treatments can be effective in individual circumstances, the only therapies currently approved by regulatory agencies for treatment of hyponatremia are vasopressin receptor antagonists.
Clinical Trial Data on Efficacy and Safety in Older Individuals
Hyperosmolality and hypernatremia
No recent clinical trials on the efficacy and safety of acute and chronic treatments for hypernatremia in older individuals have been published. However, several trials have been published on the use of desmopressin for treatment of nocturia (416). These have uniformly found that older individuals are at higher risk for the development of hyponatremia even with a single night-time low dose of desmopressin (417), which was particularly true of older females because of an enhanced response to desmopressin likely due to a sex difference in vasopressin V2 receptor expression in the kidneys (418, 419).
Hypo-osmolality and hyponatremia
Several randomized controlled clinical trials have been published on the efficacy and safety of vasopressin antagonist treatments for hyponatremia (420, 421). However, none of these have focused specifically on older individuals even though many older individuals were included in the clinical trials.
Key Points
Deficits in renal function, thirst, and AVP responses to osmotic and volume stimulation have been repeatedly demonstrated in the older population, increasing risk for disturbances of water homeostasis due to both intrinsic disease and iatrogenic causes.
These disturbances have clinical implications in terms of neurocognitive effects, falls, hospital readmission and need for long-term care, incidence of osteoporosis and bone fractures, and both inpatient and outpatient mortality.
Effective treatments for hyponatremia are available, but recommended indications for treatment of chronic hyponatremia based on demonstrated improvements in clinical outcomes are lacking.
Gaps in the Research
Clinical trials evaluating the efficacy and safety of treatments of hypernatremia and hyponatremia in older individuals are required. Studies are needed to determine the etiology of “idiopathic” hyponatremia, particularly in older individuals. Additional studies of the effects of chronic hyponatremia on the brain, bone, and other organs, and evaluation of the reversibility of these effects with correction of hyponatremia, should be performed (422). Of special interest will be studies to assess whether more effective treatment of hyponatremia can reduce the incidence of falls and fractures in older patients, the use of health care resources for both inpatients and outpatients with hyponatremia, and the increased morbidity and mortality of patients with hyponatremia associated with multiple disease states. Consequently, the indications for treatment of water-retaining disorders in patients without symptomatic hyponatremia must await further studies specifically designed to assess the effects of treatment of hyponatremic patients on clinically relevant outcomes, as well as clinical experience that better delineates efficacies and potential toxicities of all treatments for hyponatremia.
Conclusions
This Scientific Statement provides a broad overview of the research conducted to date on the hormonal changes that occur in 9 separate areas in endocrinology. It also describes specific unanswered questions where more research is needed. The potential for improved health through enhanced identification and prevention and/or treatment of the factors that impact hormonal changes with age is both exciting and substantial.
Existing knowledge of hormones and aging is largely based on results of observational and uncontrolled studies. Limitations of findings from these study designs include residual confounding, inability to make causal inferences, and the potential for reverse causality. Randomized, appropriately controlled clinical trials that are adequately powered to examine efficacy specifically in older individuals are required. Both the assessment of clinically meaningful outcomes and the risk of the older study population for these outcomes should be carefully considered in the study design. Possible outcomes include frailty, cognitive impairment, fractures, mood, patient-reported outcomes, cancer, and cardiovascular events, which should be measured using validated measures with adequate sensitivity to change.
Additional research is needed to improve understanding of the underlying mechanisms, methods of detection, and management of age-associated endocrine changes. Correlations between altered hormonal output and age-associated phenotypes have been identified in multiple hormonal axes, with decreased physical activity, sleep disruption, and increases in comorbid diseases contributing to the lower hormonal output in the growth hormone and testicular axes, for example. A thorough investigation of causal factors for age-related change is needed across all hormonal axes and endocrine diseases. In addition to these causal factors, the confounding effects of acute and chronic illness, multimorbidity, and polypharmacy on clinical manifestations, laboratory evaluation, diagnosis, monitoring, and prognosis need to be determined. Additional direct effects of aging on mitochondrial function, telomeres, and epigenetic effects, possibly mediated through inflammation and stress, require further examination across endocrine axes and organ-specific endocrine diseases. Use of humanized models in areas where animal models do not sufficiently replicate human physiology, such as for the adrenal gland, could improve understanding about human aging. Animal models should also replicate the time sequence of age-associated changes. Modern mass spectrometry assays should be used in all research studies of steroid hormones in older individuals. The use of accurate and standardized hormone assays and harmonized reference ranges is needed in research and clinical practice in all endocrine axes.
Research is needed to provide the evidence base to support when hormonal therapeutics are appropriate and, equally importantly, when they are not. Hormones have been a frequent target for the anti-aging industry, despite evidence that supports harms of GH and sex steroids outweighing benefits in unselected populations of older individuals. Researchers designing RCTs in model organisms and humans should consider the timing, dose, duration, and target population for hormonal therapeutics, in populations with and without age-associated cognitive and functional decline. The safety of treatments should be considered in the use of hormone therapeutics. Pharmacokinetics may be altered in older individuals, affecting the dosage. Whether these therapeutics should be delivered in combinations with each other, and with interventions such as exercise or senolytics that broadly target fundamental aging processes, should also be evaluated (423, 424). Hormonal modulation may also benefit nonendocrine diseases, such as cancer, especially through therapeutics with target-specific actions. Approaches to preserve or revitalize gland function should also be developed and tested. Permeating this research should be inclusion of representative populations by gender (including transgender persons), race, ethnicity, and environmental exposures.
Disclaimer Statement
The Endocrine Society develops Scientific Statements to explore the scientific basis of hormone-related conditions and disease, discuss how this knowledge can be applied in practice, and identify areas that require additional research. Topics are selected on the basis of their emerging scientific impact. Scientific Statements are developed by a Task Force of experts appointed by the Endocrine Society, with internal review by the relevant Society committees and expert external reviewers prior to a comment period open to all members of the Society. Clinicians and other users should not consider this Scientific Statement inclusive of all proper approaches or methods or exclusive of others. It cannot guarantee any specific outcome, nor does it establish a standard of care. It is not intended to dictate the treatment of a particular patient. The independent judgment of health care providers and each patient's individual circumstances must determine treatment decisions. The Endocrine Society makes no warranty, express or implied, regarding this Scientific Statement and specifically excludes any warranties of merchantability and fitness for a particular use or purpose. The Endocrine Society shall not be liable for direct, indirect, special, incidental, or consequential damages related to the use of the information contained herein.
Disclosures
R.A. has performed contracted research for Corcept Therapeutics and Sparrow Pharmaceuticals and has served as a consultant for Quest Diagnostics, Corcept Therapeutics, PhaseBio Pharmaceuticals, Crinetics Pharmaeuticals, Xeris Pharmaceuticals, and Recordati Rare Diseases. G.E.H.F. is a member of the panel of experts convening at the International Conference on Controversies in Vitamin D in September 2023. Travel and housing to the meeting are covered by Abiogen for all participants, no honorarium received. M.M. has received honorarium and consulting fees from Amgen, honorarium from Alexion, and consulting fees from Myovant. C.A.S. serves as a member of the Data and Safety Monitoring Board for Mithra Pharmaceuticals. M.O.T. is a consultant for and has an equity position in Lumos Pharma Inc. The other authors declare no conflicts.
Abbreviations
- 11βHSD1
11β-hydroxysteroid dehydrogenase type 1
- 11OHA4
11β-hydroxyandrostenedione
- 11KT
11-ketotestosterone
- 1,25(OH)2D3
1,25-dihydroxy vitamin D
- 25(OH)D
25-hydroxyvitamin D
- AMH
anti-Mullerian hormone
- APCC
aldosterone-producing cell cluster
- APM
adrenal-producing micronodules
- AVP
arginine vasopressin
- BMD
bone mineral density
- Ca/D
calcium and vitamin D
- CEE
conjugated equine estrogens
- CHD
coronary heart disease
- CVD
cardiovascular disease
- DHEA
dehydroepiandrosterone
- DHEAS
dehydroepiandrosterone sulfate
- ED
erectile dysfunction
- FDA
US Food and Drug Administration
- FSH
follicle-stimulating hormone
- GH
growth hormone
- GHRH
growth hormone-releasing hormone
- GSM
genitourinary syndrome of menopause
- HPA
hypothalamic-pituitary-adrenal
- IGHD
isolated GH deficiency
- IOM
Institute of Medicine
- IU
international units
- LC-MS
liquid chromatography–mass spectrometry
- LH
luteinizing hormone
- MHT
menopausal hormone therapy
- MPA
medroxyprogesterone acetate
- RCT
randomized controlled trial
- rhGH
recombinant human growth hormone
- SIAD
syndrome of inappropriate antidiuresis
- SWAN
Study of Women's Health Across the Nation
- T3
triiodothyronine
- T4
thyroxine
- vitamin D2
ergocalciferol
- vitamin D3
cholecalciferol
- VDR
vitamin D receptor
- VMS
vasomotor symptoms
Contributor Information
Anne R Cappola, Division of Endocrinology, Diabetes, and Metabolism, Perelman School of Medicine at the University of Pennsylvania, Philadelphia, PA 19104, USA.
Richard J Auchus, Departments of Pharmacology and Internal Medicine, Division of Metabolism, Endocrinology, and Diabetes, University of Michigan, Ann Arbor, MI 48109, USA; Endocrinology and Metabolism Section, Medical Service, LTC Charles S. Kettles Veteran Affairs Medical Center, Ann Arbor, MI 48015, USA.
Ghada El-Hajj Fuleihan, Calcium Metabolism and Osteoporosis Program, WHO Collaborating Center for Metabolic Bone Disorders, Division of Endocrinology, Department of Internal Medicine, American University of Beirut, Beirut 1107-2020, Lebanon.
David J Handelsman, ANZAC Research Institute, University of Sydney and Andrology Department, Concord Repatriation General Hospital, Sydney 2139, Australia.
Rita R Kalyani, Division of Endocrinology, Diabetes, and Metabolism, Johns Hopkins University School of Medicine, Baltimore, MD 21287, USA.
Michael McClung, Oregon Osteoporosis Center, Portland, OR 97213, USA; Mary MacKillop Institute for Health Research, Australian Catholic University, Melbourne, VIC 3000, Australia.
Cynthia A Stuenkel, Department of Medicine, University of California, San Diego, School of Medicine, La Jolla, CA 92093, USA.
Michael O Thorner, Division of Endocrinology and Metabolism, University of Virginia, Charlottesville, VA 22903, USA; Division of Endocrinology, Diabetes and Hypertension, Brigham and Women's Hospital, Boston, MA 02115, USA.
Joseph G Verbalis, Division of Endocrinology and Metabolism, Georgetown University Medical Center, Washington, DC 20057, USA.
References
- 1. United Nations, Department of Economic and Social Affairs, population division 2019. World Population Ageing 2019: Highlights (ST/ESA/SER.A/430).
- 2. Zadik Z, Chalew SA, McCarter RJ Jr, Meistas M, Kowarski AA. The influence of age on the 24-hour integrated concentration of growth hormone in normal individuals. J Clin Endocrinol Metab. 1985;60(3):513‐516. [DOI] [PubMed] [Google Scholar]
- 3. Giustina A, Veldhuis JD. Pathophysiology of the neuroregulation of growth hormone secretion in experimental animals and the human. Endocr Rev. 1998;19(6):717‐797. [DOI] [PubMed] [Google Scholar]
- 4. Nass R, Pezzoli SS, Oliveri MC, et al. Effects of an oral ghrelin mimetic on body composition and clinical outcomes in healthy older adults: a randomized trial. Ann Intern Med. 2008;149(9):601‐611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Nass R, Farhy LS, Liu J, et al. Age-dependent decline in acyl-ghrelin concentrations and reduced association of acyl-ghrelin and growth hormone in healthy older adults. J Clin Endocrinol Metab. 2014;99(2):602‐608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ho KY, Evans WS, Blizzard RM, et al. Effects of sex and age on the 24-hour profile of growth hormone secretion in man: importance of endogenous estradiol concentrations. J Clin Endocrinol Metab. 1987;64(1):51‐58. [DOI] [PubMed] [Google Scholar]
- 7. Weissberger AJ, Ho KK, Lazarus L. Contrasting effects of oral and transdermal routes of estrogen replacement therapy on 24-hour growth hormone (GH) secretion, insulin-like growth factor I, and GH-binding protein in postmenopausal women. J Clin Endocrinol Metab. 1991;72(2):374‐381. [DOI] [PubMed] [Google Scholar]
- 8. Leong GM, Moverare S, Brce J, et al. Estrogen up-regulates hepatic expression of suppressors of cytokine signaling-2 and -3 in vivo and in vitro. Endocrinology. 2004;145(12):5525‐5531. [DOI] [PubMed] [Google Scholar]
- 9. Leung KC, Johannsson G, Leong GM, Ho KK. Estrogen regulation of growth hormone action. Endocr Rev. 2004;25(5):693‐721. [DOI] [PubMed] [Google Scholar]
- 10. Aguiar-Oliveira MH, Bartke A. Growth hormone deficiency: health and longevity. Endocr Rev. 2019;40(2):575‐601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Besson A, Salemi S, Gallati S, et al. Reduced longevity in untreated patients with isolated growth hormone deficiency. J Clin Endocrinol Metab. 2003;88(8):3664‐3667. [DOI] [PubMed] [Google Scholar]
- 12. Ben-Avraham D, Govindaraju DR, Budagov T, et al. The GH receptor exon 3 deletion is a marker of male-specific exceptional longevity associated with increased GH sensitivity and taller stature. Sci Adv. 2017;3(6):e1602025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Guevara-Aguirre J, Balasubramanian P, Guevara-Aguirre M, et al. Growth hormone receptor deficiency is associated with a major reduction in pro-aging signaling, cancer, and diabetes in humans. Sci Transl Med. 2011;3(70):70ra13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. van der Spoel E, Jansen SW, Akintola AA, et al. Growth hormone secretion is diminished and tightly controlled in humans enriched for familial longevity. Aging Cell. 2016;15(6):1126‐1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Clasey JL, Weltman A, Patrie J, et al. Abdominal visceral fat and fasting insulin are important predictors of 24-hour GH release independent of age, gender, and other physiological factors. J Clin Endocrinol Metab. 2001;86(8):3845‐3852. [DOI] [PubMed] [Google Scholar]
- 16. Pollock RD, Carter S, Velloso CP, et al. An investigation into the relationship between age and physiological function in highly active older adults. J Physiol. 2015;593(3):657‐680; discussion 680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Liu H, Bravata DM, Olkin I, et al. Systematic review: the safety and efficacy of growth hormone in the healthy elderly. Ann Intern Med. 2007;146(2):104‐115. [DOI] [PubMed] [Google Scholar]
- 18. White HK, Petrie CD, Landschulz W, et al. Effects of an oral growth hormone secretagogue in older adults. J Clin Endocrinol Metab. 2009;94(4):1198‐1206. [DOI] [PubMed] [Google Scholar]
- 19. Miller WL, Auchus RJ. The molecular biology, biochemistry, and physiology of human steroidogenesis and its disorders. Endocr Rev. 2011;32(1):81‐151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Gomez-Sanchez CE, Qi X, Velarde-Miranda C, et al. Development of monoclonal antibodies against human CYP11B1 and CYP11B2. Mol Cell Endocrinol. 2014;383(1-2):111‐117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Nishimoto K, Tomlins SA, Kuick R, et al. Aldosterone-stimulating somatic gene mutations are common in normal adrenal glands. Proc Natl Acad Sci U S A. 2015;112(33):E4591‐E4599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Nanba K, Vaidya A, Williams GH, Zheng I, Else T, Rainey WE. Age-related autonomous aldosteronism. Circulation. 2017;136(4):347‐355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Iwahashi N, Umakoshi H, Seki T, et al. Characterization of aldosterone-producing cell cluster (APCC) at single-cell resolution. J Clin Endocrinol Metab. 2022;107(9):2439‐2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Deuschle M, Gotthardt U, Schweiger U, et al. With aging in humans the activity of the hypothalamus-pituitary-adrenal system increases and its diurnal amplitude flattens. Life Sci. 1997;61(22):2239‐2246. [DOI] [PubMed] [Google Scholar]
- 25. Veldhuis JD, Sharma A, Roelfsema F. Age-dependent and gender-dependent regulation of hypothalamic-adrenocorticotropic-adrenal axis. Endocrinol Metab Clin North Am. 2013;42(2):201‐225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Purnell JQ, Brandon DD, Isabelle LM, Loriaux DL, Samuels MH. Association of 24-hour cortisol production rates, cortisol-binding globulin, and plasma-free cortisol levels with body composition, leptin levels, and aging in adult men and women. J Clin Endocrinol Metab. 2004;89(1):281‐287. [DOI] [PubMed] [Google Scholar]
- 27. Born J, Ditschuneit I, Schreiber M, Dodt C, Fehm HL. Effects of age and gender on pituitary-adrenocortical responsiveness in humans. Eur J Endocrinol. 1995;132(6):705‐711. [DOI] [PubMed] [Google Scholar]
- 28. Wilkinson CW, Peskind ER, Raskind MA. Decreased hypothalamic-pituitary-adrenal axis sensitivity to cortisol feedback inhibition in human aging. Neuroendocrinology. 1997;65(1):79‐90. [DOI] [PubMed] [Google Scholar]
- 29. Le NP, Varadhan R, Fried LP, Cappola AR. Cortisol and dehydroepiandrosterone response to adrenocorticotropic hormone and frailty in older women. J Gerontol A Biol Sci Med Sci. 2021;76(5):901‐905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. van den Beld AW, Kaufman JM, Zillikens MC, Lamberts SWJ, Egan JM, van der Lely AJ. The physiology of endocrine systems with ageing. Lancet Diabetes Endocrinol. 2018;6(8):647‐658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Tiganescu A, Walker EA, Hardy RS, Mayes AE, Stewart PM. Localization, age- and site-dependent expression, and regulation of 11b-hydroxysteroid dehydrogenase type 1 in skin. J Invest Dermatol. 2011;131(1):30‐36. [DOI] [PubMed] [Google Scholar]
- 32. Kilgour AH, Gallagher IJ, MacLullich AM, et al. Increased skeletal muscle 11bHSD1 mRNA is associated with lower muscle strength in ageing. PLoS One. 2013;8(12):e84057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Bovio S, Cataldi A, Reimondo G, et al. Prevalence of adrenal incidentaloma in a contemporary computerized tomography series. J Endocrinol Invest. 2006;29(4):298‐302. [DOI] [PubMed] [Google Scholar]
- 34. Di Dalmazi G, Vicennati V, Garelli S, et al. Cardiovascular events and mortality in patients with adrenal incidentalomas that are either non-secreting or associated with intermediate phenotype or subclinical Cushing's syndrome: a 15-year retrospective study. Lancet Diabetes Endocrinol. 2014;2(5):396‐405. [DOI] [PubMed] [Google Scholar]
- 35. Morelli V, Eller-Vainicher C, Salcuni AS, et al. Risk of new vertebral fractures in patients with adrenal incidentaloma with and without subclinical hypercortisolism: a multicenter longitudinal study. J Bone Miner Res. 2011;26(8):1816‐1821. [DOI] [PubMed] [Google Scholar]
- 36. Arora VK, Schenkein E, Murali R, et al. Glucocorticoid receptor confers resistance to antiandrogens by bypassing androgen receptor blockade. Cell. 2013;155(6):1309‐1322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Li J, Alyamani M, Zhang A, et al. Aberrant corticosteroid metabolism in tumor cells enables GR takeover in enzalutamide resistant prostate cancer. Elife. 2017;6:e20183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Valle S, Sharifi N. Targeting glucocorticoid metabolism in prostate cancer. Endocrinology. 2021;162(9):bqab132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Csernansky JG, Dong H, Fagan AM, et al. Plasma cortisol and progression of dementia in subjects with Alzheimer-type dementia. Am J Psychiatry. 2006;163(12):2164‐2169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Pineau F, Canet G, Desrumaux C, et al. New selective glucocorticoid receptor modulators reverse amyloid-beta peptide-induced hippocampus toxicity. Neurobiol Aging. 2016;45:109‐122. [DOI] [PubMed] [Google Scholar]
- 41. Orentreich N, Brind JL, Rizer RL, Vogelman JH. Age changes and sex differences in serum dehydroepiandrosterone sulfate concentrations throughout adulthood. J Clin Endocrinol Metab. 1984;59(3):551‐555. [DOI] [PubMed] [Google Scholar]
- 42. Auchus RJ, Rainey WE. Adrenarche—physiology, biochemistry and human disease. Clin Endocrinol (Oxf). 2004;60(3):288‐296. [DOI] [PubMed] [Google Scholar]
- 43. Baulieu EE. Dehydroepiandrosterone (DHEA): a fountain of youth? J Clin Endocrinol Metab. 1996;81(9):3147‐3151. [DOI] [PubMed] [Google Scholar]
- 44. Burger HG. Androgen production in women. Fertil Steril. 2002;77(Suppl 4):S3‐S5. [DOI] [PubMed] [Google Scholar]
- 45. Barrett-Connor E, Khaw K, Yen SCC. A prospective study of dehydroepiandrosterone sulfate, mortality and cardiovascular disease. N Engl J Med. 1986;315(24):1519‐1524. [DOI] [PubMed] [Google Scholar]
- 46. Berr C, Lafont S, Debuire B, Dartigues JF, Baulieu EE. Relationships of dehydroepiandrosterone sulfate in the elderly with functional, psychological, and mental status, and short-term mortality: a French community-based study. Proc Natl Acad Sci U S A. 1996;93(23):13410‐13415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Turcu AF, Rege J, Auchus RJ, Rainey WE. 11-Oxygenated androgens in health and disease. Nat Rev Endocrinol. 2020;16(5):284‐296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Davio A, Woolcock H, Nanba AT, et al. Sex differences in 11-oxygenated androgen patterns across adulthood. J Clin Endocrinol Metab. 2020;105(8):e2921‐e2929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Rege J, Turcu A, Kasa-Vubu JZ, et al. 11-Ketotestosterone is the dominant circulating bioactive androgen during normal and premature adrenarche. J Clin Endocrinol Metab. 2018;103(12):4589‐4598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Labrie F, Archer DF, Martel C, Vaillancourt M, Montesino M. Combined data of intravaginal prasterone against vulvovaginal atrophy of menopause. Menopause. 2017;24(11):1246‐1256. [DOI] [PubMed] [Google Scholar]
- 51. Igwebuike A, Irving BA, Bigelow ML, Short KR, McConnell JP, Nair KS. Lack of dehydroepiandrosterone effect on a combined endurance and resistance exercise program in postmenopausal women. J Clin Endocrinol Metab. 2008;93(2):534‐538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Løvås K, Gebre-Medhin G, Trovik TS, et al. Replacement of dehydroepiandrosterone in adrenal failure: no benefit for subjective health status and sexuality in a 9-month, randomized, parallel group clinical trial. J Clin Endocrinol Metab. 2003;88(3):1112‐1118. [DOI] [PubMed] [Google Scholar]
- 53. Morales AJ, Nolan JJ, Nelson JC, Yen SS. Effects of replacement dose of dehydroepiandrosterone in men and women of advancing age. J Clin Endocrinol Metab. 1994;78(6):1360‐1367. [DOI] [PubMed] [Google Scholar]
- 54. Baulieu EE, Thomas G, Legrain S, et al. Dehydroepiandrosterone (DHEA), DHEA sulfate, and aging: contribution of the DHEAge study to a sociobiomedical issue. Proc Natl Acad Sci U S A. 2000;97(8):4279‐4284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Nair KS, Rizza RA, O’Brien P, et al. DHEA In elderly women and DHEA or testosterone in elderly men. N Engl J Med. 2006;355(16):1647‐1659. [DOI] [PubMed] [Google Scholar]
- 56. Percheron G, Hogrel JY, Denot-Ledunois S, et al. Effect of 1-year oral administration of dehydroepiandrosterone to 60- to 80-year-old individuals on muscle function and cross-sectional area: a double-blind placebo-controlled trial. Arch Intern Med. 2003;163(6):720‐727. [DOI] [PubMed] [Google Scholar]
- 57. Arlt W, Callies F, van Vlijmen JC, et al. Dehydroepiandrosterone replacement in women with adrenal insufficiency. N Engl J Med. 1999;341(14):1013‐1020. [DOI] [PubMed] [Google Scholar]
- 58. Hansen KR, Knowlton NS, Thyer AC, Charleston JS, Soules MR, Klein NA. A new model of reproductive aging: the decline in ovarian non-growing follicle number from birth to menopause. Hum Reprod. 2008;23(3):699‐708. [DOI] [PubMed] [Google Scholar]
- 59. Harlow SD, Gass M, Hall JE, et al. Executive summary of the stages of reproductive aging workshop + 10: addressing the unfinished agenda of staging reproductive aging. J Clin Endocrinol Metab. 2012;97(4):1159‐1168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Appiah D, Nwabuo CC, Ebong IA, Wellons MF, Winters SJ. Trends in age at natural menopause and reproductive life span among US women, 1959–2018. JAMA. 2021;325(13):1328‐1330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Stuenkel CA, Gompel A, Davis SR, Pinkerton JV, Lumsden MA, Santen RJ. Approach to the patient with new-onset secondary amenorrhea: is this primary ovarian insufficiency? J Clin Endocrinol Metab. 2022;107(3):825‐835. [DOI] [PubMed] [Google Scholar]
- 62. Santoro N, Johnson J. Diagnosing the onset of menopause. JAMA. 2019;322(8):775‐776. [DOI] [PubMed] [Google Scholar]
- 63. Practice Committee of the American Society for Reproductive Medicine . Testing and interpreting measures of ovarian reserve: a committee opinion. Fertil Steril. 2020;114(6):1151‐1157. [DOI] [PubMed] [Google Scholar]
- 64. Ruth KS, Day FR, Hussain J, et al. Genetic insights into biological mechanisms governing human ovarian ageing. Nature. 2021;596(7872):393‐397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Park SU, Walsh L, Berkowitz KM. Mechanisms of ovarian aging. Reproduction. 2021;162(2):R19‐R33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Gorsi B, Hernandez E, Moore MB, et al. Causal and candidate gene variants in a large cohort of women with primary ovarian insufficiency. J Clin Endocrinol Metab. 2022;107(3):685‐714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Desai S, Rajkovic A. Genetics of reproductive aging from gonadal dysgenesis through menopause. Semin Reprod Med. 2017;35(2):147‐159. [DOI] [PubMed] [Google Scholar]
- 68. Levine ME, Lu AT, Chen BH, et al. Menopause accelerates biological aging. Proc Natl Acad Sci U S A. 2016;113(33):9327‐9332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Lu AT, Xue L, Salfati EL, et al. GWAS Of epigenetic aging rates in blood reveals a critical role for TERT. Nat Commun. 2018;9(1):387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Wise PM, Kashon ML, Krajnak KM, et al. Aging of the female reproductive system: a window into brain aging. Recent Prog Horm Res. 1997;52:279‐303. discussion 303–305. [PubMed] [Google Scholar]
- 71. Kermath BA, Gore AC. Neuroendocrine control of the transition to reproductive senescence: lessons learned from the female rodent model. Neuroendocrinology. 2012;96(1):1‐12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Kermath BA, Riha PD, Woller MJ, Wolfe A, Gore AC. Hypothalamic molecular changes underlying natural reproductive senescence in the female rat. Endocrinology. 2014;155(9):3597‐3609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Bacon ER, Mishra A, Wang Y, Desai MK, Yin F, Brinton RD. Neuroendocrine aging precedes perimenopause and is regulated by DNA methylation. Neurobiol Aging. 2019;74:213‐224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Downs JL, Wise PM. The role of the brain in female reproductive aging. Mol Cell Endocrinol. 2009;299(1):32‐38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Shaw ND, Srouji SS, Histed SN, McCurnin KE, Hall JE. Aging attenuates the pituitary response to gonadotropin-releasing hormone. J Clin Endocrinol Metab. 2009;94(9):3259‐3264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Hall JE. Neuroendocrine changes with reproductive aging in women. Semin Reprod Med. 2007;25(5):344‐351. [DOI] [PubMed] [Google Scholar]
- 77. Colella M, Cuomo D, Peluso T, et al. Ovarian aging: role of pituitary-ovarian axis hormones and ncRNAs in regulating ovarian mitochondrial activity. Front Endocrinol (Lausanne). 2021;12:791071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. van der Reest J, Nardini Cecchino G, Haigis MC, Kordowitzki P. Mitochondria: their relevance during oocyte ageing. Ageing Res Rev. 2021;70:101378. [DOI] [PubMed] [Google Scholar]
- 79. Rance NE, Dacks PA, Mittelman-Smith MA, Romanovsky AA, Krajewski-Hall SJ. Modulation of body temperature and LH secretion by hypothalamic KNDy (kisspeptin, neurokinin B and dynorphin) neurons: a novel hypothesis on the mechanism of hot flushes. Front Neuroendocrinol. 2013;34(3):211‐227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Prague JK, Roberts RE, Comninos AN, et al. Neurokinin 3 receptor antagonism as a novel treatment for menopausal hot flushes: a phase 2, randomised, double-blind, placebo-controlled trial. Lancet. 2017;389(10081):1809‐1820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Modi M, Dhillo WS. The neuroendocrinology of the preoptic area in menopause: symptoms and therapeutic strategies. Handb Clin Neurol. 2021;179:455‐460. [DOI] [PubMed] [Google Scholar]
- 82. Zhu LL, Blair H, Cao J, et al. Blocking antibody to the β-subunit of FSH prevents bone loss by inhibiting bone resorption and stimulating bone synthesis. Proc Natl Acad Sci U S A. 2012;109(36):14574‐14579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Liu P, Ji Y, Yuen T, et al. Blocking FSH induces thermogenic adipose tissue and reduces body fat. Nature. 2017;546(7656):107‐112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Kohrt WM, Wierman ME. Preventing fat gain by blocking follicle-stimulating hormone. N Engl J Med. 2017;377(3):293‐295. [DOI] [PubMed] [Google Scholar]
- 85. Allan CM, Kalak R, Dunstan CR, et al. Follicle-stimulating hormone increases bone mass in female mice. Proc Natl Acad Sci U S A. 2010;107(52):22629‐22634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Samargandy S, Matthews KA, Brooks MM, et al. Trajectories of blood pressure in midlife women: does menopause matter? Circ Res. 2022;130(3):312‐322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Welt CK, Jimenez Y, Sluss PM, Smith PC, Hall JE. Control of estradiol secretion in reproductive ageing. Hum Reprod. 2006;21(8):2189‐2193. [DOI] [PubMed] [Google Scholar]
- 88. Shaw ND, Srouji SS, Welt CK, et al. Compensatory increase in ovarian aromatase in older regularly cycling women. J Clin Endocrinol Metab. 2015;100(9):3539‐3547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Alberico HC, Woods DC. Role of granulosa cells in the aging ovarian landscape: a focus on mitochondrial and metabolic function. Front Physiol. 2021;12:800739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Ofori EK, Conde Alonso S, Correas-Gomez L, et al. Thigh and abdominal adipose tissue depot associations with testosterone levels in postmenopausal females. Clin Endocrinol (Oxf). 2019;90(3):433‐439. [DOI] [PubMed] [Google Scholar]
- 91. Rariy CM, Ratcliffe SJ, Weinstein R, et al. Higher serum free testosterone concentration in older women is associated with greater bone mineral density, lean body mass, and total fat mass: the cardiovascular health study. J Clin Endocrinol Metab. 2011;96(4):989‐996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Wierman ME, Arlt W, Basson R, et al. Androgen therapy in women: a reappraisal: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2014;99(10):3489‐3510. [DOI] [PubMed] [Google Scholar]
- 93. Laughlin GA, Barrett-Connor E, Kritz-Silverstein D, von Mühlen D. Hysterectomy, oophorectomy, and endogenous sex hormone levels in older women: the Rancho Bernardo study. J Clin Endocrinol Metab. 2000;85(2):645‐651. [DOI] [PubMed] [Google Scholar]
- 94. Davison SL, Bell R, Donath S, Montalto JG, Davis SR. Androgen levels in adult females: changes with age, menopause, and oophorectomy. J Clin Endocrinol Metab. 2005;90(7):3847‐3853. [DOI] [PubMed] [Google Scholar]
- 95. Cappola AR, Ratcliffe SJ, Bhasin S, et al. Determinants of serum total and free testosterone levels in women over the age of 65 years. J Clin Endocrinol Metab. 2007;92(2):509‐516. [DOI] [PubMed] [Google Scholar]
- 96. Patel SM, Ratcliffe SJ, Reilly MP, et al. Higher serum testosterone concentration in older women is associated with insulin resistance, metabolic syndrome, and cardiovascular disease. J Clin Endocrinol Metab. 2009;94(12):4776‐4784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. The NAMS 2020 GSM Position Statement Editorial Panel . The 2020 genitourinary syndrome of menopause position statement of the North American Menopause Society. Menopause. 2020;27(9):976‐992. [DOI] [PubMed] [Google Scholar]
- 98. Santoro N, Roeca C, Peters BA, Neal-Perry G. The menopause transition: signs, symptoms, and management options. J Clin Endocrinol Metab. 2021;106(1):1‐15. [DOI] [PubMed] [Google Scholar]
- 99. McKinlay SM, Brambilla DJ, Posner JG. The normal menopause transition. Am J Hum Biol. 1992;4(1):37‐46. [DOI] [PubMed] [Google Scholar]
- 100. Burger HG, Dudley EC, Hopper JL, et al. Prospectively measured levels of serum follicle-stimulating hormone, estradiol, and the dimeric inhibins during the menopausal transition in a population-based cohort of women. J Clin Endocrinol Metab. 1999;84(11):4025‐4030. [DOI] [PubMed] [Google Scholar]
- 101. Gracia CR, Freeman EW. Onset of the menopause transition: the earliest signs and symptoms. Obstet Gynecol Clin North Am. 2018;45(4):585‐597. [DOI] [PubMed] [Google Scholar]
- 102. Woods NF, Mitchell ES. The Seattle Midlife Women's Health study: a longitudinal prospective study of women during the menopausal transition and early postmenopause. Womens Midlife Health. 2016;2:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Matthews KA, Kuller LH, Wing RR, Meilahn EN. Biobehavioral aspects of menopause: lessons from the healthy women study. Exp Gerontol. 1994;29(3-4):337‐342. [DOI] [PubMed] [Google Scholar]
- 104. El Khoudary SR, Greendale G, Crawford SL, et al. The menopause transition and women's Health at midlife: a progress report from the study of Women's Health Across the Nation (SWAN). Menopause. 2019;26(10):1213‐1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. El Khoudary SR, Aggarwal B, Beckie TM, et al. Menopause transition and cardiovascular disease risk: implications for timing of early prevention: a scientific statement from the American Heart Association. Circulation. 2020;142(25):e506‐e532. [DOI] [PubMed] [Google Scholar]
- 106. Thurston RC, Karvonen-Gutierrez CA, Derby CA, El Khoudary SR, Kravitz HM, Manson JE. Menopause versus chronologic aging: their roles in women's health. Menopause. 2018;25(8):849‐854. [DOI] [PubMed] [Google Scholar]
- 107. Rocca WA, Gazzuola Rocca L, Smith CY, et al. Loss of ovarian hormones and accelerated somatic and mental aging. Physiology (Bethesda). 2018;33(6):374‐383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Manson JE, Aragaki AK, Bassuk SS, et al. Menopausal estrogen-alone therapy and health outcomes in women with and without bilateral oophorectomy: a randomized trial. Ann Intern Med. 2019;171(6):406‐414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Mishra SR, Chung HF, Waller M, Mishra GD. Duration of estrogen exposure during reproductive years, age at menarche and age at menopause, and risk of cardiovascular disease events, all-cause and cardiovascular mortality: a systematic review and meta-analysis. BJOG. 2021;128(5):809‐821. [DOI] [PubMed] [Google Scholar]
- 110. Manson JE, Woodruff TK. Reproductive health as a marker of subsequent cardiovascular disease: the role of estrogen. JAMA Cardiol. 2016;1(7):776‐777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Freeman EW, Sammel MD, Sanders RJ. Risk of long-term hot flashes after natural menopause: evidence from the Penn Ovarian Aging Study cohort. Menopause. 2014;21(9):924‐932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Tepper PG, Brooks MM, Randolph JF Jr, et al. Characterizing the trajectories of vasomotor symptoms across the menopausal transition. Menopause. 2016;23(10):1067‐1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Thurston RC. Vasomotor symptoms: natural history, physiology, and links with cardiovascular health. Climacteric. 2018;21(2):96‐100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Thurston RC, Aslanidou Vlachos HE, Derby CA, et al. Menopausal vasomotor symptoms and risk of incident cardiovascular disease events in SWAN. J Am Heart Assoc. 2021;10(3):e017416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Stuenkel CA. Managing menopausal vasomotor symptoms in older women. Maturitas. 2021;143:36‐40. [DOI] [PubMed] [Google Scholar]
- 116. Huang AJ, Sawaya GF, Vittinghoff E, Lin F, Grady D. Hot flushes, coronary heart disease, and hormone therapy in postmenopausal women. Menopause. 2009;16(4):639‐643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Manson JE, Chlebowski RT, Stefanick ML, et al. Menopausal hormone therapy and health outcomes during the intervention and extended poststopping phases of the Women's Health Initiative randomized trials. JAMA. 2013;310(13):1353‐1368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Stuenkel CA, Davis SR, Gompel A, et al. Treatment of symptoms of the menopause: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2015;100(11):3975‐4011. [DOI] [PubMed] [Google Scholar]
- 119. Reed SD, LaCroix AZ, Anderson GL, et al. Lights on MsFLASH: a review of contributions. Menopause. 2020;27(4):473‐484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. The 2017 hormone therapy position statement of the North American Menopause Society. Menopause. 2018;25(11):1362‐1387. [DOI] [PubMed] [Google Scholar]
- 121. Santen RJ, Heitjan DF, Gompel A, et al. Approach to managing a postmenopausal patient. J Clin Endocrinol Metab. 2020;105(12):3792‐3806. [DOI] [PubMed] [Google Scholar]
- 122. Davis SR, Baber R, Panay N, et al. Global consensus position statement on the use of testosterone therapy for women. J Clin Endocrinol Metab. 2019;104(10):4660‐4666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Parish SJ, Simon JA, Davis SR, et al. International Society for the study of women's sexual health clinical practice guideline for the use of systemic testosterone for hypoactive sexual desire disorder in women. J Womens Health (Larchmt). 2021;30(4):474‐491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Grossman DC, Curry SJ, Owens DK, et al. Hormone therapy for the primary prevention of chronic conditions in postmenopausal women: US Preventive Services Task Force recommendation statement. JAMA. 2017;318(22):2224‐2233. [DOI] [PubMed] [Google Scholar]
- 125. Stuenkel CA. Deciphering the complex relationship between menopause and heart disease: 25 years and counting. Menopause. 2018;25(9):955‐962. [DOI] [PubMed] [Google Scholar]
- 126. Manson JE, Bassuk SS, Kaunitz AM, Pinkerton JV. The Women's Health Initiative trials of menopausal hormone therapy: lessons learned. Menopause. 2020;27(8):918‐928. [DOI] [PubMed] [Google Scholar]
- 127. Rossouw JE, Prentice RL, Manson JE, et al. Postmenopausal hormone therapy and risk of cardiovascular disease by age and years since menopause. JAMA. 2007;297(13):1465‐1477. [DOI] [PubMed] [Google Scholar]
- 128. Manson JE, Aragaki AK, Rossouw JE, et al. Menopausal hormone therapy and long-term all-cause and cause-specific mortality: the Women's Health Initiative randomized trials. JAMA. 2017;318(10):927‐938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Chlebowski RT, Anderson GL, Aragaki AK, et al. Association of menopausal hormone therapy with breast cancer incidence and mortality during long-term follow-up of the Women's Health Initiative randomized clinical trials. JAMA. 2020;324(4):369‐380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Santen RJ, Stuenkel CA, Davis SR, Pinkerton JV, Gompel A, Lumsden MA. Managing menopausal symptoms and associated clinical issues in breast cancer survivors. J Clin Endocrinol Metab. 2017;102(10):3647‐3661. [DOI] [PubMed] [Google Scholar]
- 131. Clarkson TB, Meléndez GC, Appt SE. Timing hypothesis for postmenopausal hormone therapy: its origin, current status, and future. Menopause. 2013;20(3):342‐353. [DOI] [PubMed] [Google Scholar]
- 132. Somani YB, Pawelczyk JA, De Souza MJ, Kris-Etherton PM, Proctor DN. Aging women and their endothelium: probing the relative role of estrogen on vasodilator function. Am J Physiol Heart Circ Physiol. 2019;317(2):H395‐H404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Pereira RI, Casey BA, Swibas TA, Erickson CB, Wolfe P, Van Pelt RE. Timing of estradiol treatment after menopause may determine benefit or harm to insulin action. J Clin Endocrinol Metab. 2015;100(12):4456‐4462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Schierbeck LL, Rejnmark L, Tofteng CL, et al. Effect of hormone replacement therapy on cardiovascular events in recently postmenopausal women: randomised trial. BMJ. 2012;345:e6409. [DOI] [PubMed] [Google Scholar]
- 135. Harman SM, Black DM, Naftolin F, et al. Arterial imaging outcomes and cardiovascular risk factors in recently menopausal women: a randomized trial. Ann Intern Med. 2014;161(4):249‐260. [DOI] [PubMed] [Google Scholar]
- 136. Hodis HN, Mack WJ, Henderson VW, et al. Vascular effects of early versus late postmenopausal treatment with estradiol. N Engl J Med. 2016;374(13):1221‐1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Cho L, Davis M, Elgendy I, et al. Summary of updated recommendations for primary prevention of cardiovascular disease in women: JACC state-of-the-art review. J Am Coll Cardiol. 2020;75(20):2602‐2618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Shufelt CL, Manson JE. Menopausal hormone therapy and cardiovascular disease: the role of formulation, dose, and route of delivery. J Clin Endocrinol Metab. 2021;106(5):1245‐1254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. ACOG Practice bulletin No. 141: management of menopausal symptoms. Obstet Gynecol. 2014;123(1):202‐216. [DOI] [PubMed] [Google Scholar]
- 140. Harlow SD, Burnett-Bowie SM, Greendale GA, et al. Disparities in reproductive aging and midlife health between black and white women: the study of Women's Health Across the Nation (SWAN). Womens Midlife Health. 2022;8(1):3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Herbert M, Surani A. Oocytes from stem cells. N Engl J Med. 2022;386(2):188‐190. [DOI] [PubMed] [Google Scholar]
- 142. Rosario R, Anderson RA. Novel approaches to fertility restoration in women with premature ovarian insufficiency. Climacteric. 2021;24(5):491‐497. [DOI] [PubMed] [Google Scholar]
- 143. Zhang J, Chen Q, Du D, et al. Can ovarian aging be delayed by pharmacological strategies? Aging (Albany NY). 2019;11(2):817‐832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Llarena N, Hine C. Reproductive longevity and aging: geroscience approaches to maintain long-term ovarian fitness. J Gerontol A Biol Sci Med Sci. 2021;76(9):1551‐1560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Johnson SL, Dunleavy J, Gemmell NJ, Nakagawa S. Consistent age-dependent declines in human semen quality: a systematic review and meta-analysis. Ageing Res Rev. 2015;19:22‐33. [DOI] [PubMed] [Google Scholar]
- 146. Kidd SA, Eskenazi B, Wyrobek AJ. Effects of male age on semen quality and fertility: a review of the literature. Fertil Steril. 2001;75(2):237‐248. [DOI] [PubMed] [Google Scholar]
- 147. Sartorius GA, Nieschlag E. Paternal age and reproduction. Hum Reprod Update. 2010;16(1):65‐79. [DOI] [PubMed] [Google Scholar]
- 148. Barratt CLR, Bjorndahl L, De Jonge CJ, et al. The diagnosis of male infertility: an analysis of the evidence to support the development of global WHO guidance-challenges and future research opportunities. Hum Reprod Update. 2017;23(6):660‐680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Ray PF, Toure A, Metzler-Guillemain C, Mitchell MJ, Arnoult C, Coutton C. Genetic abnormalities leading to qualitative defects of sperm morphology or function. Clin Genet. 2017;91(2):217‐232. [DOI] [PubMed] [Google Scholar]
- 150. Laurentino S, Borgmann J, Gromoll J. On the origin of sperm epigenetic heterogeneity. Reproduction. 2016;151(5):R71‐R78. [DOI] [PubMed] [Google Scholar]
- 151. Chen Q, Yan W, Duan E. Epigenetic inheritance of acquired traits through sperm RNAs and sperm RNA modifications. Nat Rev Genet. 2016;17(12):733‐743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152. Levine H, Jorgensen N, Martino-Andrade A, et al. Temporal trends in sperm count: a systematic review and meta-regression analysis. Hum Reprod Update. 2017;23(6):646‐659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153. Handelsman DJ. Sperm output of healthy men in Australia: magnitude of bias due to self-selected volunteers. Hum Reprod. 1997;12(12):2701‐2705. [DOI] [PubMed] [Google Scholar]
- 154. Setchell BP. Sperm counts in semen of farm animals 1932–1995. Int J Androl. 1997;20(4):209‐214. [DOI] [PubMed] [Google Scholar]
- 155. Bonde JP, Flachs EM, Rimborg S, et al. The epidemiologic evidence linking prenatal and postnatal exposure to endocrine disrupting chemicals with male reproductive disorders: a systematic review and meta-analysis. Hum Reprod Update. 2016;23(1):104‐125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156. D’Onofrio BM, Rickert ME, Frans E, et al. Paternal age at childbearing and offspring psychiatric and academic morbidity. JAMA Psychiatry. 2014;71(4):432‐438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157. Goriely A, Wilkie AO. Paternal age effect mutations and selfish spermatogonial selection: causes and consequences for human disease. Am J Hum Genet. 2012;90(2):175‐200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158. Maher GJ, Ralph HK, Ding Z, et al. Selfish mutations dysregulating RAS-MAPK signaling are pervasive in aged human testes. Genome Res. 2018;28(12):1779‐1790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159. Vlachopoulos CV, Terentes-Printzios DG, Ioakeimidis NK, Aznaouridis KA, Stefanadis CI. Prediction of cardiovascular events and all-cause mortality with erectile dysfunction: a systematic review and meta-analysis of cohort studies. Circ Cardiovasc Qual Outcomes. 2013;6(1):99‐109. [DOI] [PubMed] [Google Scholar]
- 160. Hsu B, Cumming RG, Blyth FM, et al. The longitudinal relationship of sexual function and androgen status in older men: the concord health and ageing in men project. J Clin Endocrinol Metab. 2015;100(4):1350‐1358. [DOI] [PubMed] [Google Scholar]
- 161. Spitzer M, Basaria S, Travison TG, et al. Effect of testosterone replacement on response to sildenafil citrate in men with erectile dysfunction: a parallel, randomized trial. Ann Intern Med. 2012;157(10):681‐691. [DOI] [PubMed] [Google Scholar]
- 162. Handelsman DJ. Global trends in testosterone prescribing, 2000–2011: expanding the spectrum of prescription drug misuse. Med J Aust. 2013;199(8):548‐551. [DOI] [PubMed] [Google Scholar]
- 163. Hatzimouratidis K, Salonia A, Adaikan G, et al. Pharmacotherapy for erectile dysfunction: recommendations from the Fourth International Consultation for Sexual Medicine (ICSM 2015). J Sex Med. 2016;13(4):465‐488. [DOI] [PubMed] [Google Scholar]
- 164. Goldman AL, Bhasin S, Wu FCW, Krishna M, Matsumoto AM, Jasuja R. A reappraisal of testosterone's binding in circulation: physiological and clinical implications. Endocr Rev. 2017;38(4):302‐324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165. Handelsman DJ. Free testosterone: pumping up the tires or ending the free ride? Endocr Rev. 2017;38(4):297‐301. [DOI] [PubMed] [Google Scholar]
- 166. Hsu B, Cumming RG, Blyth FM, et al. Evaluating calculated free testosterone as a predictor of morbidity and mortality independent of testosterone for cross-sectional and 5-year longitudinal health outcomes in older men: the Concord Health and Ageing in Men Project. J Gerontol A Biol Sci Med Sci. 2018;73(6):729‐736. [DOI] [PubMed] [Google Scholar]
- 167. Travison TG, Vesper HW, Orwoll E, et al. Harmonized reference ranges for circulating testosterone levels in men of four cohort studies in the United States and Europe. J Clin Endocrinol Metab. 2017;102(4):1161‐1173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168. Handelsman DJ, Yeap B, Flicker L, Martin S, Wittert GA, Ly LP. Age-specific population centiles for androgen status in men. Eur J Endocrinol. 2015;173(6):809‐817. [DOI] [PubMed] [Google Scholar]
- 169. Wu FC, Tajar A, Beynon JM, et al. Identification of late-onset hypogonadism in middle-aged and elderly men. N Engl J Med. 2010;363(2):123‐135. [DOI] [PubMed] [Google Scholar]
- 170. Harman SM, Metter EJ, Tobin JD, Pearson J, Blackman MR, Baltimore Longitudinal Study of Aging . Longitudinal effects of aging on serum total and free testosterone levels in healthy men. Baltimore Longitudinal Study of Aging. J Clin Endocrinol Metab. 2001;86(2):724‐731. [DOI] [PubMed] [Google Scholar]
- 171. Gray A, Feldman HA, McKinlay JB, Longcope C. Age, disease, and changing sex hormone levels in middle-aged men: results of the Massachussetts Male Aging Study. J Clin Endocrinol Metab. 1991;73(5):1016‐1025. [DOI] [PubMed] [Google Scholar]
- 172. Taya M, Koh E, Izumi K, et al. Comparison of testosterone fractions between Framingham Heart Study participants and Japanese participants. Int J Urol. 2014;21(7):689‐695. [DOI] [PubMed] [Google Scholar]
- 173. Xia F, Wang N, Han B, et al. Hypothalamic-pituitary-gonadal axis in aging men and women: increasing total testosterone in aging men. Neuroendocrinology. 2017;104(3):291‐301. [DOI] [PubMed] [Google Scholar]
- 174. Kelsey TW, Li LQ, Mitchell RT, Whelan A, Anderson RA, Wallace WH. A validated age-related normative model for male total testosterone shows increasing variance but no decline after age 40 years. PLoS One. 2014;9(10):e109346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175. Camacho EM, Huhtaniemi IT, O’Neill TW, et al. Age-associated changes in hypothalamic-pituitary-testicular function in middle-aged and older men are modified by weight change and lifestyle factors: longitudinal results from the European Male Ageing Study. Eur J Endocrinol. 2013;168(3):445‐455. [DOI] [PubMed] [Google Scholar]
- 176. Sartorius G, Spasevska S, Idan A, et al. Serum testosterone, dihydrotestosterone and estradiol concentrations in older men self-reporting very good health: the healthy man study. Clin Endocrinol (Oxf). 2012;77(5):755‐763. [DOI] [PubMed] [Google Scholar]
- 177. Shi Z, Araujo AB, Martin S, O’Loughlin P, Wittert GA. Longitudinal changes in testosterone over five years in community-dwelling men. J Clin Endocrinol Metab. 2013;98(8):3289‐3297. [DOI] [PubMed] [Google Scholar]
- 178. Townsley DM, Dumitriu B, Liu D, et al. Danazol treatment for telomere diseases. N Engl J Med. 2016;374(20):1922‐1931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179. Yeap BB, Knuiman MW, Divitini ML, et al. Epidemiological and Mendelian randomization studies of dihydrotestosterone and estradiol and leukocyte telomere length in men. J Clin Endocrinol Metab. 2016;101(3):1299‐1306. [DOI] [PubMed] [Google Scholar]
- 180. Calado RT, Yewdell WT, Wilkerson KL, et al. Sex hormones, acting on the TERT gene, increase telomerase activity in human primary hematopoietic cells. Blood. 2009;114(11):2236‐2243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181. Handelsman DJ. The illusory case for treatment of an invented disease. Front Endocrinol (Lausanne). 2022;12:682620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182. Handelsman DJ. Irrational exuberance in testosterone prescribing: when will the bubble burst? Med Care. 2015;53(9):743‐745. [DOI] [PubMed] [Google Scholar]
- 183. Baillargeon J, Kuo YF, Westra JR, Urban RJ, Goodwin JS. Testosterone prescribing in the United States, 2002–2016. JAMA. 2018;320(2):200‐202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184. Morden NE, Woloshin S, Brooks CG, Schwartz LM. Trends in testosterone prescribing for age-related hypogonadism in men with and without heart disease. JAMA Intern Med. 2018;179(3):446‐448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185. Handelsman DJ. Androgen physiology, pharmacology, use and misuse. In: Feingold KR, Anawalt B, Boyce A, et al., eds. Endotext [Internet]. South Dartmouth (MA): MDText.com, Inc.; 2000. [PubMed] [Google Scholar]
- 186. Ventimiglia E, Capogrosso P, Montorsi F, Salonia A. The safety of phosphodiesterase type 5 inhibitors for erectile dysfunction. Expert Opin Drug Saf. 2016;15(2):141‐152. [DOI] [PubMed] [Google Scholar]
- 187. Basaria S, Coviello AD, Travison TG, et al. Adverse events associated with testosterone administration. N Engl J Med. 2010;363(2):109‐122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188. Srinivas-Shankar U, Roberts SA, Connolly MJ, et al. Effects of testosterone on muscle strength, physical function, body composition, and quality of life in intermediate-frail and frail elderly men: a randomized, double-blind, placebo-controlled study. J Clin Endocrinol Metab. 2010;95(2):639‐650. [DOI] [PubMed] [Google Scholar]
- 189. Kenny AM, Prestwood KM, Gruman CA, Marcello KM, Raisz LG. Effects of transdermal testosterone on bone and muscle in older men with low bioavailable testosterone levels. J Gerontol A Biol Sci Med Sci. 2001;56(5):M266‐M272. [DOI] [PubMed] [Google Scholar]
- 190. Emmelot-Vonk MH, Verhaar HJ, Nakhai Pour HR, et al. Effect of testosterone supplementation on functional mobility, cognition, and other parameters in older men: a randomized controlled trial. JAMA. 2008;299(1):39‐52. [DOI] [PubMed] [Google Scholar]
- 191. Snyder PJ, Bhasin S, Cunningham GR, et al. Effects of testosterone treatment in older men. N Engl J Med. 2016;374(7):611‐624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192. Snyder PJ, Bhasin S, Cunningham GR, et al. Lessons from the testosterone trials. Endocr Rev. 2018;39(3):369‐386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193. Budoff MJ, Ellenberg SS, Lewis CE, et al. Testosterone treatment and coronary artery plaque volume in older men with low testosterone. JAMA. 2017;317(7):708‐716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194. Hudson J, Cruickshank M, Quinton R, et al. Adverse cardiovascular events and mortality in men during testosterone treatment: an individual patient and aggregate data meta-analysis. Lancet Healthy Longev. 2022;3(6):e381‐e393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195. Bhasin S, Lincoff AM, Basaria S, et al. Effects of long-term testosterone treatment on cardiovascular outcomes in men with hypogonadism: rationale and design of the TRAVERSE study. Am Heart J. 2022;245:41‐50. [DOI] [PubMed] [Google Scholar]
- 196. Albert SG, Morley JE. Testosterone therapy, association with age, initiation and mode of therapy with cardiovascular events: a systematic review. Clin Endocrinol (Oxf). 2016;85(3):436‐443. [DOI] [PubMed] [Google Scholar]
- 197. Alexander GC, Iyer G, Lucas E, Lin D, Singh S. Cardiovascular risks of exogenous testosterone use among men: a systematic review and meta-analysis. Am J Med. 2017;130(3):293‐305. [DOI] [PubMed] [Google Scholar]
- 198. Onasanya O, Iyer G, Lucas E, Lin D, Singh S, Alexander GC. Association between exogenous testosterone and cardiovascular events: an overview of systematic reviews. Lancet Diabetes Endocrinol. 2016;4(11):943‐956. [DOI] [PubMed] [Google Scholar]
- 199. Martinez C, Suissa S, Rietbrock S, et al. Testosterone treatment and risk of venous thromboembolism: population based case-control study. BMJ. 2016;355:i5968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200. Xu L, Freeman G, Cowling BJ, Schooling CM. Testosterone therapy and cardiovascular events among men: a systematic review and meta-analysis of placebo-controlled randomized trials. BMC Med. 2013;11:108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201. Wittert G, Bracken K, Robledo KP, et al. Testosterone treatment to prevent or revert type 2 diabetes in men enrolled in a lifestyle programme (T4DM): a randomised, double-blind, placebo-controlled, 2-year, phase 3b trial. Lancet Diabetes Endocrinol. 2021;9(1):32‐45. [DOI] [PubMed] [Google Scholar]
- 202. Handelsman DJ, Desai R, Conway AJ, et al. Recovery of male reproductive endocrine function after ceasing prolonged testosterone undecanoate injections. Eur J Endocrinol. 2022;186(3):307‐318. [DOI] [PubMed] [Google Scholar]
- 203. Roddam AW, Allen NE, Appleby P, Key TJ. Endogenous sex hormones and prostate cancer: a collaborative analysis of 18 prospective studies. J Natl Cancer Inst. 2008;100(3):170‐183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204. Boyle P, Koechlin A, Bota M, et al. Endogenous and exogenous testosterone and the risk of prostate cancer and increased prostate specific antigen (PSA): a meta-analysis. BJU Int. 2016;118(5):731‐741. [DOI] [PubMed] [Google Scholar]
- 205. Cui Y, Zong H, Yan H, Zhang Y. The effect of testosterone replacement therapy on prostate cancer: a systematic review and meta-analysis. Prostate Cancer Prostatic Dis. 2014;17(2):132‐143. [DOI] [PubMed] [Google Scholar]
- 206. Gregerman RI, Gaffney GW, Shock NW, Crowder SE. Thyroxine turnover in euthyroid man with special reference to changes with age. J Clin Invest. 1962;41(11):2065‐2074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207. Herrmann J, Heinen E, Kroll HJ, Rudorff KH, Kruskemper HL. Thyroid function and thyroid hormone metabolism in elderly people. Low T3–syndrome in old age? Klin Wochenschr. 1981;59(7):315‐323. [DOI] [PubMed] [Google Scholar]
- 208. Waring AC, Arnold AM, Newman AB, Bùžková P, Hirsch C, Cappola AR. Longitudinal changes in thyroid function in the oldest old and survival: the Cardiovascular Health Study All-Stars Study. J Clin Endocrinol Metab. 2012;97(11):3944‐3950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209. Mariotti S, Barbesino G, Caturegli P, et al. Complex alteration of thyroid function in healthy centenarians. J Clin Endocrinol Metab. 1993;77(5):1130‐1134. [DOI] [PubMed] [Google Scholar]
- 210. Surks MI, Hollowell JG. Age-specific distribution of serum thyrotropin and antithyroid antibodies in the US population: implications for the prevalence of subclinical hypothyroidism. J Clin Endocrinol Metab. 2007;92(12):4575‐4582. [DOI] [PubMed] [Google Scholar]
- 211. Bremner AP, Feddema P, Leedman PJ, et al. Age-related changes in thyroid function: a longitudinal study of a community-based cohort. J Clin Endocrinol Metab. 2012;97(5):1554‐1562. [DOI] [PubMed] [Google Scholar]
- 212. Stott DJ, Rodondi N, Kearney PM, et al. Thyroid hormone therapy for older adults with subclinical hypothyroidism. N Engl J Med. 2017;376(26):2534‐2544. [DOI] [PubMed] [Google Scholar]
- 213. Rodondi N, den Elzen WP, Bauer DC, et al. Subclinical hypothyroidism and the risk of coronary heart disease and mortality. JAMA. 2010;304(12):1365‐1374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214. Chaker L, Baumgartner C, den Elzen WP, et al. Subclinical hypothyroidism and the risk of stroke events and fatal stroke: an individual participant data analysis. J Clin Endocrinol Metab. 2015;100(6):2181‐2191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215. van Vliet NA, van Heemst D, Almeida OP, et al. Association of thyroid dysfunction with cognitive function: an individual participant data analysis. JAMA Intern Med. 2021;181(11):1440‐1450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216. Gussekloo J, van Exel E, de Craen AJ, Meinders AE, Frölich M, Westendorp RG. Thyroid status, disability and cognitive function, and survival in old age. JAMA. 2004;292(21):2591‐2599. [DOI] [PubMed] [Google Scholar]
- 217. Gencer B, Collet TH, Virgini V, et al. Subclinical thyroid dysfunction and the risk of heart failure events: an individual participant data analysis from 6 prospective cohorts. Circulation. 2012;126(9):1040‐1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218. Simonsick EM, Newman AB, Ferrucci L, et al. Subclinical hypothyroidism and functional mobility in older adults. Arch Intern Med. 2009;169(21):2011‐2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219. Pearce SH, Razvi S, Yadegarfar ME, et al. Serum thyroid function, mortality and disability in advanced old age: the Newcastle 85+ study. J Clin Endocrinol Metab. 2016;101(11):4385‐4394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220. Razvi S, Weaver JU, Butler TJ, Pearce SH. Levothyroxine treatment of subclinical hypothyroidism, fatal and nonfatal cardiovascular events, and mortality. Arch Intern Med. 2012;172(10):811‐817. [DOI] [PubMed] [Google Scholar]
- 221. Andersen MN, Olsen AM, Madsen JC, et al. Levothyroxine substitution in patients with subclinical hypothyroidism and the risk of myocardial infarction and mortality. PLoS One. 2015;10(6):e0129793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222. Somwaru LL, Arnold AM, Joshi N, Fried LP, Cappola AR. High frequency of and factors associated with thyroid hormone over-replacement and under-replacement in men and women aged 65 and over. J Clin Endocrinol Metab. 2009;94(4):1342‐1345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223. Collet TH, Gussekloo J, Bauer DC, et al. Subclinical hyperthyroidism and the risk of coronary heart disease and mortality. Arch Intern Med. 2012;172(10):799‐809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224. Blum MR, Bauer DC, Collet TH, et al. Subclinical thyroid dysfunction and fracture risk: a meta-analysis. JAMA. 2015;313(20):2055‐2065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225. Rieben C, Segna D, da Costa BR, et al. Subclinical thyroid dysfunction and the risk of cognitive decline: a meta-analysis of prospective cohort studies. J Clin Endocrinol Metab. 2016;101(12):4945‐4954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226. Yeap BB, Alfonso H, Chubb SA, et al. Higher free thyroxine levels predict increased incidence of dementia in older men: the health in men study. J Clin Endocrinol Metab. 2012;97(12):E2230‐E2237. [DOI] [PubMed] [Google Scholar]
- 227. Cappola AR, Arnold AM, Wulczyn K, Carlson M, Robbins J, Psaty BM. Thyroid function in the euthyroid range and adverse outcomes in older adults. J Clin Endocrinol Metab. 2015;100(3):1088‐1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228. Chaker L, Cappola AR, Mooijaart SP, Peeters RP. Clinical aspects of thyroid function during ageing. Lancet Diabetes Endocrinol. 2018;6(9):733‐742. [DOI] [PubMed] [Google Scholar]
- 229. De Leo S, Lee SY, Braverman LE. Hyperthyroidism. Lancet. 2016;388(10047):906‐918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230. Doucet J, Trivalle C, Chassagne P, et al. Does age play a role in clinical presentation of hypothyroidism? J Am Geriatr Soc. 1994;42(9):984‐986. [DOI] [PubMed] [Google Scholar]
- 231. Trivalle C, Doucet J, Chassagne P, et al. Differences in the signs and symptoms of hyperthyroidism in older and younger patients. J Am Geriatr Soc. 1996;44(1):50‐53. [DOI] [PubMed] [Google Scholar]
- 232. Carle A, Pedersen IB, Knudsen N, et al. Hypothyroid symptoms fail to predict thyroid insufficiency in old people: a population-based case-control study. Am J Med. 2016;129(10):1082‐1092. [DOI] [PubMed] [Google Scholar]
- 233. Burch HB. Drug effects on the thyroid. N Engl J Med. 2019;381(8):749‐761. [DOI] [PubMed] [Google Scholar]
- 234. Mooijaart SP, Du Puy RS, Stott DJ, et al. Association between levothyroxine treatment and thyroid-related symptoms among adults aged 80 years and older with subclinical hypothyroidism. JAMA. 2019;322(20):1977‐1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235. Eastell R, Rosen CJ, Black DM, Cheung AM, Murad MH, Shoback D. Pharmacological management of osteoporosis in postmenopausal women: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2019;104(5):1595‐1622. [DOI] [PubMed] [Google Scholar]
- 236. Shoback D, Rosen CJ, Black DM, Cheung AM, Murad MH, Eastell R. Pharmacological management of osteoporosis in postmenopausal women: an Endocrine Society guideline update. J Clin Endocrinol Metab. 2020;105(3):587‐594. [DOI] [PubMed] [Google Scholar]
- 237. Compston JE, McClung MR, Leslie WD. Osteoporosis. Lancet. 2019;393(10169):364‐376. [DOI] [PubMed] [Google Scholar]
- 238. Wright NC, Looker AC, Saag KG, et al. The recent prevalence of osteoporosis and low bone mass in the United States based on bone mineral density at the femoral neck or lumbar spine. J Bone Miner Res. 2014;29(11):2520‐2526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239. Center JR, Nguyen TV, Schneider D, Sambrook PN, Eisman JA. Mortality after all major types of osteoporotic fracture in men and women: an observational study. Lancet. 1999;353(9156):878‐882. [DOI] [PubMed] [Google Scholar]
- 240. Hernlund E, Svedbom A, Ivergård M, et al. Osteoporosis in the European Union: medical management, epidemiology and economic burden. A report prepared in collaboration with the International Osteoporosis Foundation (IOF) and the European Federation of Pharmaceutical Industry Associations (EFPIA). Arch Osteoporos. 2013;8(1):136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241. Hansen D, Bazell C, Pellizari P, Peynson P. Medicare cost of osteoporotic fractures. 2019. Accessed May 5, 2023. https://www.milliman.com/-/media/Milliman/importedfiles/ektron/medicare_cost_of_osteoporotic_fractures.ashx
- 242. Gold DT. The nonskeletal consequences of osteoporotic fractures. Psychologic and social outcomes. Rheum Dis Clin North Am. 2001;27(1):255‐262. [DOI] [PubMed] [Google Scholar]
- 243. Fink HA, Ensrud KE, Nelson DB, et al. Disability after clinical fracture in postmenopausal women with low bone density: the Fracture Intervention Trial (FIT). Osteoporos Int. 2003;14(1):69‐76. [DOI] [PubMed] [Google Scholar]
- 244. Brauer CA, Coca-Perraillon M, Cutler DM, Rosen AB. Incidence and mortality of hip fractures in the United States. JAMA. 2009;302(14):1573‐1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245. Kendler DL, Bauer DC, Davison KS, et al. Vertebral fractures: clinical importance and management. Am J Med. 2016;129(2):221.e1‐221.e10. [DOI] [PubMed] [Google Scholar]
- 246. Dyer SM, Crotty M, Fairhall N, et al. A critical review of the long-term disability outcomes following hip fracture. BMC Geriatr. 2016;16(1):158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247. Yusuf AA, Matlon TJ, Grauer A, Barron R, Chandler D, Peng Y. Utilization of osteoporosis medication after a fragility fracture among elderly Medicare beneficiaries. Arch Osteoporos. 2016;11(1):31. [DOI] [PubMed] [Google Scholar]
- 248. Lewiecki EM, Chastek B, Sundquist K, et al. Osteoporotic fracture trends in a population of US managed care enrollees from 2007 to 2017. Osteoporos Int. 2020;31(7):1299‐1304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249. Sepúlveda-Loyola W, Phu S, Bani Hassan E, et al. The joint occurrence of osteoporosis and sarcopenia (osteosarcopenia): definitions and characteristics. J Am Med Dir Assoc. 2020;21(2):220‐225. [DOI] [PubMed] [Google Scholar]
- 250. Sherrington C, Fairhall NJ, Wallbank GK, et al. Exercise for preventing falls in older people living in the community. Cochrane Database Syst Rev. 2019;1(1):Cd012424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251. Kanis JA, Johnell O, Oden A, Dawson A, De Laet C, Jonsson B. Ten year probabilities of osteoporotic fractures according to BMD and diagnostic thresholds. Osteoporos Int. 2001;12(12):989‐995. [DOI] [PubMed] [Google Scholar]
- 252. van Geel TA, Huntjens KM, van den Bergh JP, Dinant GJ, Geusens PP. Timing of subsequent fractures after an initial fracture. Curr Osteoporos Rep. 2010;8(3):118‐122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253. Ahmed LA, Center JR, Bjørnerem A, et al. Progressively increasing fracture risk with advancing age after initial incident fragility fracture: the Tromsø study. J Bone Miner Res. 2013;28(10):2214‐2221. [DOI] [PubMed] [Google Scholar]
- 254. US Preventive Services Task Force . Screening for osteoporosis: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2011;154(5):356‐364. [DOI] [PubMed] [Google Scholar]
- 255. Cosman F, de Beur SJ, LeBoff MS, et al. Clinician's guide to prevention and treatment of osteoporosis. Osteoporos Int. 2014;25(10):2359‐2381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256. Camacho PM, Petak SM, Binkley N, et al. American Association of Clinical Endocrinologists /American College of Endocrinology clinical practice guidelines for the diagnosis and treatment of postmenopausal osteoporosis—2020 update. Endocr Pract. 2020;26(Suppl 1):1‐46. [DOI] [PubMed] [Google Scholar]
- 257. International Society for Clinical Densitometry . ISCD adult official positions: indications for Bone Mineral Density (BMD) testing. 2019. Accessed May 5, 2023. https://iscd.org/learn/official-positions/adult-positions/
- 258. Kanis JA, Hans D, Cooper C, et al. Interpretation and use of FRAX in clinical practice. Osteoporos Int. 2011;22(9):2395‐2411. [DOI] [PubMed] [Google Scholar]
- 259. Kendler DL, Marin F, Zerbini CAF, et al. Effects of teriparatide and risedronate on new fractures in post-menopausal women with severe osteoporosis (VERO): a multicentre, double-blind, double-dummy, randomised controlled trial. Lancet. 2018;391(10117):230‐240. [DOI] [PubMed] [Google Scholar]
- 260. Kanis JA, Harvey NC, McCloskey E, et al. Algorithm for the management of patients at low, high and very high risk of osteoporotic fractures. Osteoporos Int. 2020;31(1):1‐12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261. Black DM, Bauer DC, Vittinghoff E, et al. Treatment-related changes in bone mineral density as a surrogate biomarker for fracture risk reduction: meta-regression analyses of individual patient data from multiple randomised controlled trials. Lancet Diabetes Endocrinol. 2020;8(8):672‐682. [DOI] [PubMed] [Google Scholar]
- 262. Lewiecki EM, Kendler DL, Davison KS, et al. Western Osteoporosis Alliance clinical practice series: treat-to-target for osteoporosis. Am J Med. 2019;132(11):e771‐e777. [DOI] [PubMed] [Google Scholar]
- 263. Knopp-Sihota JA, Newburn-Cook CV, Homik J, Cummings GG, Voaklander D. Calcitonin for treating acute and chronic pain of recent and remote osteoporotic vertebral compression fractures: a systematic review and meta-analysis. Osteoporos Int. 2012;23(1):17‐38. [DOI] [PubMed] [Google Scholar]
- 264. Tsourdi E, Zillikens MC, Meier C, et al. Fracture risk and management of discontinuation of denosumab therapy: a systematic review and position statement by ECTS. J Clin Endocrinol Metab. 2021;106(1):264‐281. [DOI] [PubMed] [Google Scholar]
- 265. Cummings SR, McCulloch C. Explanations for the difference in rates of cardiovascular events in a trial of alendronate and romosozumab. Osteoporos Int. 2020;31(6):1019‐1021. [DOI] [PubMed] [Google Scholar]
- 266. Ross AC, Manson JE, Abrams SA, et al. The 2011 report on dietary reference intakes for calcium and vitamin D from the institute of medicine: what clinicians need to know. J Clin Endocrinol Metab. 2011;96(1):53‐58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267. Bauer JM, Diekmann R. Protein supplementation with aging. Curr Opin Clin Nutr Metab Care. 2015;18(1):24‐31. [DOI] [PubMed] [Google Scholar]
- 268. Zoltick ES, Sahni S, McLean RR, Quach L, Casey VA, Hannan MT. Dietary protein intake and subsequent falls in older men and women: the Framingham study. J Nutr Health Aging. 2011;15(2):147‐152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269. Martyn-St James M, Carroll S. Meta-analysis of walking for preservation of bone mineral density in postmenopausal women. Bone. 2008;43(3):521‐531. [DOI] [PubMed] [Google Scholar]
- 270. de Bot R, Veldman HD, Witlox AM, van Rhijn LW, Hiligsmann M. Hip protectors are cost-effective in the prevention of hip fractures in patients with high fracture risk. Osteoporos Int. 2020;31(7):1217‐1229. [DOI] [PubMed] [Google Scholar]
- 271. Centers for Disease Control and Prevention/STEADI—Older Adult Fall Prevention. 2020. Accessed May 5, 2023. https://www.cdc.gov/steadi/index.html
- 272. Caitriona C, Mark MG, Elaine H, et al. Management of hospitalised osteoporotic vertebral fractures. Arch Osteoporos. 2020;15(1):14. [DOI] [PubMed] [Google Scholar]
- 273. Dyer SM, Perracini MR, Smith T, et al. Rehabilitation following hip fracture. In: Falaschi P, Marsh D, eds. Orthogeriatrics: The Management of Older Patients with Fragility Fractures. Springer; 2021:183‐222. [PubMed] [Google Scholar]
- 274. Sinaki M. Exercise for patients with osteoporosis: management of vertebral compression fractures and trunk strengthening for fall prevention. PM R. 2012;4(11):882‐888. [DOI] [PubMed] [Google Scholar]
- 275. Boonen S, McClung MR, Eastell R, El-Hajj Fuleihan G, Barton IP, Delmas P. Safety and efficacy of risedronate in reducing fracture risk in osteoporotic women aged 80 and older: implications for the use of antiresorptive agents in the old and oldest old. J Am Geriatr Soc. 2004;52(11):1832‐1839. [DOI] [PubMed] [Google Scholar]
- 276. Boonen S, Black DM, Colon-Emeric CS, et al. Efficacy and safety of a once-yearly intravenous zoledronic acid 5 mg for fracture prevention in elderly postmenopausal women with osteoporosis aged 75 and older. J Am Geriatr Soc. 2010;58(2):292‐299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277. Boonen S, Adachi JD, Man Z, et al. Treatment with denosumab reduces the incidence of new vertebral and hip fractures in postmenopausal women at high risk. J Clin Endocrinol Metab. 2011;96(6):1727‐1736. [DOI] [PubMed] [Google Scholar]
- 278. Boonen S, Marin F, Mellstrom D, et al. Safety and efficacy of teriparatide in elderly women with established osteoporosis: bone anabolic therapy from a geriatric perspective. J Am Geriatr Soc. 2006;54(5):782‐789. [DOI] [PubMed] [Google Scholar]
- 279. Niimi R, Kono T, Nishihara A, et al. Usefulness of daily teriparatide treatment in elderly patients over 80 years of age. Osteoporos Int. 2016;27(5):1869‐1874. [DOI] [PubMed] [Google Scholar]
- 280. McClung MR, Harvey NC, Fitzpatrick LA, et al. Effects of abaloparatide on bone mineral density and risk of fracture in postmenopausal women aged 80 years or older with osteoporosis. Menopause. 2018;25(7):767‐771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281. Greenspan SL, Perera S, Ferchak MA, Nace DA, Resnick NM. Efficacy and safety of single-dose zoledronic acid for osteoporosis in frail elderly women: a randomized clinical trial. JAMA Intern Med. 2015;175(6):913‐921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282. Lyles KW, Colon-Emeric CS, Magaziner JS, et al. Zoledronic acid and clinical fractures and mortality after hip fracture. N Engl J Med. 2007;357(18):1799‐1809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283. Fuleihan Gel H, Bouillon R, Clarke B, et al. Serum 25-hydroxyvitamin D levels: variability, knowledge gaps, and the concept of a desirable range. J Bone Miner Res. 2015;30(7):1119‐1133. [DOI] [PubMed] [Google Scholar]
- 284. Bouillon R, Bikle D. Vitamin D metabolism revised: fall of dogmas. J Bone Miner Res. 2019;34(11):1985‐1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285. Pusceddu I, Farrell CJ, Di Pierro AM, Jani E, Herrmann W, Herrmann M. The role of telomeres and vitamin D in cellular aging and age-related diseases. Clin Chem Lab Med. 2015;53(11):1661‐1678. [DOI] [PubMed] [Google Scholar]
- 286. Autier P, Boniol M, Pizot C, Mullie P. Vitamin D status and ill health: a systematic review. Lancet Diabetes Endocrinol. 2014;2(1):76‐89. [DOI] [PubMed] [Google Scholar]
- 287. Caristia S, Filigheddu N, Barone-Adesi F, et al. Vitamin D as a biomarker of ill health among the over-50s: a systematic review of cohort studies. Nutrients. 2019;11(10):2384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288. Bouillon R, Marcocci C, Carmeliet G, et al. Skeletal and extraskeletal actions of vitamin D: current evidence and outstanding questions. Endocr Rev. 2019;40(4):1109‐1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289. Ordóñez-Morán P, Muñoz A. Nuclear receptors: genomic and non-genomic effects converge. Cell Cycle. 2009;8(11):1675‐1680. [DOI] [PubMed] [Google Scholar]
- 290. Christakos S, Dhawan P, Porta A, Mady LJ, Seth T. Vitamin D and intestinal calcium absorption. Mol Cell Endocrinol. 2011;347(1-2):25‐29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291. Hill TR, Granic A, Aspray TJ. Vitamin D and ageing. Subcell Biochem. 2018;90:191‐220. [DOI] [PubMed] [Google Scholar]
- 292. de Jongh RT, van Schoor NM, Lips P. Changes in vitamin D endocrinology during aging in adults. Mol Cell Endocrinol. 2017;453:144‐150. [DOI] [PubMed] [Google Scholar]
- 293. Gonçalves de Carvalho CM, Ribeiro SM. Aging, low-grade systemic inflammation and vitamin D: a mini-review. Eur J Clin Nutr. 2017;71(4):434‐440. [DOI] [PubMed] [Google Scholar]
- 294. Wacker M, Holick MF. Sunlight and vitamin D: a global perspective for health. Dermatoendocrinol. 2013;5(1):51‐108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295. Chalcraft JR, Cardinal LM, Wechsler PJ, et al. Vitamin D synthesis following a single bout of sun exposure in older and younger men and women. Nutrients. 2020;12(8):2237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 296. Theodoratou E, Tzoulaki I, Zgaga L, Ioannidis JP. Vitamin D and multiple health outcomes: umbrella review of systematic reviews and meta-analyses of observational studies and randomised trials. BMJ. 2014;348:g2035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 297. Chapuy MC, Arlot ME, Duboeuf F, et al. Vitamin D3 and calcium to prevent hip fractures in elderly women. N Engl J Med. 1992;327(23):1637‐1642. [DOI] [PubMed] [Google Scholar]
- 298. Remelli F, Vitali A, Zurlo A, Volpato S. Vitamin D deficiency and sarcopenia in older persons. Nutrients. 2019;11(12):2861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 299. Ju SY, Lee JY, Kim DH. Low 25-hydroxyvitamin D levels and the risk of frailty syndrome: a systematic review and dose-response meta-analysis. BMC Geriatr. 2018;18(1):206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 300. Brøndum-Jacobsen P, Benn M, Jensen GB, Nordestgaard BG. 25-hydroxyvitamin D levels and risk of ischemic heart disease, myocardial infarction, and early death: population-based study and meta-analyses of 18 and 17 studies. Arterioscler Thromb Vasc Biol. 2012;32(11):2794‐2802. [DOI] [PubMed] [Google Scholar]
- 301. Brøndum-Jacobsen P, Nordestgaard BG, Schnohr P, Benn M. 25-hydroxyvitamin D and symptomatic ischemic stroke: an original study and meta-analysis. Ann Neurol. 2013;73(1):38‐47. [DOI] [PubMed] [Google Scholar]
- 302. Gandini S, Boniol M, Haukka J, et al. Meta-analysis of observational studies of serum 25-hydroxyvitamin D levels and colorectal, breast and prostate cancer and colorectal adenoma. Int J Cancer. 2011;128(6):1414‐1424. [DOI] [PubMed] [Google Scholar]
- 303. Heine-Bröring RC, Winkels RM, Renkema JM, et al. Dietary supplement use and colorectal cancer risk: a systematic review and meta-analyses of prospective cohort studies. Int J Cancer. 2015;136(10):2388‐2401. [DOI] [PubMed] [Google Scholar]
- 304. Goodwill AM, Szoeke C. A systematic review and meta-analysis of the effect of low vitamin D on cognition. J Am Geriatr Soc. 2017;65(10):2161‐2168. [DOI] [PubMed] [Google Scholar]
- 305. Gaksch M, Jorde R, Grimnes G, et al. Vitamin D and mortality: individual participant data meta-analysis of standardized 25–hydroxyvitamin D in 26916 individuals from a European consortium. PLoS One. 2017;12(2):e0170791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 306. Cashman KD, Seamans KM, Lucey AJ, et al. Relative effectiveness of oral 25-hydroxyvitamin D3 and vitamin D3 in raising wintertime serum 25-hydroxyvitamin D in older adults. Am J Clin Nutr. 2012;95(6):1350‐1356. [DOI] [PubMed] [Google Scholar]
- 307. Pérez-Castrillón JL, Dueñas-Laita A, Brandi ML, et al. Calcifediol is superior to cholecalciferol in improving vitamin D status in postmenopausal women: a randomized trial. J Bone Miner Res. 2021;36(10):1967‐1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 308. Minisola S, Cianferotti L, Biondi P, et al. Correction of vitamin D status by calcidiol: pharmacokinetic profile, safety, and biochemical effects on bone and mineral metabolism of daily and weekly dosage regimens. Osteoporos Int. 2017;28(11):3239‐3249. [DOI] [PubMed] [Google Scholar]
- 309. Quesada-Gomez JM, Bouillon R. Is calcifediol better than cholecalciferol for vitamin D supplementation? Osteoporosis Int. 2018;29(8):1697‐1711. [DOI] [PubMed] [Google Scholar]
- 310. Tripkovic L, Lambert H, Hart K, et al. Comparison of vitamin D2 and vitamin D3 supplementation in raising serum 25-hydroxyvitamin D status: a systematic review and meta-analysis. Am J Clin Nutr. 2012;95(6):1357‐1364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 311. Ish-Shalom S, Segal E, Salganik T, Raz B, Bromberg IL, Vieth R. Comparison of daily, weekly, and monthly vitamin D3 in ethanol dosing protocols for two months in elderly hip fracture patients. J Clin Endocrinol Metab. 2008;93(9):3430‐3435. [DOI] [PubMed] [Google Scholar]
- 312. Trivedi DP, Doll R, Khaw KT. Effect of four monthly oral vitamin D3 (cholecalciferol) supplementation on fractures and mortality in men and women living in the community: randomised double blind controlled trial. BMJ. 2003;326(7387):469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 313. Sanders KM, Seibel MJ. Therapy: new findings on vitamin D3 supplementation and falls—when more is perhaps not better. Nat Rev Endocrinol. 2016;12(4):190‐191. [DOI] [PubMed] [Google Scholar]
- 314. Navaneethan SD, Zoungas S, Caramori ML, et al. Diabetes management in chronic kidney disease: synopsis of the KDIGO 2022 Clinical Practice Guideline Update. Ann Intern Med. 2023;176(3):381‐387. [DOI] [PubMed] [Google Scholar]
- 315. Ferri E, Casati M, Cesari M, Vitale G, Arosio B. Vitamin D in physiological and pathological aging: lesson from centenarians. Rev Endocr Metab Disord. 2019;20(3):273‐282. [DOI] [PubMed] [Google Scholar]
- 316. Grant AM, Avenell A, Campbell MK, et al. Oral vitamin D3 and calcium for secondary prevention of low-trauma fractures in elderly people (randomised evaluation of calcium or vitamin D, RECORD): a randomised placebo-controlled trial. Lancet. 2005;365(9471):1621‐1628. [DOI] [PubMed] [Google Scholar]
- 317. Jackson RD, LaCroix AZ, Gass M, et al. Calcium plus vitamin D supplementation and the risk of fractures. N Engl J Med. 2006;354(7):669‐683. [DOI] [PubMed] [Google Scholar]
- 318. Lappe J, Watson P, Travers-Gustafson D, et al. Effect of vitamin D and calcium supplementation on cancer incidence in older women: a randomized clinical trial. JAMA. 2017;317(12):1234‐1243. [DOI] [PubMed] [Google Scholar]
- 319. Scragg R, Stewart AW, Waayer D, et al. Effect of monthly high-dose vitamin D supplementation on cardiovascular disease in the vitamin D assessment study : a randomized clinical trial. JAMA Cardiol. 2017;2(6):608‐616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 320. Pittas AG, Dawson-Hughes B, Sheehan P, et al. Vitamin D supplementation and prevention of type 2 diabetes. N Engl J Med. 2019;381(6):520‐530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 321. Manson JE, Cook NR, Lee IM, et al. Vitamin D supplements and prevention of cancer and cardiovascular disease. N Engl J Med. 2019;380(1):33‐44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 322. Bischoff-Ferrari HA, Vellas B, Rizzoli R, et al. Effect of vitamin D supplementation, omega-3 fatty acid supplementation, or a strength-training exercise program on clinical outcomes in older adults: the DO-HEALTH randomized clinical trial. JAMA. 2020;324(18):1855‐1868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 323. Neale RE, Baxter C, Romero BD, et al. The D-health trial: a randomised controlled trial of the effect of vitamin D on mortality. Lancet Diabetes Endocrinol. 2022;10(2):120‐128. [DOI] [PubMed] [Google Scholar]
- 324. Virtanen JK, Nurmi T, Aro A, et al. Vitamin D supplementation and prevention of cardiovascular disease and cancer in the Finnish vitamin D trial-a randomized controlled trial. Am J Clin Nutr. 2022;115(5):1300‐1310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 325. Chakhtoura M, Bacha DS, Gharios C, et al. Vitamin D supplementation and fractures in adults: a systematic umbrella review of meta-analyses of controlled trials. J Clin Endocrinol Metab. 2022;107(3):882‐898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 326. Avenell A, Mak JC, O’Connell D. Vitamin D and vitamin D analogues for preventing fractures in post-menopausal women and older men. Cochrane Database Syst Rev. 2014;2014(4):Cd000227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 327. Chung M, Lee J, Terasawa T, Lau J, Trikalinos TA. Vitamin D with or without calcium supplementation for prevention of cancer and fractures: an updated meta-analysis for the U.S. preventive services task force. Ann Intern Med. 2011;155(12):827‐838. [DOI] [PubMed] [Google Scholar]
- 328. Yao P, Bennett D, Mafham M, et al. Vitamin D and calcium for the prevention of fracture: a systematic review and meta-analysis. JAMA Netw Open. 2019;2(12):e1917789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 329. Bolland MJ, Grey A, Gamble GD, Reid IR. Vitamin D supplementation and falls: a trial sequential meta-analysis. Lancet Diabetes Endocrinol. 2014;2(7):573‐580. [DOI] [PubMed] [Google Scholar]
- 330. LeBoff MS, Chou SH, Ratliff KA, et al. Supplemental vitamin D and incident fractures in midlife and older adults. N Engl J Med. 2022;387(4):299‐309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 331. Guirguis-Blake JM, Michael YL, Perdue LA, Coppola EL, Beil TL. Interventions to prevent falls in older adults: updated evidence report and systematic review for the US Preventive Services Task Force. JAMA. 2018;319(16):1705‐1716. [DOI] [PubMed] [Google Scholar]
- 332. Gillespie LD, Robertson MC, Gillespie WJ, et al. Interventions for preventing falls in older people living in the community. Cochrane Database Syst Rev. 2012:2012(9):Cd007146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 333. Mao PJ, Zhang C, Tang L, et al. Effect of calcium or vitamin D supplementation on vascular outcomes: a meta-analysis of randomized controlled trials. Int J Cardiol. 2013;169(2):106‐111. [DOI] [PubMed] [Google Scholar]
- 334. Barbarawi M, Kheiri B, Zayed Y, et al. Vitamin D supplementation and cardiovascular disease risks in more than 83 000 individuals in 21 randomized clinical trials: a meta-analysis. JAMA Cardiol. 2019;4(8):765‐776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 335. Rutjes AW, Denton DA, Di Nisio M, et al. Vitamin and mineral supplementation for maintaining cognitive function in cognitively healthy people in mid and late life. Cochrane Database Syst Rev. 2018;12(12):Cd011906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 336. Bjelakovic G, Gluud LL, Nikolova D, et al. Vitamin D supplementation for prevention of cancer in adults. Cochrane Database Syst Rev. 2014;(6):Cd007469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 337. Bjelakovic G, Gluud LL, Nikolova D, et al. Vitamin D supplementation for prevention of mortality in adults. Cochrane Database Syst Rev. 2014;(1):Cd007470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 338. Holick MF, Binkley NC, Bischoff-Ferrari HA, et al. Evaluation, treatment, and prevention of vitamin D deficiency: an Endocrine Society Clinical Practice Guideline. J Clin Endocrinol Metab. 2011;96(7):1911‐1930. [DOI] [PubMed] [Google Scholar]
- 339. Mo M, Wang S, Chen Z, et al. A systematic review and meta-analysis of the response of serum 25-hydroxyvitamin D concentration to vitamin D supplementation from RCTs from around the globe. Eur J Clin Nutr. 2019;73(6):816‐834. [DOI] [PubMed] [Google Scholar]
- 340. Bassatne A, Chakhtoura M, Saad R, Fuleihan GE. Vitamin D supplementation in obesity and during weight loss: a review of randomized controlled trials. Metabolism. 2019;92:193‐205. [DOI] [PubMed] [Google Scholar]
- 341. Aloia J, Fazzari M, Islam S, et al. Vitamin D supplementation in elderly black women does not prevent bone loss: a randomized controlled trial. J Bone Miner Res. 2018;33(11):1916‐1922. [DOI] [PubMed] [Google Scholar]
- 342. National Diabetes Statistics Report A, GA: Centers for Disease Control and Prevention, U.S. Dept of Health and Human Services; 2020.
- 343. Ogurtsova K, Guariguata L, Barengo NC, et al. IDF diabetes atlas: global estimates of undiagnosed diabetes in adults for 2021. Diabetes Res Clin Pract. 2022;183:109118. [DOI] [PubMed] [Google Scholar]
- 344. Kalyani RR, Egan JM. Diabetes and altered glucose metabolism with aging. Endocrinol Metab Clin North Am. 2013;42(2):333‐347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 345. Meigs JB, Muller DC, Nathan DM, Blake DR, Andres R, Baltimore Longitudinal Study of Aging . The natural history of progression from normal glucose tolerance to type 2 diabetes in the Baltimore Longitudinal Study of Aging. Diabetes. 2003;52(6):1475‐1484. [DOI] [PubMed] [Google Scholar]
- 346. Defronzo RA. Glucose intolerance and aging: evidence for tissue insensitivity to insulin. Diabetes. 1979;28(12):1095‐1101. [DOI] [PubMed] [Google Scholar]
- 347. Al-Sofiani ME, Ganji SS, Kalyani RR. Body composition changes in diabetes and aging. J Diabetes Complications. 2019;33(6):451‐459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 348. Chang AM, Halter JB. Aging and insulin secretion. Am J Physiol Endocrinol Metab. 2003;284(1):E7‐E12. [DOI] [PubMed] [Google Scholar]
- 349. Gregg EW, Li Y, Wang J, et al. Changes in diabetes-related complications in the United States, 1990–2010. N Engl J Med. 2014;370(16):1514‐1523. [DOI] [PubMed] [Google Scholar]
- 350. Selvin E, Coresh J, Brancati FL. The burden and treatment of diabetes in elderly individuals in the U.S. Diabetes Care. 2006;29(11):2415‐2419. [DOI] [PubMed] [Google Scholar]
- 351. Paolisso G, Gambardella A, Ammendola S, et al. Glucose tolerance and insulin action in healthy centenarians. Am J Physiol. 1996;270(5 Pt 1):E890‐E894. [DOI] [PubMed] [Google Scholar]
- 352. Corriere M, Rooparinesingh N, Kalyani RR. Epidemiology of diabetes and diabetes complications in the elderly: an emerging public health burden. Curr Diab Rep. 2013;13(6):805‐813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 353. Sinclair AJ, Conroy SP, Bayer AJ. Impact of diabetes on physical function in older people. Diabetes Care. 2008;31(2):233‐235. [DOI] [PubMed] [Google Scholar]
- 354. Kalyani RR, Metter EJ, Ramachandran R, Chia CW, Saudek CD, Ferrucci L. Glucose and insulin measurements from the oral glucose tolerance test and relationship to muscle mass. J Gerontol A Biol Sci Med Sci. 2012;67(1):74‐81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 355. Kalyani RR, Varadhan R, Weiss CO, Fried LP, Cappola AR. Frailty status and altered glucose-insulin dynamics. J Gerontol A Biol Sci Med Sci. 2012;67(12):1300‐1306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 356. Park SW, Goodpaster BH, Lee JS, et al. Excessive loss of skeletal muscle mass in older adults with type 2 diabetes. Diabetes Care. 2009;32(11):1993‐1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 357. Kalyani RR, Metter EJ, Egan J, Golden SH, Ferrucci L. Hyperglycemia predicts persistently lower muscle strength with aging. Diabetes Care. 2015;38(1):82‐90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 358. Barzilay JI, Cotsonis GA, Walston J, et al. Insulin resistance is associated with decreased quadriceps muscle strength in nondiabetic adults aged ≥70 years. Diabetes Care. 2009;32(4):736‐738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 359. Kalyani RR, Golden SH, Cefalu WT. Diabetes and aging: unique considerations and goals of care. Diabetes Care. 2017;40(4):440‐443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 360. Knowler WC, Barrett-Connor E, Fowler SE, et al. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. 2002;346(6):393‐403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 361. Quartuccio M, Buta B, Kalyani RR. Comparative effectiveness for glycemic control in older adults with diabetes. Curr Geriatr Rep. 2017;6(3):175‐186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 362. Action to Control Cardiovascular Risk in Diabetes Study Group, Gerstein HC, Miller ME, et al. Effects of intensive glucose lowering in type 2 diabetes. N Engl J Med. 2008;358(24):2545‐2559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 363. Lipska KJ, Ross JS, Miao Y, Shah ND, Lee SJ, Steinman MA. Potential overtreatment of diabetes mellitus in older adults with tight glycemic control. JAMA Intern Med. 2015;175(3):356‐362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 364. Rawlings AM, Sharrett AR, Albert MS, et al. The association of late-life diabetes status and hyperglycemia with incident mild cognitive impairment and dementia: the ARIC study. Diabetes Care. 2019;42(7):1248‐1254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 365. Kim WJ, Lee SJ, Lee E, Lee EY, Han K. Risk of incident dementia according to glycemic status and comorbidities of hyperglycemia: a nationwide population-based cohort study. Diabetes Care. 2022;45(1):134‐141. [DOI] [PubMed] [Google Scholar]
- 366. Munshi M, Neumiller JJ. Liberalisation, deintensification, and simplification in diabetes management: words matter. Lancet Diabetes Endocrinol. 2020;8(2):95‐97. [DOI] [PubMed] [Google Scholar]
- 367. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). UK prospective diabetes study (UKPDS) group. Lancet. 1998;352(9131):837‐853. [PubMed] [Google Scholar]
- 368. Holman RR, Paul SK, Bethel MA, Matthews DR, Neil HA. 10-year follow-up of intensive glucose control in type 2 diabetes. N Engl J Med. 2008;359(15):1577‐1589. [DOI] [PubMed] [Google Scholar]
- 369. Duckworth W, Abraira C, Moritz T, et al. Glucose control and vascular complications in veterans with type 2 diabetes. N Engl J Med. 2009;360(2):129‐139. [DOI] [PubMed] [Google Scholar]
- 370. Group AC, Patel A, MacMahon S, et al. Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. N Engl J Med. 2008;358(24):2560‐2572. [DOI] [PubMed] [Google Scholar]
- 371. American Diabetes Association . 12. Older adults: standards of medical care in diabetes-2021. Diabetes Care. 2021;44(Suppl 1):S168‐S179. [DOI] [PubMed] [Google Scholar]
- 372. LeRoith D, Biessels GJ, Braithwaite SS, et al. Treatment of diabetes in older adults: an Endocrine Society Clinical Practice Guideline. J Clin Endocrinol Metab. 2019;104(5):1520‐1574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 373. Cowen LE, Hodak SP, Verbalis JG. Age-associated abnormalities of water homeostasis. Endocrinol Metab Clin North Am. 2013;42(2):349‐370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 374. Phillips PA, Rolls BJ, Ledingham JG, Forsling ML, Morton JJ. Osmotic thirst and vasopressin release in humans: a double-blind crossover study. Am J Physiol. 1985;248(6 Pt 2):R645‐R650. [DOI] [PubMed] [Google Scholar]
- 375. Phillips PA, Johnston CI, Gray L. Disturbed fluid and electrolyte homoeostasis following dehydration in elderly people. Age Ageing. 1993;22(Suppl 1):S26‐S33. [DOI] [PubMed] [Google Scholar]
- 376. Mack GW, Weseman CA, Langhans GW, Scherzer H, Gillen CM, Nadel ER. Body fluid balance in dehydrated healthy older men: thirst and renal osmoregulation. J Appl Physiol (1985). 1994;76(4):1615‐1623. [DOI] [PubMed] [Google Scholar]
- 377. Stachenfeld NS, DiPietro L, Nadel ER, Mack GW. Mechanism of attenuated thirst in aging: role of central volume receptors. Am J Physiol. 1997;272(1 Pt 2):R148‐R157. [DOI] [PubMed] [Google Scholar]
- 378. Lindeman RD. Assessment of renal function in the old. Special considerations. Clin Lab Med. 1993;13(1):269‐277. [PubMed] [Google Scholar]
- 379. Lindeman RD, Tobin J, Shock NW. Longitudinal studies on the rate of decline in renal function with age. J Am Geriatr Soc. 1985;33(4):278‐285. [DOI] [PubMed] [Google Scholar]
- 380. Faull CM, Holmes C, Baylis PH. Water balance in elderly people: is there a deficiency of vasopressin? Age Ageing. 1993;22(2):114‐120. [DOI] [PubMed] [Google Scholar]
- 381. Clark BA, Shannon RP, Rosa RM, Epstein FH. Increased susceptibility to thiazide-induced hyponatremia in the elderly. J Am Soc Nephrol. 1994;5(4):1106‐1111. [DOI] [PubMed] [Google Scholar]
- 382. Wong LL, Verbalis JG. Systemic diseases associated with disorders of water homeostasis. Endocrinol Metab Clin North Am. 2002;31(1):121‐140. [DOI] [PubMed] [Google Scholar]
- 383. Davies I, O’Neill PA, McLean KA, Catania J, Bennett D. Age-associated alterations in thirst and arginine vasopressin in response to a water or sodium load. Age Ageing. 1995;24(2):151‐159. [DOI] [PubMed] [Google Scholar]
- 384. Fried LF, Palevsky PM. Hyponatremia and hypernatremia. Med Clin North Am. 1997;81(3):585‐609. [DOI] [PubMed] [Google Scholar]
- 385. Hawkins RC. Age and gender as risk factors for hyponatremia and hypernatremia. Clin Chim Acta. 2003;337(1-2):169‐172. [DOI] [PubMed] [Google Scholar]
- 386. Beck LH. Changes in renal function with aging. Clin Geriatr Med. 1998;14(2):199‐209. [PubMed] [Google Scholar]
- 387. Palevsky PM. Hypernatremia. Semin Nephrol. 1998;18(1):20‐30. [PubMed] [Google Scholar]
- 388. Lindner G, Schwarz C, Funk GC. Osmotic diuresis due to urea as the cause of hypernatraemia in critically ill patients. Nephrol Dial Transplant. 2012;27(3):962‐967. [DOI] [PubMed] [Google Scholar]
- 389. Snyder NA, Feigal DW, Arieff AI. Hypernatremia in elderly patients. A heterogeneous, morbid, and iatrogenic entity. Ann Intern Med. 1987;107(3):309‐319. [DOI] [PubMed] [Google Scholar]
- 390. Beck LH. The aging kidney. Defending a delicate balance of fluid and electrolytes. Geriatrics. 2000;55(4):26‐28, 31-22. [PubMed] [Google Scholar]
- 391. Leung AA, McAlister FA, Finlayson SR, Bates DW. Preoperative hypernatremia predicts increased perioperative morbidity and mortality. Am J Med. 2013;126(10):877‐886. [DOI] [PubMed] [Google Scholar]
- 392. Janicic N, Verbalis JG. Evaluation and management of hypo-osmolality in hospitalized patients. Endocrinol Metab Clin North Am. 2003;32(2):459‐481, vii. [DOI] [PubMed] [Google Scholar]
- 393. Verbalis J. Hyponatremia and hypoosmolar disorders. In: GIlbert SJ, Weiner DE, eds. Primer on Kidney Diseases. Elsevier; 2018:68‐76. [Google Scholar]
- 394. Miller M, Hecker MS, Friedlander DA, Carter JM. Apparent idiopathic hyponatremia in an ambulatory geriatric population. J Am Geriatr Soc. 1996;44(4):404‐408. [DOI] [PubMed] [Google Scholar]
- 395. Anpalahan M. Chronic idiopathic hyponatremia in older people due to syndrome of inappropriate antidiuretic hormone secretion (SIADH) possibly related to aging. J Am Geriatr Soc. 2001;49(6):788‐792. [DOI] [PubMed] [Google Scholar]
- 396. Hirshberg B, Ben-Yehuda A. The syndrome of inappropriate antidiuretic hormone secretion in the elderly. Am J Med. 1997;103(4):270‐273. [DOI] [PubMed] [Google Scholar]
- 397. Liamis G, Milionis H, Elisaf M. A review of drug-induced hyponatremia. Am J Kidney Dis. 2008;52(1):144‐153. [DOI] [PubMed] [Google Scholar]
- 398. Filippatos T, Tzavella E, Rizos C, Elisaf M, Liamis G. Acid-base and electrolyte disorders associated with the use of antidiabetic drugs. Expert Opin Drug Saf. 2017;16(10):1121‐1132. [DOI] [PubMed] [Google Scholar]
- 399. Cohen DL, Townsend RR. Hyponatremia and thiazides. J Clin Hypertens (Greenwich). 2012;14(9):653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 400. Clayton JA, Rodgers S, Blakey J, Avery A, Hall IP. Thiazide diuretic prescription and electrolyte abnormalities in primary care. Br J Clin Pharmacol. 2006;61(1):87‐95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 401. Thaler SM, Teitelbaum I, Berl T. “Beer potomania” in non-beer drinkers: effect of low dietary solute intake. Am J Kidney Dis. 1998;31(6):1028‐1031. [DOI] [PubMed] [Google Scholar]
- 402. Rittenhouse KJ, To T, Rogers A, et al. Hyponatremia as a fall predictor in a geriatric trauma population. Injury. 2015;46(1):119‐123. [DOI] [PubMed] [Google Scholar]
- 403. Vandergheynst F, Gombeir Y, Bellante F, et al. Impact of hyponatremia on nerve conduction and muscle strength. Eur J Clin Invest. 2016;46(4):328‐333. [DOI] [PubMed] [Google Scholar]
- 404. Wald R, Jaber BL, Price LL, Upadhyay A, Madias NE. Impact of hospital-associated hyponatremia on selected outcomes. Arch Intern Med. 2010;170(3):294‐302. [DOI] [PubMed] [Google Scholar]
- 405. Gankam Kengne F, Andres C, Sattar L, Melot C, Decaux G. Mild hyponatremia and risk of fracture in the ambulatory elderly. QJM. 2008;101(7):583‐588. [DOI] [PubMed] [Google Scholar]
- 406. Verbalis JG, Barsony J, Sugimura Y, et al. Hyponatremia-induced osteoporosis. J Bone Miner Res. 2010;25(3):554‐563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 407. Terzian C, Frye EB, Piotrowski ZH. Admission hyponatremia in the elderly: factors influencing prognosis. J Gen Intern Med. 1994;9(2):89‐91. [DOI] [PubMed] [Google Scholar]
- 408. Hoorn EJ, Liamis G, Zietse R, Zillikens MC. Hyponatremia and bone: an emerging relationship. Nat Rev Endocrinol. 2011;8(1):33‐39. [DOI] [PubMed] [Google Scholar]
- 409. Renneboog B, Musch W, Vandemergel X, Manto MU, Decaux G. Mild chronic hyponatremia is associated with falls, unsteadiness, and attention deficits. Am J Med. 2006;119(1):71.e1‐71.e8. [DOI] [PubMed] [Google Scholar]
- 410. Usala RL, Fernandez SJ, Mete M, et al. Hyponatremia is associated with increased osteoporosis and bone fractures in a large US health system population. J Clin Endocrinol Metab. 2015;100(8):3021‐3031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 411. Usala RL, Verbalis JG. Disorders of water and sodium homeostasis and bone. Curr Opin Endocr Metab Res. 2018;3:83‐92. [Google Scholar]
- 412. Chauhan K, Pattharanitima P, Patel N, et al. Rate of correction of hypernatremia and health outcomes in critically ill patients. Clin J Am Soc Nephrol. 2019;14(5):656‐663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 413. Christ-Crain M, Bichet DG, Fenske WK, et al. Diabetes insipidus. Nat Rev Dis Primers. 2019;5(1):54. [DOI] [PubMed] [Google Scholar]
- 414. Garrahy A, Galloway I, Hannon AM, et al. Fluid restriction therapy for chronic SIAD; results of a prospective randomized controlled trial. J Clin Endocrinol Metab. 2020;105(12):e4360–e4369. [DOI] [PubMed] [Google Scholar]
- 415. Verbalis JG, Goldsmith SR, Greenberg A, et al. Diagnosis, evaluation, and treatment of hyponatremia: expert panel recommendations. Am J Med. 2013;126(10 Suppl 1):S1‐S42. [DOI] [PubMed] [Google Scholar]
- 416. Alford N, Hashim H. Desmopressin acetate the first sublingual tablet to treat nocturia due to nocturnal polyuria. Expert Rev Clin Pharmacol. 2021;14(8):939‐954. [DOI] [PubMed] [Google Scholar]
- 417. Juul KV, Malmberg A, van der Meulen E, Walle JV, Norgaard JP. Low-dose desmopressin combined with serum sodium monitoring can prevent clinically significant hyponatraemia in patients treated for nocturia. BJU Int. 2017;119(5):776‐784. [DOI] [PubMed] [Google Scholar]
- 418. Juul KV, Klein BM, Sandstrom R, Erichsen L, Norgaard JP. Gender difference in antidiuretic response to desmopressin. Am J Physiol Renal Physiol. 2011;300(5):F1116‐F1122. [DOI] [PubMed] [Google Scholar]
- 419. Liu J, Sharma N, Zheng W, et al. Sex differences in vasopressin V(2) receptor expression and vasopressin-induced antidiuresis. Am J Physiol Renal Physiol. 2011;300(2):F433‐F440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 420. Verbalis JG, Zeltser D, Smith N, Barve A, Andoh M. Assessment of the efficacy and safety of intravenous conivaptan in patients with euvolaemic hyponatraemia: subgroup analysis of a randomized, controlled study. Clin Endocrinol (Oxf). 2008;69(1):159‐168. [DOI] [PubMed] [Google Scholar]
- 421. Schrier RW, Gross P, Gheorghiade M, et al. Tolvaptan, a selective oral vasopressin V2-receptor antagonist, for hyponatremia. N Engl J Med. 2006;355(20):2099‐2112. [DOI] [PubMed] [Google Scholar]
- 422. Verbalis JG, Peri A, Thompson CJ. Future of hyponatremia research. Front Horm Res. 2019;52:200‐203. [DOI] [PubMed] [Google Scholar]
- 423. Khosla S, Farr JN, Tchkonia T, Kirkland JL. The role of cellular senescence in ageing and endocrine disease. Nat Rev Endocrinol. 2020;16(5):263‐275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 424. Tchkonia T, Palmer AK, Kirkland JL. New horizons: novel approaches to enhance healthspan through targeting cellular senescence and related aging mechanisms. J Clin Endocrinol Metab. 2021;106(3):e1481‐e1487. [DOI] [PMC free article] [PubMed] [Google Scholar]