Abstract
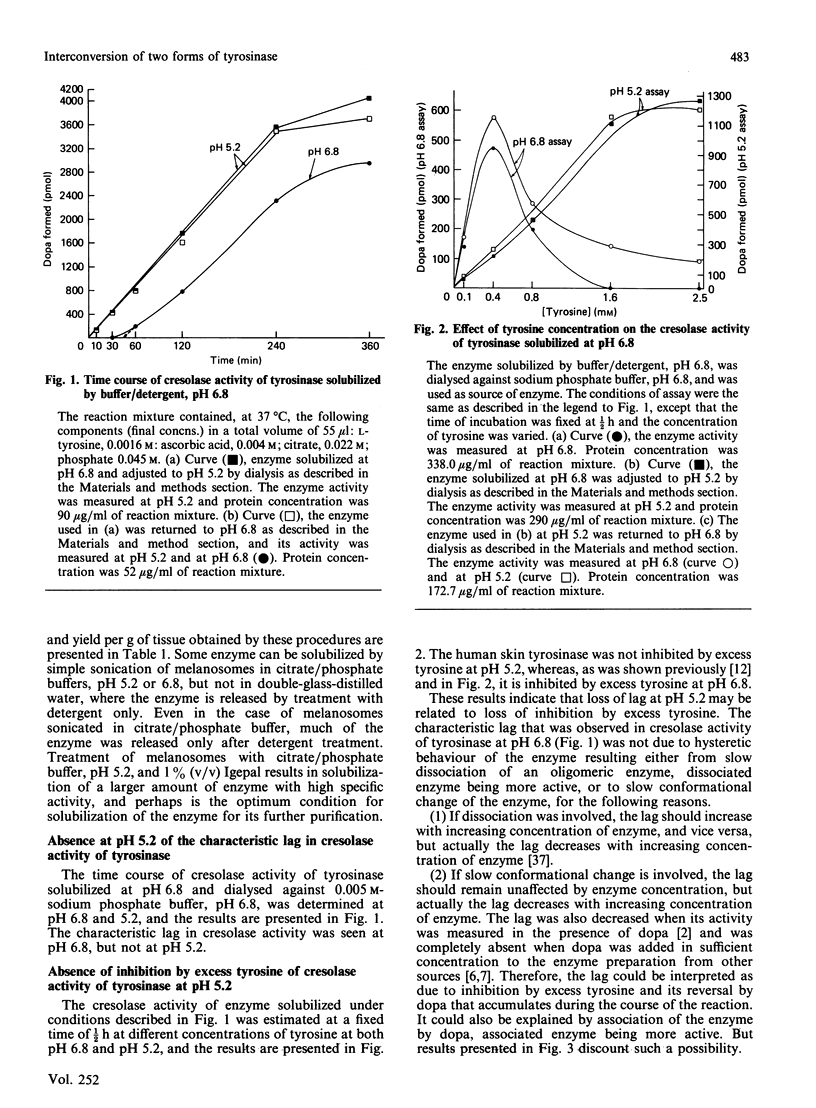
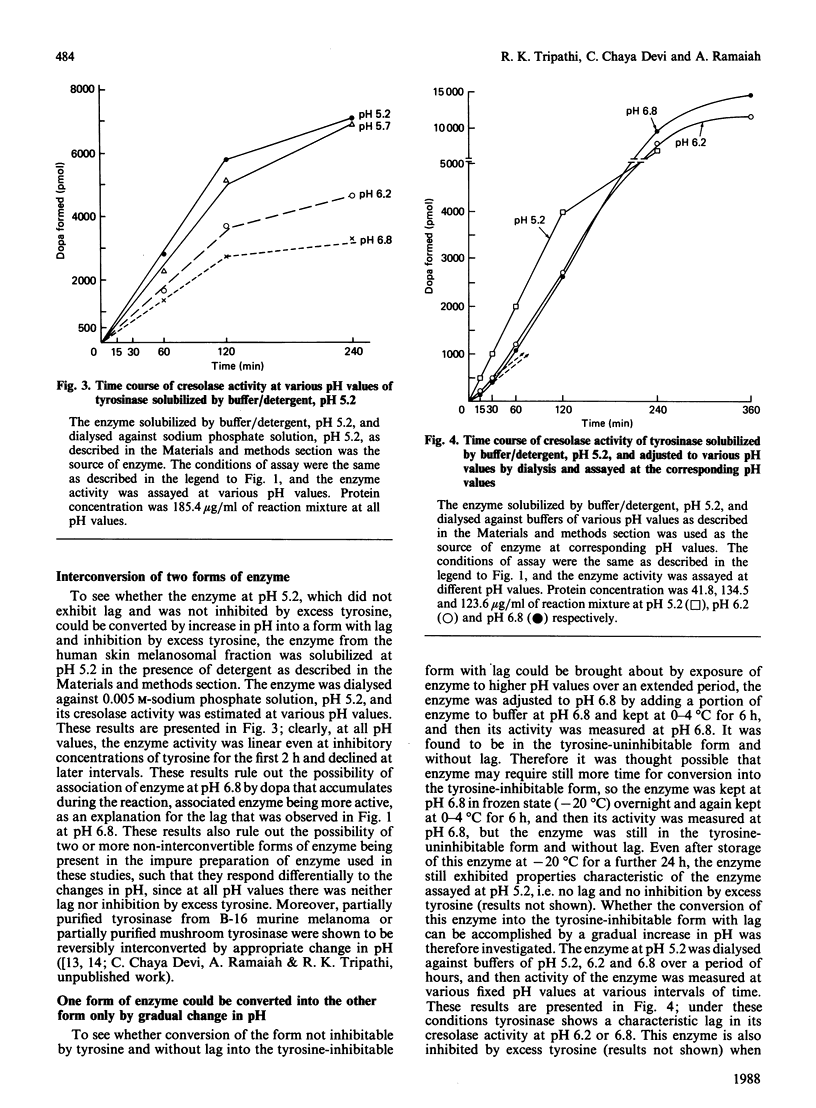
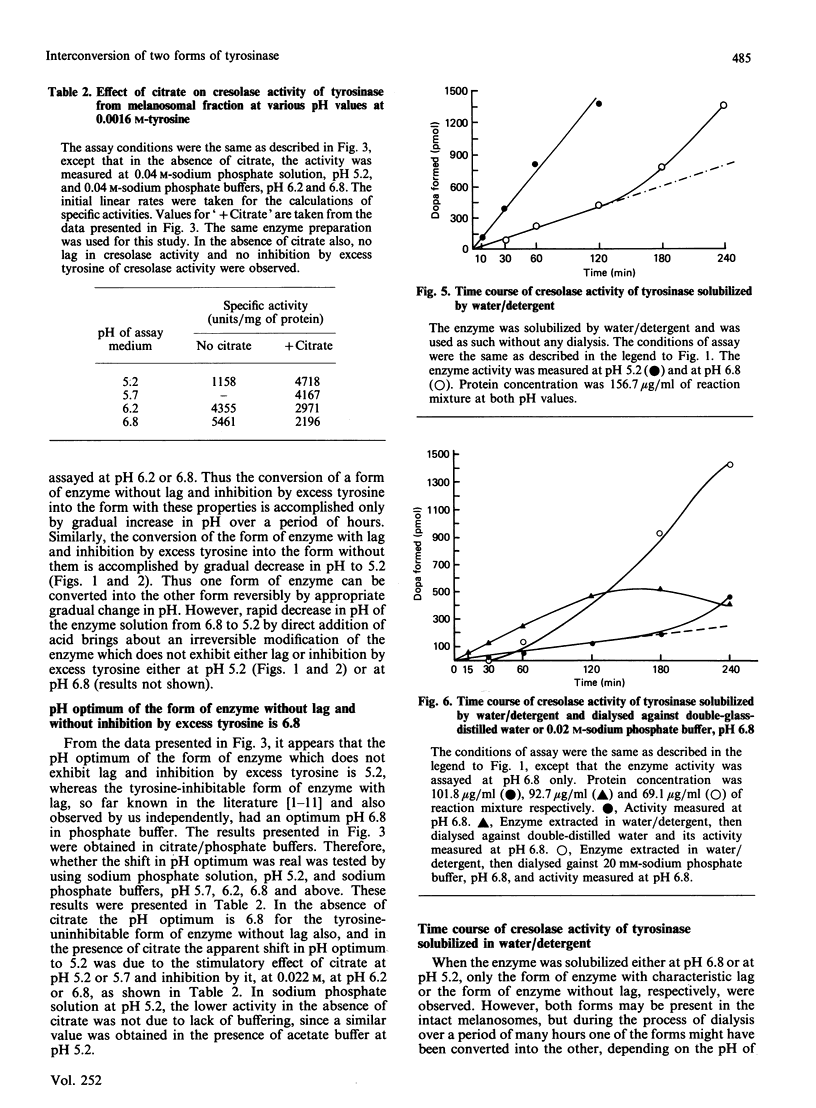
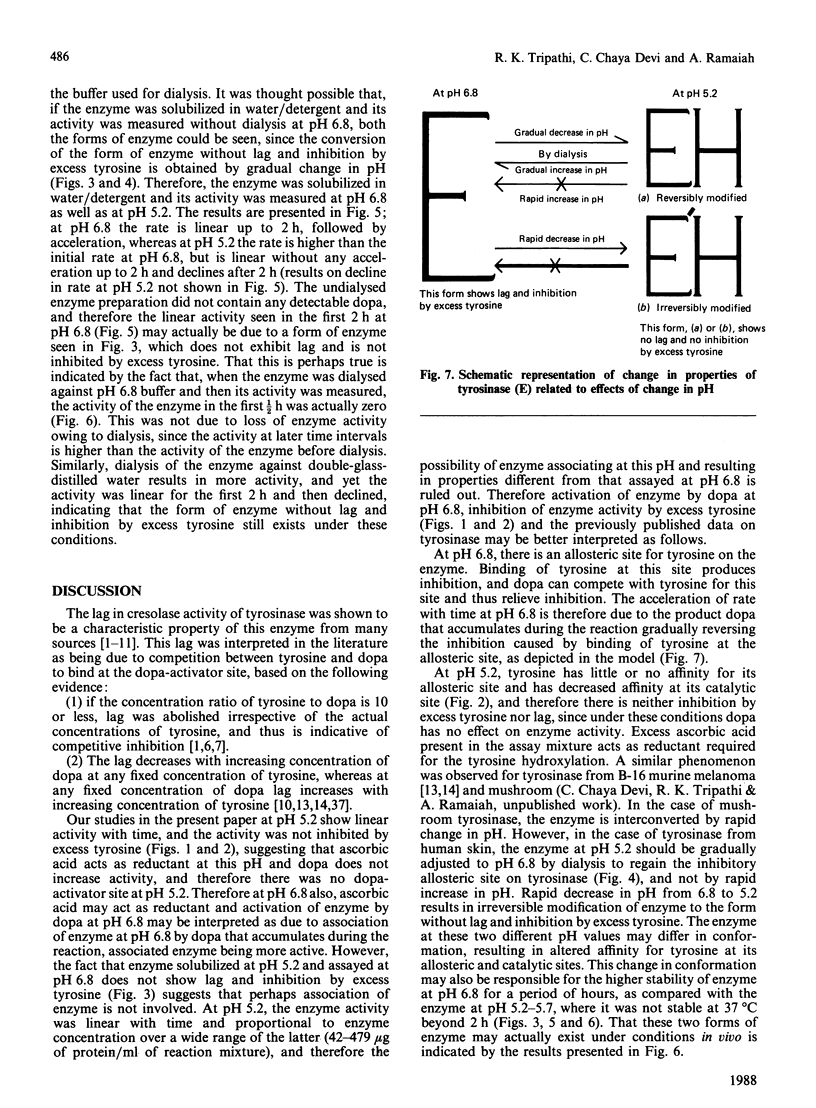
1. We have shown that the characteristic lag in cresolase activity of human skin tyrosinase at inhibitory concentration of tyrosine was absent at all pH values studied, i.e. pH 5.2, 5.7, 6.2 and 6.8, if the enzyme solubilized at low pH was used as the source of enzyme, but the same enzyme when dialysed against buffers of various pH values showed linear activity only at pH 5.2 and was not inhibited by excess tyrosine, whereas at higher pH values it exhibited a lag and inhibition by excess tyrosine. 2. However, the enzyme solubilized in buffer/detergent, pH 6.8, when dialysed against buffer of the same pH showed linear activity at pH 5.2 and non-linear activity at pH 6.8. 3. The water/detergent-solubilized enzyme from human skin melanosomes showed linear activity even at inhibitory concentrations of tyrosine at pH 5.2 and 6.8 up to 2 h, but acceleration of rate was observed after 2 h for the enzyme measured at pH 6.8. 4. After dialysis of the water/detergent-solubilized enzyme against double-glass-distilled water, it still exhibits linear activity at inhibitory concentration of tyrosines at pH 6.8 for the first 2 h, but the same enzyme when dialysed against 0.02 M-sodium phosphate buffer, pH 6.8, exhibits negligible activity up to 1/2 h, in contrast with considerable activity before dialysis during the same interval of time, but without any loss of activity at later intervals of incubation time. 5. On the basis of these results, it is concluded that the enzyme exists in at least two interconvertible forms, one without lag and inhibition by excess tyrosine and the other with lag and inhibition by excess tyrosine. These two forms are interconvertible only by gradual change in pH over a period of hours.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adachi K., Halprin K. M. A sensitive fluorometric assay method for mammalian tyrosinase. Biochem Biophys Res Commun. 1967 Jan 23;26(2):242–246. doi: 10.1016/0006-291x(67)90241-0. [DOI] [PubMed] [Google Scholar]
- Burke R. C., Lee T. H., Buettner-Janusch V. Free amino acids and water soluble peptides in stratum corneum and skin surface film in human beings. Yale J Biol Med. 1966 Feb;38(4):355–373. [PMC free article] [PubMed] [Google Scholar]
- Devi C. C., Tripathi R. K., Ramaiah A. pH-dependent interconvertible allosteric forms of murine melanoma tyrosinase. Physiological implications. Eur J Biochem. 1987 Aug 3;166(3):705–711. doi: 10.1111/j.1432-1033.1987.tb13569.x. [DOI] [PubMed] [Google Scholar]
- Duckworth H. W., Coleman J. E. Physicochemical and kinetic properties of mushroom tyrosinase. J Biol Chem. 1970 Apr 10;245(7):1613–1625. [PubMed] [Google Scholar]
- FITZPATRICK T. B., BECKER S. W., Jr, LERNER A. B., MONTGOMERY H. Tyrosinase in human skin: demonstration of its presence and of its role in human melanin formation. Science. 1950 Aug 25;112(2904):223–225. doi: 10.1126/science.112.2904.223. [DOI] [PubMed] [Google Scholar]
- Fuller B. B., Lunsford J. B., Iman D. S. Alpha-melanocyte-stimulating hormone regulation of tyrosinase in Cloudman S-91 mouse melanoma cell cultures. J Biol Chem. 1987 Mar 25;262(9):4024–4033. [PubMed] [Google Scholar]
- Hearing V. J., Ekel T. M. Mammalian tyrosinase. A comparison of tyrosine hydroxylation and melanin formation. Biochem J. 1976 Sep 1;157(3):549–557. doi: 10.1042/bj1570549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hearing V. J., Jr, Ekel T. M., Montague P. M., Hearing E. D., Nicholson J. M. Mammalian tyrosinase: isolation by a simple new procedure and characterization of its steric requirements for cofactor activity. Arch Biochem Biophys. 1978 Jan 30;185(2):407–418. doi: 10.1016/0003-9861(78)90183-2. [DOI] [PubMed] [Google Scholar]
- Hori Y., Toda K., Pathak M. A., Clark W. H., Jr, Fitzpatrick T. B. A fine-structure study of the human epidermal melanosome complex and its acid phosphatase activity. J Ultrastruct Res. 1968 Oct;25(1):109–120. doi: 10.1016/s0022-5320(68)80064-4. [DOI] [PubMed] [Google Scholar]
- Husain I., Vijayan E., Ramaiah A., Pasricha J. S., Madan N. C. Demonstration of tyrosinase in the vitiligo skin of human beings by a sensitive fluorometric method as well as by 14C(U)-L-tyrosine incorporation into melanin. J Invest Dermatol. 1982 Mar;78(3):243–252. doi: 10.1111/1523-1747.ep12506603. [DOI] [PubMed] [Google Scholar]
- Jergil B., Lindbladh C., Rorsman H., Rosengren E. Dopa oxidation and tyrosine oxygenation by human melanoma tyrosinase. Acta Derm Venereol. 1983;63(6):468–475. [PubMed] [Google Scholar]
- KREUGER R. C. The role of reducing agents in the action of tyrosinase. Arch Biochem Biophys. 1958 Jul;76(1):87–96. doi: 10.1016/0003-9861(58)90122-x. [DOI] [PubMed] [Google Scholar]
- LERNER A. B., SHIZUME K., BUNDING I. The mechanism of endocrine control of melanin pigmentation. J Clin Endocrinol Metab. 1954 Dec;14(12):1463–1490. doi: 10.1210/jcem-14-12-1463. [DOI] [PubMed] [Google Scholar]
- Ohkuma S., Poole B. Fluorescence probe measurement of the intralysosomal pH in living cells and the perturbation of pH by various agents. Proc Natl Acad Sci U S A. 1978 Jul;75(7):3327–3331. doi: 10.1073/pnas.75.7.3327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oikawa A., Nakayasu M. Stimulation of melanogenesis in cultured melanoma cells by calciferols. FEBS Lett. 1974 May 15;42(1):32–35. doi: 10.1016/0014-5793(74)80272-3. [DOI] [PubMed] [Google Scholar]
- Pathak M. A., Sinesi S. J., Szabó G. The effect of a single dose of ultraviolet radiation on epidermal melanocytes. J Invest Dermatol. 1965 Dec;45(6):520–528. doi: 10.1038/jid.1965.168. [DOI] [PubMed] [Google Scholar]
- Pau R. N., Kelly C. The hydroxylation of tyrosine by an enzyme from third-instar larvae of the blowfly Calliphora erythrocephala. Biochem J. 1975 Jun;147(3):565–573. doi: 10.1042/bj1470565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson G. L. A simplification of the protein assay method of Lowry et al. which is more generally applicable. Anal Biochem. 1977 Dec;83(2):346–356. doi: 10.1016/0003-2697(77)90043-4. [DOI] [PubMed] [Google Scholar]
- Pomerantz S. H. The tyrosine hydroxylase activity of mammalian tyrosinase. J Biol Chem. 1966 Jan 10;241(1):161–168. [PubMed] [Google Scholar]
- Pomerantz S. H., Warner M. C. 3,4-dihydroxy-L-phenylalanine as the tyrosinase cofactor. Occurrence in melanoma and binding constant. J Biol Chem. 1967 Nov 25;242(22):5308–5314. [PubMed] [Google Scholar]
- Saeki H., Oikawa A. Stimulation by ionophores of tyrosinase activity of mouse melanoma cells in culture. J Invest Dermatol. 1985 Nov;85(5):423–425. doi: 10.1111/1523-1747.ep12277091. [DOI] [PubMed] [Google Scholar]
- Saeki H., Oikawa A. Stimulation of tyrosinase activity of cultured melanoma cells by lysosomotropic agents. J Cell Physiol. 1983 Jul;116(1):93–97. doi: 10.1002/jcp.1041160114. [DOI] [PubMed] [Google Scholar]
- Seiji M., Iwashita S. Intracellular localization of tyrosinase and site of melanin formation in melanocyte. J Invest Dermatol. 1965 Nov;45(5):305–314. doi: 10.1038/jid.1965.135. [DOI] [PubMed] [Google Scholar]
- Seiji M., Kikuchi A. Acid phosphatase activity in melanosomes. J Invest Dermatol. 1969 Feb;52(2):212–216. doi: 10.1038/jid.1969.33. [DOI] [PubMed] [Google Scholar]
- Seiji M., Otaki N. Lysosomes in mouse melanoma. J Invest Dermatol. 1971 Jun;56(6):436–440. doi: 10.1111/1523-1747.ep12261356. [DOI] [PubMed] [Google Scholar]
- Tabachnick J., LaBadie J. H. Studies on the biochemistry of epidermis. IV. The free amino acids, ammonia, urea, and pyrrolidone carboxylic acid content of conventional and germ-free albino guina pig epidermia. J Invest Dermatol. 1970 Jan;54(1):24–31. doi: 10.1111/1523-1747.ep12551492. [DOI] [PubMed] [Google Scholar]
- Vijayan E., Husain I., Ramaiah A., Madan N. C. Purification of human skin tyrosinase and its protein inhibitor: properties of the enzyme and the mechanism of inhibition by protein. Arch Biochem Biophys. 1982 Sep;217(2):738–747. doi: 10.1016/0003-9861(82)90555-0. [DOI] [PubMed] [Google Scholar]
- Wolff K., Hönigsmann H. Are melanosome complexes lysosomes? J Invest Dermatol. 1972 Aug;59(2):170–176. doi: 10.1111/1523-1747.ep12625960. [DOI] [PubMed] [Google Scholar]
- Wolff K., Schreiner E. Melanosomal acid phosphatase. Arch Dermatol Forsch. 1971;241(3):255–272. doi: 10.1007/BF00595442. [DOI] [PubMed] [Google Scholar]
- Wong G., Pawelek J. Melanocyte-stimulating hormone promotes activation of pre-existing tyrosinase molecules in Cloudman S91 melanoma cells. Nature. 1975 Jun 19;255(5510):644–646. doi: 10.1038/255644a0. [DOI] [PubMed] [Google Scholar]


