Abstract
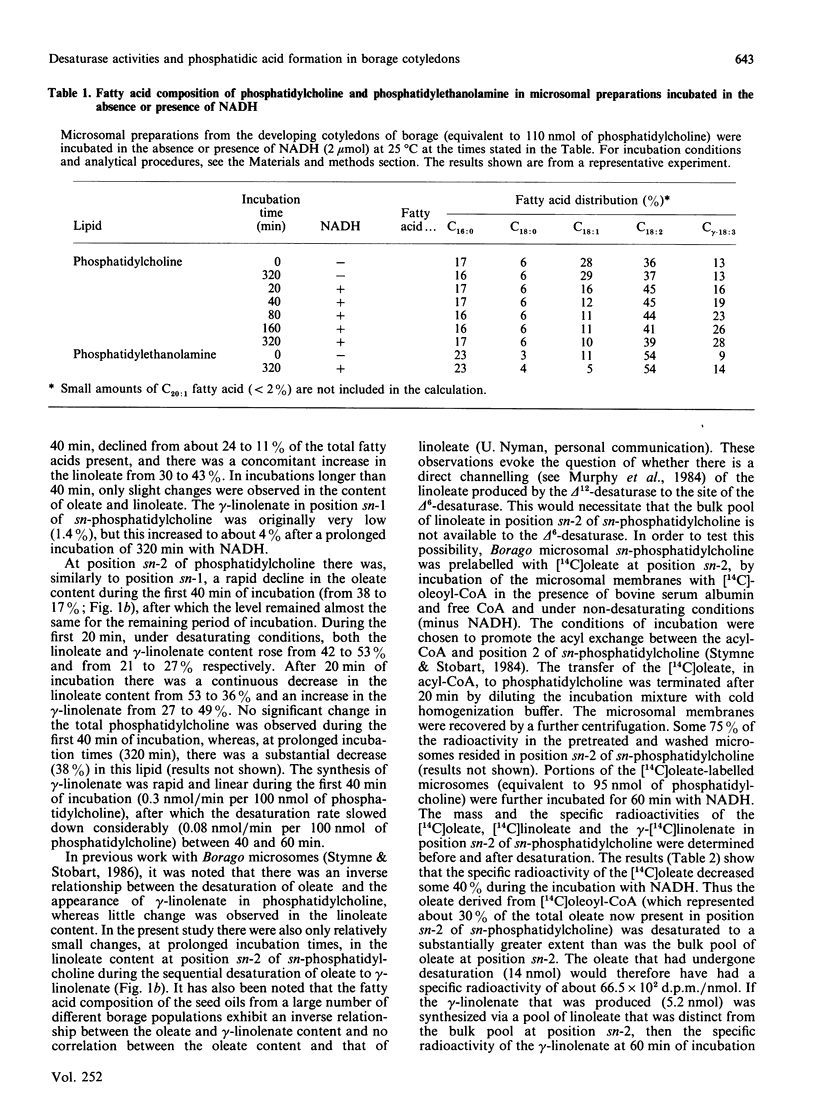
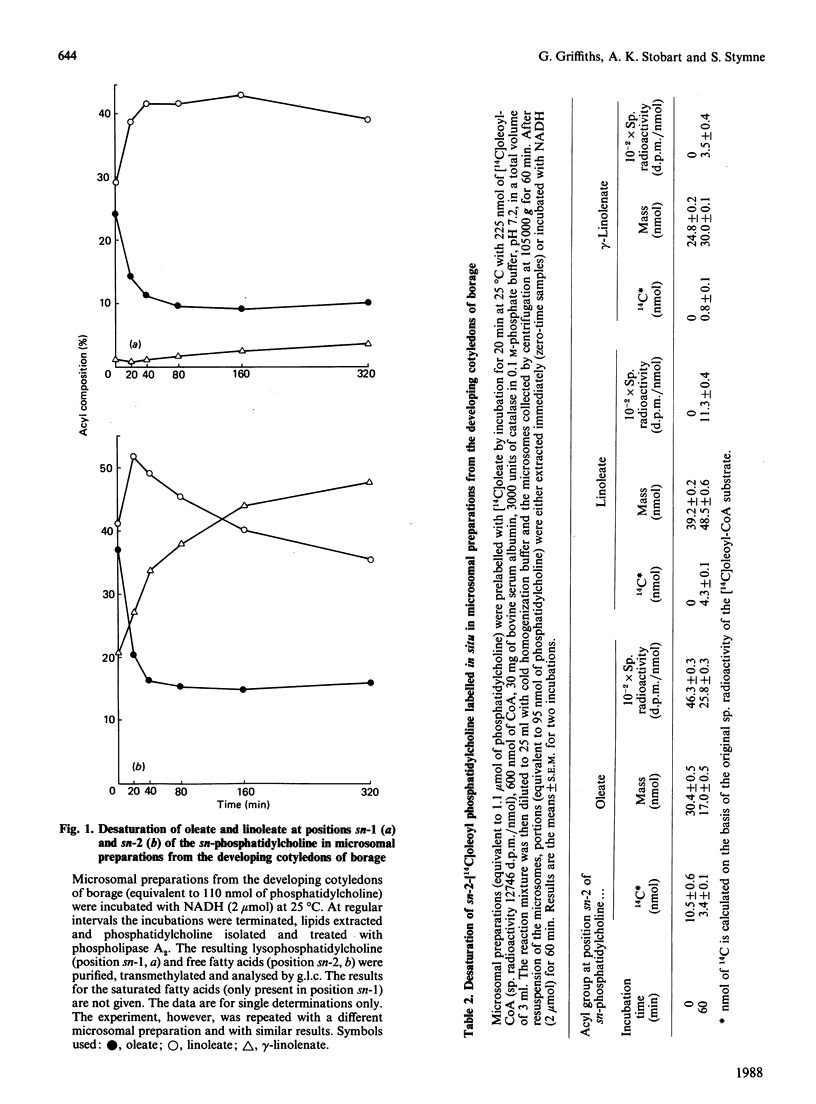
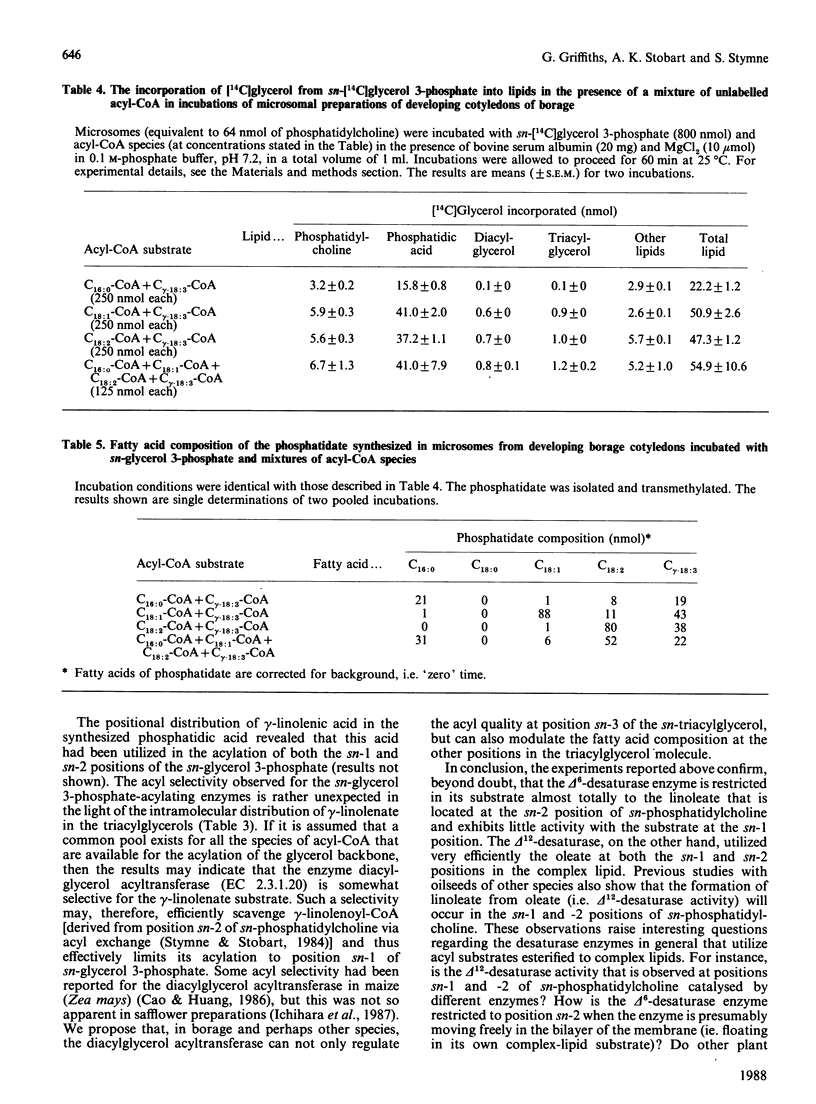
Microsomal membrane preparations from the maturing cotyledons of common borage (Borago officinalis) exhibit delta 12- and delta 6-desaturase activities, which resulted in the synthesis of linoleate and gamma-linolenate respectively. The desaturase enzymes utilized the complex lipid substrate phosphatidylcholine. The activity of these enzymes was sufficiently high to allow the monitoring of the mass changes in the endogenous oleate, linoleate and gamma-linolenate in the microsomal phosphatidylcholine in the presence of NADH (i.e. under desaturating conditions). The results illustrate that the delta 12-desaturase uses the oleate substrate at both the sn-1 and -2 positions of sn-phosphatidylcholine, whereas the delta 6-desaturase is almost totally restricted to the linoleate at position 2 of the complex lipid. Estimate of the acyl-substrate pool size at position 2 of sn-phosphatidylcholine for both desaturases indicated that some 50% of the oleate and linoleate was available to the enzymes. The microsomes (microsomal fractions) had a somewhat impaired Kennedy [(1961) Fed. Proc. Fed. Am. Soc. Exp. Biol. 20, 934-940] pathway for the formation of triacylglycerols when compared with other oil-rich plant species that have been studied [Stymne & Stobart (1987) The Biochemistry of Plants: a Comprehensive Treatise (Stumpf, P.K., ed.), vol. 10, chapter 8, pp. 175-214, Academic Press, New York]. In the presence of sn-glycerol 3-phosphate and acyl-CoA, large quantities of phosphatidic acid accumulated in the membranes. Acyl-selectivity studies on the glycerol-acylating enzymes showed that gamma-linolenate could be acylated to both the sn-1 and sn-2 positions of sn-glycerol 3-phosphate. However, stereochemical analysis of the acyl components of the sn-triacylglycerol obtained from mature seeds indicated that, whereas no gamma-linolenate was present at the sn-1 position, it accounted for over 50% of the fatty acids at position sn-3. The results indicate that the diacylglycerol acyltransferase (EC 2.3.1.20) may show a strong selectivity for gamma-linolenoyl-CoA and hence result in the efficient removal of this fatty acid from the acyl-CoA pool in vivo, leaving negligible substrate for utilization by the sn-glycerol 3-phosphate acyltransferase (EC 2.3.1.15).
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BLIGH E. G., DYER W. J. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959 Aug;37(8):911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- Cao Y. Z., Huang A. H. Diacylglycerol acyltransferase in maturing oil seeds of maize and other species. Plant Physiol. 1986 Nov;82(3):813–820. doi: 10.1104/pp.82.3.813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffiths G., Stobart A. K., Stymne S. The acylation of sn-glycerol 3-phosphate and the metabolism of phosphatidate in microsomal preparations from the developing cotyledons of safflower (Carthamus tinctorius L.) seed. Biochem J. 1985 Sep 1;230(2):379–388. doi: 10.1042/bj2300379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ichihara K., Asahi T., Fujii S. 1-Acyl-sn-glycerol-3-phosphate acyltransferase in maturing safflower seeds and its contribution to the non-random fatty acid distribution of triacylglycerol. Eur J Biochem. 1987 Sep 1;167(2):339–347. doi: 10.1111/j.1432-1033.1987.tb13342.x. [DOI] [PubMed] [Google Scholar]
- KATES M. SIMPLIFIED PROCEDURES FOR HYDROLYSIS OR METHANOLYSIS OF LIPIDS. J Lipid Res. 1964 Jan;5:132–135. [PubMed] [Google Scholar]
- KENNEDY E. P. Biosynthesis of complex lipids. Fed Proc. 1961 Dec;20:934–940. [PubMed] [Google Scholar]
- Murphy D. J., Mukherjee K. D., Woodrow I. E. Functional association of a monoacylglycerophosphocholine acyltransferase and the oleoylglycerophosphocholine desaturase in microsomes from developing leaves. Eur J Biochem. 1984 Mar 1;139(2):373–379. doi: 10.1111/j.1432-1033.1984.tb08016.x. [DOI] [PubMed] [Google Scholar]
- Myher J. J., Kuksis A. Stereospecific analysis of triacylglycerols via racemic phosphatidylcholines and phospholipase C. Can J Biochem. 1979 Feb;57(2):117–124. doi: 10.1139/o79-015. [DOI] [PubMed] [Google Scholar]
- Slack C. R., Roughan P. G., Browse J. Evidence for an oleoyl phosphatidylcholine desaturase in microsomal preparations from cotyledons of safflower (Carthamus tinctorius) seed. Biochem J. 1979 Jun 1;179(3):649–656. doi: 10.1042/bj1790649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stymne S., Stobart A. K. Biosynthesis of gamma-linolenic acid in cotyledons and microsomal preparations of the developing seeds of common borage (Borago officinalis). Biochem J. 1986 Dec 1;240(2):385–393. doi: 10.1042/bj2400385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stymne S., Stobart A. K. Evidence for the reversibility of the acyl-CoA:lysophosphatidylcholine acyltransferase in microsomal preparations from developing safflower (Carthamus tinctorius L.) cotyledons and rat liver. Biochem J. 1984 Oct 15;223(2):305–314. doi: 10.1042/bj2230305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sánchez M., Nicholls D. G., Brindley D. N. [The relationship between palmitoyl-coenzyme A synthetase activity and esterification of sn-glycerol 3-phosphate in rat liver mitochondria]. Biochem J. 1973 Apr;132(4):697–706. doi: 10.1042/bj1320697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trotter J., Ferber E. CoA-dependent cleavage of arachidonic acid from phosphatidylcholine and transfer to phosphatidylethanolamine in homogenates of murine thymocytes. FEBS Lett. 1981 Jun 15;128(2):237–241. doi: 10.1016/0014-5793(81)80089-0. [DOI] [PubMed] [Google Scholar]


