Abstract
Background
Patients with metastatic pancreatic ductal adenocarcinoma (PDAC) have poor 5-year survival. Pharmacological ascorbate (P-AscH-, high dose, intravenous, vitamin C) has shown promise as an adjunct to chemotherapy. We hypothesized adding P-AscH- to gemcitabine and nab-paclitaxel would increase survival in patients with metastatic PDAC.
Methods
Patients diagnosed with stage IV pancreatic cancer randomized 1:1 to gemcitabine and nab-paclitaxel only (SOC, control) or to SOC with concomitant P-AscH−, 75 g three times weekly (ASC, investigational). The primary outcome was overall survival with secondary objectives of determining progression-free survival and adverse event incidence. Quality of life and patient reported outcomes for common oncologic symptoms were captured as an exploratory objective. Thirty-six participants were randomized; of this 34 received their assigned study treatment. All analyses were based on data frozen on December 11, 2023.
Results
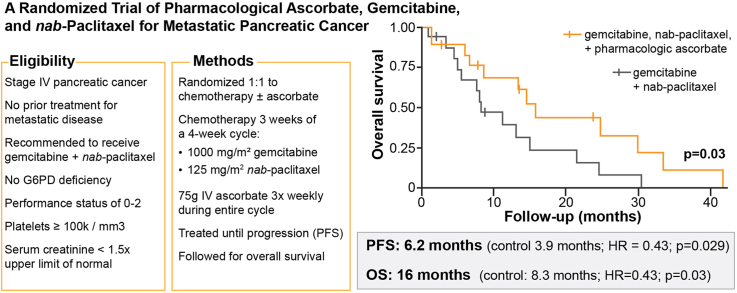
Intravenous P-AscH- increased serum ascorbate levels from micromolar to millimolar levels. P-AscH- added to the gemcitabine + nab-paclitaxel (ASC) increased overall survival to 16 months compared to 8.3 months with gemcitabine + nab-paclitaxel (SOC) (HR = 0.46; 90 % CI 0.23, 0.92; p = 0.030). Median progression free survival was 6.2 (ASC) vs. 3.9 months (SOC) (HR = 0.43; 90 % CI 0.20, 0.92; p = 0.029). Adding P-AscH- did not negatively impact quality of life or increase the frequency or severity of adverse events.
Conclusions
P-AscH− infusions of 75 g three times weekly in patients with metastatic pancreatic cancer prolongs overall and progression free survival without detriment to quality of life or added toxicity (ClinicalTrials.gov number NCT02905578).
MeSH KeyWords: Pancreatic neoplasms, Ascorbic acid, Controlled clinical trial, Gemcitabine, Nab-paclitaxel
Graphical abstract
1. Background
Pancreatic ductal adenocarcinoma (PDAC) is the 3rd leading cause of cancer death in the US with increasing 0.5 % per year [1,2]. An estimated 80–85 % of patients have unresectable disease at initial presentation [3]; of those who undergo surgery, about 75 % develop metastatic disease [4]. The average overall survival (OS) at 5 years is currently 12 %, with an abysmal 3 % for those diagnosed with distant disease [5]. Despite promising results with immunotherapy in other cancers, the unique characteristics of PDAC engender poor response to immunotherapy [4,6,7]. Thus, new treatment paradigms must be identified and examined for efficacy.
There has been renewed interest in the use of ascorbate (vitamin C) in cancer treatment. Clinical data demonstrate that when ascorbate is given orally, plasma concentrations are tightly controlled at < 100 μM [8]. In contrast, when administered IV, plasma concentrations as high as 30 mM (pharmacologic ascorbate, P-AscH–) are safely achieved with few side effects [[9], [10], [11], [12], [13]]. Resulting pharmacologic concentrations from IV infusion distribute rapidly into the extracellular water space, generating the ascorbate radical and H2O2 [14,15]. Thus, P-AscH−, exploits the foundational differences in redox biology within the cancer cell, resulting in preferential cell killing [[16], [17], [18]].
Although used as a complementary and alternative medicine for decades [19], information regarding drug interactions, safety, tolerability, and impact of P-AscH– on cancer treatment were limited and anecdotal. In 2011 the FDA deemed IV ascorbate to be a pharmaceutical agent requiring the same robust oversight as a chemotherapeutic [20]. Two phase I trials were completed, both identifying 75 g of P-AscH− as safe when administered concurrently with gemcitabine for the treatment of stage IV PDAC [9,21]. Both trials also suggested a potential improvement for overall survival (OS) at this dose, identifying 75g as the recommended phase 2 dose for further trials [9,21]. To further examine if P-AscH− improved OS, we conducted a randomized trial in patients diagnosed with stage IV PDAC who had not yet received gemcitabine and/or nab-paclitaxel for the treatment of their metastatic disease. This is the first randomized controlled trial utilizing P-AscH− to manipulate the redox metabolism of cancer cells as a strategy to increase OS and progression free survival (PFS).
2. Methods
Trial Oversight–An investigator-initiated, multisite, unblinded, randomized controlled trial was overseen, and independently monitored, by the Data and Safety Monitoring Committee (DSMC) of the Holden Comprehensive Cancer Center at the University of Iowa. The trial protocol was approved by NIH and each site's Institutional Review Board of record (funding was obtained prior to the single-IRB policy). Trial subjects were enrolled at Medical College of Wisconsin (Milwaukee, Wisconsin, USA) and University of Iowa (Iowa City, Iowa, USA). An investigational new drug application was applied for, and granted by the U.S. FDA (J. J. Cullen, sponsor-investigator); annual reports were filed as required.
Sample size. Primary trial endpoint was OS defined as time from treatment initiation to death from any cause. The trial was designed to have 80 % power to detect an OS hazard ratio (HR) of 0.625 for standard of care cohort (SOC: gemcitabine, nab-paclitaxel only) vs. the ascorbate cohort (ASC: SOC with concomitant P-AscH−) using a one-sided log-rank statistical test stratified by study site, assessed for significance at the 0.2 level, and performed after randomizing 31 patients per cohort and observing a total of 52 deaths. The design also included an interim analysis to estimate conditional power after half of the target deaths (n = 26) had been observed and to stop the trial for futility if the estimated power was less than 25 %. Conditional power is the probability that the treatment effect will be statistically significant at the end of the study, given the hypothesized hazard ratio of 0.625 and the observed interim data [22,23]. Bayesian predictive power was additionally calculated at the interim as conditional power averaged over the posterior distribution of the log-hazard ratio. It can be interpreted as the expected predictive probability that the treatment effect would be significant at study end, given the uncertainty in the hazard ratio and the observed interim data [22,24].
Patients–Individuals with pathologically confirmed stage IV PDAC recommended to receive gemcitabine and nab-paclitaxel were screened for eligibility. Thus, those eligible for targeted therapy were not considered for this trial. Additional key criteria included measurable disease as per RECIST 1.1 [25], platelet count of at least 100,000 cells/mm3 and creatinine of <1.5x the institutional limit of normal or a creatinine clearance of at least 60 mL/min. Potential participants were required to provide independent consent and have an ECOG performance status of 0, 1, or 2 at screening. Patients were excluded from consideration if they had prior chemotherapy to treat their metastatic disease or had glucose-6-phosphatase dehydrogenase (G6PD) deficiency, Patients receiving insulin, or who were prescribed to check blood glucose with fingerstick assessments, were enrolled only with the approval of the principal investigator and DSMC due to an interaction between high concentrations of ascorbate with fingerstick glucometers. Detailed eligibility criteria are provided in the protocol and at ClinicalTrials.gov (NCT02905578) [26].
Trial Procedures–A two step enrollment process was employed due to the protracted result time for G6PD testing. Participants initiated gemcitabine/nab-paclitaxel when step 1 eligibility was confirmed, while step 2 confirmed G6PD status and then participants were 1:1 randomized (SOC vs ASC). Randomization was performed by the investigational pharmacy under the direction of the statistician, utilizing block-randomization with a block size of four patients and stratified by the study site.
All participants received IV gemcitabine and nab-paclitaxel weekly for three weeks of a four week cycle. Participants randomized to ASC received the same gemcitabine and nab-paclitaxel regimen in addition to P-AscH- (75 g, IV) three times weekly during all weeks of the cycle. Dose adjustments to P-AscH– were not allowed. Due to the fact that the current study involved stage IV pancreatic cancer patients, the diagnosis was made with needle biopsies or brushings yielding only enough material for diagnosis and not enough tissue to measure any redox parameters. Hematologic indices were required weekly with a comprehensive metabolic panel drawn prior to the start of each cycle. The EORTC QLQ-C30 was administered at the start of each cycle. Imaging was conducted every two cycles for response assessment; treatment response, based upon RECIST v1.1 principles, was conducted by American College of Radiology board-certified radiation oncologists specializing in PDAC and blinded to treatment assignment. Treatment continued until disease progression (clinical or radiographic), death, patient withdrawal, or unacceptable toxicity. Due to the 2-step eligibility process, participants were followed for adverse events from the first dose of any therapy (gemcitabine, nab-paclitaxel, or P-AscH−) through 30 days after the last infusion of P-AscH- and then followed for OS. Adverse events were graded using the Common Terminology Criteria for Adverse Events (CTCAE) version 4.03 [27]. All serious adverse events were independently reviewed by the assigned DSMC medical monitor at occurrence and re-reviewed in aggregate no less than yearly. Medical records were reviewed at monitoring to confirm adverse events were collected and reported appropriately. This trial was opened to accrual on November 2, 2018, and closed to accrual on September 8, 2023.
Outcomes–The primary outcome was OS. Secondary objectives included evaluating the impact on progression-free survival (PFS) as well as further categorizing the adverse event profile of the P-AscH−, gemcitabine, and nab-paclitaxel regimen. Quality of life (QoL) and patient reported symptoms were assessed using the EORTC QLQ-C30 version 3 during treatment as an exploratory objective [28]. Participants completed the QoL questionnaire within 28 days of the first day of therapy and then at the start of each subsequent cycle. Completion of the questionnaire was not required at the end-of-treatment visit because treatment was typically discontinued at the start of a cycle where discussion of disease progression would confound patient responses. Quality of life, functionality and symptom burden were scored as per the EORTC version 3 scoring manual [29]. Patients were excluded from statistical analysis of a score if their baseline value was missing. Other missing scores were linearly interpolated from their adjacent non-missing scores. Less than 1 % of the values for each score were imputed. For the functional scores and quality of life, minimal clinically important deterioration was defined as a 10-point decrease; for symptom burden, deterioration was defined as a 10-point increase.
Statistical Analysis-Patient demographics (Table 1, Supplementary Table S7) were summarized with descriptive statistics according to treatment arms. OS and PFS were estimated and plotted with the method of Kaplan-Meier. OS was defined as described previously. Patients with unobserved death dates were censored at the last known dates alive in the OS analysis. Progression free survival was defined as the time from treatment initiation to the earlier of RECIST-based disease progression or death. Participants were censored in the PFS analysis if their follow up for progression ended prior to death or if neither progression nor death were observed. Improved survival for ASC vs. SOC treated patients was assessed with one-sided log-rank tests stratified by study site, and HRs were computed with stratified Cox regression models. Included in the supplement is an expanded Cox regression analysis with univariable and multivariable adjustments for patient characteristics which may have been unbalanced in the trial randomization and thus potentially confounded the estimated treatment effect. Survival estimates are reported along with 90 % confidence intervals (CIs) which correspond to one-sided statistical testing at the 0.05 level.
Table 1.
Demographic and disease characteristics of randomized participants at baseline.
| Characteristic | SOC (n = 16) | ASC (n = 18) |
|---|---|---|
| Median age, yr (IQR) | 65 (56, 72) | 58.5 (54.5, 69) |
| Female | 8 (50.0 %) | 6 (33.3 %) |
| Enrolling Site | ||
| University of Iowa | 15 (93.8 %) | 17 (94.4 %) |
| Medical College of Wisconsin | 1 (6.3 %) | 1 (5.6 %) |
| Race | ||
| White | 15 (93.8 %) | 17 (94.4 %) |
| Unknown/declined to answer | 1 (6.3 %) | 1 (5.6 %) |
| Ethnicity | ||
| Hispanic or Latinx ethnic group | 0 (0.0 %) | 0 (0.0 %) |
| ECOG performance status | ||
| 0 | 8 (50.0 %) | 5 (27.8 %) |
| 1 | 6 (37.5 %) | 11 (61.1 %) |
| 2 | 2 (12.5 %) | 2 (11.1 %) |
| Newly diagnosed with metastatic disease | 14 (87.5 %) | 15 (83.3 %) |
| Number of metastases at study entry | ||
| 1 | 2 (12.5 %) | 2 (11.1 %) |
| 2 or 3 | 3 (18.8 %) | 2 (11.1 %) |
| 4 or 5 | 0 (0.0 %) | 2 (11.1 %) |
| 8 | 3 (18.8 %) | 0 (0.0 %) |
| ≥10 | 8 (50.0 %) | 12 (66.7 %) |
| Number of metastatic sites at study entry | ||
| 1 | 5 (31.3 %) | 11 (61.1 %) |
| 2 | 6 (37.5 %) | 1 (5.6 %) |
| 3 | 3 (18.8 %) | 5 (27.8 %) |
| 4+ | 2 (12.5 %) | 1 (5.6 %) |
| Sites of metastatic involvement | ||
| Liver | 13 (81.3 %) | 14 (77.8 %) |
| Peritoneal | 6 (37.5 %) | 3 (16.7 %) |
| Non-regional adenopathy | 6 (37.5 %) | 4 (22.2 %) |
| Lungs | 5 (31.3 %) | 6 (33.3 %) |
| Bone | 2 (12.5 %) | 0 (0 %) |
| Othera | 3 (18.8 %) | 1 (5.6 %) |
| Prior radiation therapya | 1 (6.3 %) | 1 (5.6 %) |
| Prior chemotherapya | 2 (12.5 %) | 2 (11.1 %) |
| Prior surgerya | 0 (0.0 %) | 3 (16.7 %) |
| Median total gemcitabine dose per participant, mg | 14,100 | 32,713 |
| Median total nab-paclitaxel dose per participant, mg | 1398 | 3123 |
| Median relative dose intensity, C1 & C2, gemcitabine | 87.7 % | 95.7 % |
| Median relative dose intensity, C1 & C2, nab-paclitaxel | 95.6 % | 95.6 % |
| Mean number of days between clinical visitb | 12.48 | 15.06 |
| Median number of days between clinical visitb | 11.18 | 15.27 |
Granular details regarding metastatic sites and treatment are available in Supplementary Table S7.
Any clinical visit within medical oncology, including on-demand, special complaints, or routine, with a physician or advanced practice provider. Does not include infusion-only visits.
Times to definitive deterioration (TDD2) in QLQ-C30 domain scores and global health status were compared between the two treatment arms with the survival methods described previously. TDD2 was defined as the time from treatment initiation to the earlier of a minimal clinically important deterioration that persisted for two cycles or disease progression occurring within 6 months of the last completed QoL questionnaire. Patients not experiencing either were treated as censored observations. Estimated median TDD2 are reported along with HRs, 95 % CIs, and two-sided p-values.
3. Results
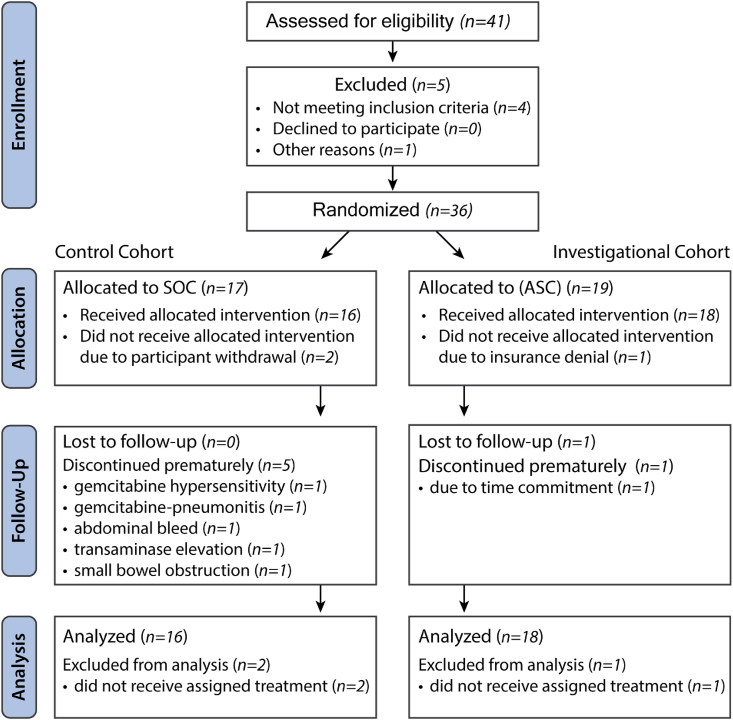
Participants—At the time of the interim analysis, thirty-six participants had been randomized across two academic sites (Fig. 1). Participants were considered evaluable for analysis of the primary objective if one dose of their assigned treatment regimen was received, resulting in an analysis cohort of 34 participants. Their demographic information is provided in Table 1. Five participants in the SOC cohort were discontinued from treatment prematurely (i.e., ended treatment absent of progression) due to adverse events whereas none were discontinued in the ASC cohort.
Fig. 1.
CONSORT participant flow diagram.
The interim analysis estimated a conditional power of 97 %, decidedly above the futility threshold of 25 %, and a Bayesian predictive power of 96.5 %. Based on the strong signal of efficacy conveyed by the large predictive power, independent DSMC review of the interim analysis results, and their support of a decision to close the trial, the trial was stopped at the interim analysis and final efficacy analysis was conducted.
Safety–Adverse events were consistent with the anticipated events associated with concomitant gemcitabine and nab-paclitaxel therapy (Table 2 and Supplementary Table S1). The rates of serious adverse events were lower in the ASC arm and the rate of grade 3 and 4 hematologic events (anemia, neutropenia, thrombocytopenia, and leukopenia) was lower in the ASC cohort compared to SOC (1.1 vs. 1.6).
Table 2.
Serious adverse events and selected routine grade 3 or 4 adverse events.
| Number of participants at risk: | SOC (n = 16) | ASC (n = 18) | ||
|---|---|---|---|---|
| Serious Adverse Eventsa, total (rate) | 27 (1.7) | 23 (1.2) | ||
| Fever | 5 | 3 | ||
| Cholangitis | 2 | 3 | ||
| Sepsis | 0 | 3 | ||
| Atrial Fibrillation | 0 | 2 | ||
| Clostridium Difficile Enteritis | 2 | 0 | ||
| Death, not otherwise specified | 0 | 2 | ||
| Febrile Neutropenia | 2 | 0 | ||
| Pneumonia | 1 | 1 | ||
| Small Intestinal Obstruction | 2 | 0 | ||
| Thrombosis | 1 | 1 | ||
| Vomiting | 2 | 0 | ||
| Abdominal Pain | 0 | 1 | ||
| Bile Duct Stenosis | 0 | 1 | ||
| Biliary Obstruction | 0 | 1 | ||
| Cardiac Arrest | 0 | 1 | ||
| Cather Infection | 1 | 0 | ||
| Colonic Hemorrhage | 1 | 0 | ||
| Diverticulitis, Perforated | 0 | 1 | ||
| Endocarditis Infective | 1 | 0 | ||
| Gastric Obstruction | 1 | 0 | ||
| Hyperkalemia | 1 | 0 | ||
| Hypovolemic Shock | 0 | 1 | ||
| Inguinal Hernia | 1 | 0 | ||
| Lung Infection | 1 | 0 | ||
| Nausea | 1 | 0 | ||
| Platelet Count Decreased | 1 | 0 | ||
| Prolonged Qtc | 1 | 0 | ||
| Transient ischemic attack | 0 | 1 | ||
| SOC (n = 16) | ASC (n = 18) | |||
| Selected Grade 3 or 4 Adverse Eventsb | 3 | 4 | 3 | 4 |
| Neutrophil count decreased | 5 | 5 | 4 | 2 |
| Platelet count decreased | 1 | 2 | 1 | 0 |
| Febrile neutropenia | 2 | 0 | 0 | 0 |
| Anorexia | 1 | 0 | 0 | 0 |
| Constipation | 0 | 0 | 2 | 0 |
| Diarrhea | 2 | 0 | 2 | 0 |
| Fatigue | 1 | 0 | 3 | 0 |
| Nausea | 2 | 0 | 0 | 0 |
| Peripheral sensory neuropathy | 0 | 0 | 1 | 0 |
| Vomiting | 2 | 0 | 0 | 0 |
| Weight loss | 0 | 0 | 1 | 0 |
| Hypertension | 1 | 0 | 1 | 0 |
| Painc | 3 | 0 | 2 | 0 |
| Infectionc | 11 | 0 | 6 | 2 |
Number (n) of serious adverse events as defined by 21 CFR 312.32.
Maximum toxicity experienced per subject.
Granular details are available in the full adverse event listing in the Supplementary Table S1.
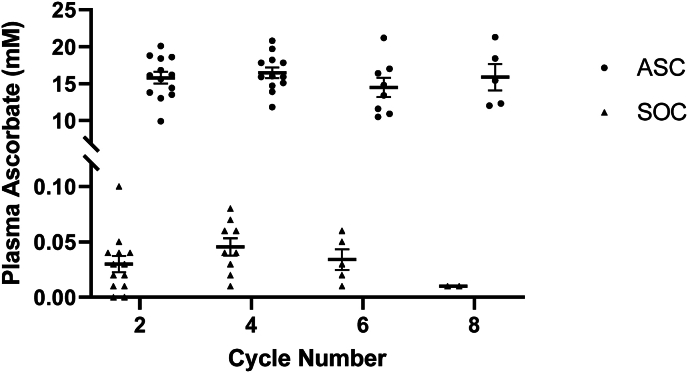
Ascorbate Levels–Ascorbate levels were measured at baseline and at the end of every 2 cycles using methods published previously [9]. As seen in Fig. 2, intravenous administration of P-AscH- resulted in a nearly 500-fold increase in plasma ascorbate levels with the average ascorbate concentration being 16 ± 3 mM in the ASC cohort compared to 0.034 ± 0.02 mM in the SOC cohort.
Fig. 2.
Plasma levels of ascorbate achieved in subjects over time. In subjects receiving standard of care and ascorbate (ASC), peak ascorbate levels post-infusion were approximately 500-fold higher than in patients receiving standard of care (SOC).
Chemotherapy compliance–The ASC cohort had a longer median treatment time per subject (179 days vs. 94 days), a higher median total dose of nab-paclitaxel per subject (3123 mg vs. 1398 mg) and a higher median total dose of gemcitabine per subject (32,713 mg vs. 14,100 mg). The relative dose intensity (RDI) was calculated as per the literature [30] and demonstrated the median RDI was higher in the ASC cohort for gemcitabine (Table 1: ASC 96 % vs. 88 % SOC) and comparable for nab-paclitaxel (Table 1: ASC 96 % vs 96 % SOC).
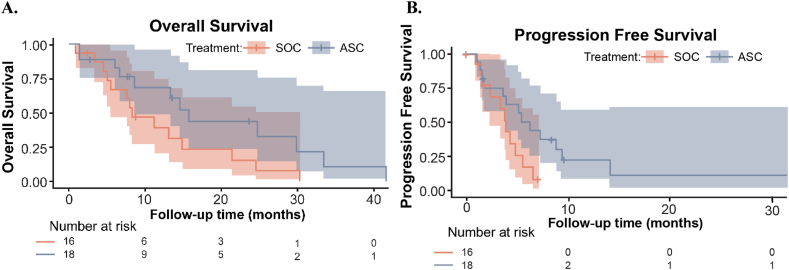
Efficacy–In the primary endpoint analysis, the OS HR was 0.46 (90 % CI 0.23, 0.92; p = 0.030) indicating a significant benefit of P-AscH- in the ASC cohort. Median OS was 16 months (90 % CI 8.6, upper bound not achieved NA) in the ASC cohort vs 8.3 (90 % CI 5.6, NA) months in the SOC cohort (Fig. 3A). Median OS of the SOC cohort aligned with the historic value (8.5 months) that was used to power the study [31]. In analysis of secondary endpoints, the progression free survival (PFS) HR was 0.43 (90 % CI 0.20, 0.92; p = 0.029) with a median PFS of 6.2 months (90 % CI 3.9, NA) in the ASC cohort vs. 3.9 months (90 % CI 2.3, NA) in the SOC cohort (Fig. 3B). The hazard ratios are comparable between PFS and OS. In Cox regression analysis with univariable adjustments for potential confounders, the OS HR ranged from 0.27 (90 % CI 0.12, 0.59) with gemcitabine RDI adjustment to 0.53 (90 % CI 0.24, 1.14) with age adjustment, whereas the PFS HR ranged from 0.35 (90 % CI 0.16, 0.77) to 0.49 (0.23, 1.07) with the same adjustments (Supplementary Table S2). With multivariable regression adjustments, the HR was 0.31 (90 % CI 0.14, 0.70) for OS and 0.23 (90 % CI 0.09, 0.57) for PFS (Supplementary Table S3). Objective response rate was 23 % for SOC cohort vs. 38 % for ASC.
Fig. 3.
Kaplan-Meier estimates and 90 % confidence intervals for (A) overall survival and (B) progression free survival in the treatment arms. The investigational cohort (ASC) demonstrated statistically significant increases in overall survival (median: 16 months ASC vs. 8.3 months SOC; p = 0.030) and progression free survival (median: 6.2 months ASC vs. 3.9 months SOC; p = 0.029).
Subsequent Therapy. Post-study treatments were similar across both arms, with the most commonly prescribed regimens being the NAPOLI regimen and mFOLFIRINOX. Nine subjects transitioned to hospice immediately (SOC = 5, ASC 4).
Quality of Life–Overall, quality of life (QoL) analysis and interpretation are limited by the small sample size as well as the nature of quantifying subjective data resulting in participant variation. The QoL data were analyzed as time to definitive deterioration (TDD2) (Supplementary Table S4) and did not demonstrate statistical significance between the two cohorts for global health status. However, participants in the ASC cohort generally had longer TDD2 when compared to the SOC cohort, which is favorable. The ASC cohort took longer to experience negative impact from insomnia (ASC 6.2 vs. SOC 3.8 months, HR = 0.43; p = 0.047), constipation (ASC 6.2 vs. SOC 3.8 months, HR = 0.39; p = 0.032), and financial difficulties (ASC 5.4 vs. SOC 3.8 months, HR = 0.38; p = 0.022).
4. Discussion
First fully described in 1933, vitamin C has been found to play many roles in biochemical processes based on its ability to act as an enzyme cofactor and as a donor antioxidant [32]. Vitamin C became an early unorthodox therapy for cancer treatment, with initial clinical studies demonstrating increased OS in terminal cancer patients treated with high-dose ascorbate [[33], [34], [35]]. Subsequent randomized controlled trials found no benefit in administering ascorbate to cancer patients [36,37]. However, when comparing these divergent results, the initial trials included both oral and high dose IV ascorbate whereas the latter randomized trials administered only oral ascorbate. The differences between oral and IV ascorbate bioavailability were then unknown, but as seen in Fig. 2, the pharmacokinetics of intravenous ascorbate demonstrate a significant increase in achievable plasma levels compared to orally administered ascorbate [38,39]. Pre-clinical data suggested a therapeutic benefit at an ascorbate concentration of >10 mM [9]. Two phase I clinical trials identified this was achievable in the PDAC patient population with an infusion of 75 g of ascorbate [9,13]. These same trials suggested a benefit to adding P-AscH- to a gemcitabine-based regimen for PDAC [9,13]. In 1997, Burris and colleagues evaluated gemcitabine against fluorouracil as a first-line therapy for advanced pancreatic cancer in a randomized clinical trial [40]. Although gemcitabine was identified as the superior anti-neoplastic therapy, overall survival was abysmal in either arm: with a median survival of 5.65 months for gemcitabine and 4.41 months for fluorouracil [40]. In 2011, Conroy et al. identified FOLFIRINOX as superior over gemcitabine alone (11.1 months vs. 6.8 months) but at the expense of an increase in the incidence of adverse events [41]. The phase 1 safety study adding pharmacologic ascorbate to the Burris gemcitabine regimen suggested an improvement in OS to 13 months but any inference is significantly hampered by the sample size [9].
The mechanism of P-AscH--induced toxicity is due to H2O2 which has been demonstrated by our group and others [8,[12], [13], [14]]. These studies clearly demonstrate addition of various forms of both extracellular and intracellular catalase completely reverses P-AscH--induced cytotoxicity in a variety of cancer cell lines, while normal cells are resistant. Our previous studies suggest catalase activity in the tumors may explain differences in patient responding to high dose ascorbate [15]. Other investigators have suggested that P-AscH--induced toxicity is due to KRAS or BRAF mutations and possibly GLUT1 status [42]. However, our preclinical studies have demonstrated that KRAS or BRAF mutations have no influence in the mechanism of P-AscH- [14].
Per NCCN guidelines, category 1 recommendations for metastatic PDAC are either fluorouracil (5-FU), leucovorin, irinotecan and oxaliplatin (FOLFIRINOX) regimen, established by the PRODIGE 4/ACCORD 11 clinical trial or gemcitabine combined with nab-paclitaxel, established by the MPACT trial [31,41]. The PRODIGE 4/ACCORD 11 clinical trial reported significant toxicity with the FOLFIRINOX regimen with increases in grade 3 and 4 events compared to its gemcitabine-only arm: neutropenia (45.7 % vs. 21.0 %), febrile neutropenia (5.4 % vs. 1.2 %) and thrombocytopenia (9.1 % vs. 3.6 %). The incidence of grade 3 and 4 events for the MPACT trial's gemcitabine and nab-paclitaxel cohort was 38 % for neutropenia, 3 % for febrile neutropenia, and 13 % for thrombocytopenia [31].
Subjects who received P-AscH- had a reduction in chemotherapy-related toxicities which has been seen in other P-AscH- clinical studies [9,10,13,43]. This study's investigational ASC cohort had a reduction in chemotherapy-related toxicities compared to the SOC cohort: neutropenia (33.3 % vs. 62.5 %), febrile neutropenia (0 %–12.5 %), and thrombocytopenia (5.6 % vs.12.5 %) (Table 2). This study utilized a study-specific dose-reduction table designed by the study's medical oncologists (Supplementary Table S5). The differences in dose reduction strategies of the cytotoxic regimen could explain the differences in these toxicities for the gemcitabine and nab-paclitaxel regimens. Days between clinic visits are similar (Table 1), negating concern for additional medical oversight during therapy.
The relative dose intensity for the first two cycles of therapy is higher in the ASC cohort (Table 1). Doses were held 9 times for SOC cohort (cycle 1 = 4, cycle 2 = 5) and 3 times for ASC cohort (cycle 1 = 2, cycle 2 = 1). Independent monitoring confirmed protocol-dictated dose modifications and holds were applied as required per protocol (Supplementary Table S5). This could be due to ascorbate increasing tolerability of chemotherapy. However, this inference may be hampered by the small sample size.
Although the chemotherapy regimens were intended to be administered for 6 cycles in the PRODIGE 4/ACCORD 11 trial, the reported median PFS was 6.4 months for the FOLFRINOX group and 3.3 months for the gemcitabine-only group. In our current study, PFS was 3.9 months in the SOC cohort and 6.2 months in the ASC cohort. Another promising result is that the anticipated level of grade 3 and 4 hematologic toxicities was not realized, although the sample size is small. The observed PFS of 3.9 months for the SOC cohort was shorter compared to the MPACT clinical trial, which established the gemcitabine and nab-paclitaxel regimen, but the observed OS of 8.3 months for the SOC cohort did align [31]. In addition, hazard ratios are not disproportionate between PFS and OS. The improvement in OS is consistent with single cohort trials investigating ascorbate in PDAC and is now demonstrated in our current, randomized trial [9,13,44].
The dosing for nab-paclitaxel utilized in this study was 125 mg/m2, infused over 30–40 min during weeks 1, 2, and 3 of the 4 week cycle as per the MPACT trial, which reported a combined incidence of grade 3 and 4 nab-paclitaxel-induced peripheral neuropathy of 17 % [31]. In comparison, this study did not have any grade 4 nab-paclitaxel-induced peripheral neuropathy and had an incidence of only 5.5 % grade 3 nab-paclitaxel-induced peripheral neuropathy in the ASC cohort and 0 % in the SOC cohort. Risks for developing peripheral neuropathy include a cumulative dose of 1400 mg/m2 of paclitaxel, with up to 60 % of these patients having the toxicity transition to a chronic condition [45]. The overall cumulative nab-paclitaxel dose was 967 mg/m2 for the SOC cohort and 1944 mg/m2 for the ASC cohort, well above the increased risk threshold, with less than expected toxicity.
Despite the total dose of paclitaxel and the prolonged periods of administration, there was a delay in time to deterioration when using QLQ-C30. Although exploratory and limited by sample size, these data provide insight into the participant's experience during treatment. Participants in the ASC cohort did not experience a decrease in the time to deterioration; rather, hazard ratios suggest a delay in deterioration in functionality, experienced symptoms, and overall quality of life (Supplementary Tables S4 and S6, Supplementary Figs. S1 and S2). Areas of interest for future focus include further evaluating the domains of physical function and pain symptomology, which demonstrated a promising, yet not significant, improvement in the ASC cohort. Data from the QLQ-C30, combined with the frequency and severity of treatment emergent adverse events, suggest increased chemotherapy tolerance in the ASC investigational arm may contribute to the improved OS and PFS.
This phase 2 randomized trial of P-AscH- indicates a benefit in both OS as well as quality of life for patients with advanced PDAC. This benefit is tempered by the time investiture necessary for treatment. Treatment is three times weekly with a 2 h infusion; this does not include travel time or time spent in the waiting room.
In conclusion, this randomized trial demonstrated adding P-AscH- to gemcitabine and nab-paclitaxel yielded increased OS and PFS without the added hematologic toxicity [31,41]. Quality of life as assessed by the EORTC QLQ-C30 remained consistent between arms, without an apparent treatment associated degradation (Supplementary Table S4) [41]. Although limited by small sample size and a lack of diversity (Supplementary Table S8), this randomized, actively controlled trial provides key data regarding effect size to design a phase 3 trial to assess effectiveness of P-AscH-with metastatic PDAC as well as its generalizability to a larger population. Additionally, it provides data suggesting P-AscH– increasing the tolerability of cytotoxic chemotherapy.
Data Availability statement
The data generated in this study are not publicly available due to protect our patient participants’ privacy and confidentiality. Data are available upon reasonable request from the corresponding author and execution of an appropriate data usage agreement. Data are available only from those participants who opted into data sharing.
The protocol, informed consent, and statistical analysis plan are immediately available.
Funding
This work was supported by the National Institutes of Health P01 CA217797 as well as the Holden Comprehensive Cancer Center support grant 1P30CA086862-23. The funders did not play a role in the design of the study; the collection, analysis, and interpretation of the data; the writing of the manuscript; and the decision to submit the manuscript for publication.
CRediT authorship contribution statement
Kellie L. Bodeker: Writing – review & editing, Writing – original draft, Project administration, Methodology, Investigation, Formal analysis, Data curation. Brian J. Smith: Writing – review & editing, Writing – original draft, Formal analysis, Conceptualization. Daniel J. Berg: Writing – review & editing, Investigation, Funding acquisition, Conceptualization. Chandrikha Chandrasekharan: Writing – review & editing, Investigation. Saima Sharif: Writing – review & editing, Investigation. Naomi Fei: Project administration. Sandy Vollstedt: Writing – review & editing, Investigation, Data curation. Heather Brown: Writing – review & editing, Project administration. Meghan Chandler: Writing – review & editing, Project administration, Data curation. Amanda Lorack: Writing – review & editing, Project administration, Data curation. Stacy McMichael: Writing – review & editing, Project administration, Data curation. Jared Wulfekuhle: Writing – review & editing, Project administration, Data curation. Brett A. Wagner: Writing – review & editing, Methodology, Data curation. Garry R. Buettner: Writing – review & editing, Project administration, Methodology, Investigation, Funding acquisition, Conceptualization. Bryan G. Allen: Writing – review & editing, Project administration, Investigation, Funding acquisition, Conceptualization. Joseph M. Caster: Writing – review & editing, Investigation, Formal analysis, Data curation. Barbara Dion: Writing – review & editing, Project administration, Data curation. Mandana Kamgar: Writing – review & editing, Project administration, Investigation, Data curation. John M. Buatti: Writing – review & editing, Project administration, Investigation, Funding acquisition, Conceptualization. Joseph J. Cullen: Writing – original draft, Resources, Project administration, Methodology, Investigation, Funding acquisition, Data curation.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
The authors thank the brave patients, as well as their caregivers and support network, for participating in this clinical trial to improve cancer research for us all. We appreciate the diligent efforts of the assigned independent monitors J. Wegmann, J. Sieren, J. Geick, A. Farmer, and E. Seig throughout the conduct of this trial.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.redox.2024.103375.
Appendix A. Supplementary data
The following is the Supplementary data to this article.
Data availability
Data will be made available on request.
References
- 1.American Cancer Society . 2023. Cancer Facts & Figures 2023. Atlanta, GA. [Google Scholar]
- 2.Ilic I., Ilic M. International patterns in incidence and mortality trends of pancreatic cancer in the last three decades: a joinpoint regression analysis. World J. Gastroenterol. 2022;28(32):4698–4715. doi: 10.3748/wjg.v28.i32.4698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rawla P., Sunkara T., Gaduputi V. Epidemiology of pancreatic cancer: global trends, etiology and risk factors. World J. Oncol. 2019;10(1):10–27. doi: 10.14740/wjon1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Halbrook C.J., Lyssiotis C.A., Pasca di Magliano M., et al. Pancreatic cancer: advances and challenges. Cell. 2023;186(8):1729–1754. doi: 10.1016/j.cell.2023.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.American Cancer Society. Survival rates for pancreatic cancer. https://www.cancer.org/cancer/types/pancreatic-cancer/detection-diagnosis-staging/survival-rates.html (02 March; date last accessed).
- 6.Gautam S.K., Batra S.K., Jain M. Molecular and metabolic regulation of immunosuppression in metastatic pancreatic ductal adenocarcinoma. Mol. Cancer. 2023;22(1):118. doi: 10.1186/s12943-023-01813-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hester R., Mazur P.K., McAllister F. Immunotherapy in pancreatic adenocarcinoma: beyond "Copy/Paste". Clin. Cancer Res. 2021;27(23):6287–6297. doi: 10.1158/1078-0432.CCR-18-0900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen Q., Espey M.G., Sun A.Y., et al. Ascorbate in pharmacologic concentrations selectively generates ascorbate radical and hydrogen peroxide in extracellular fluid in vivo. Proc Natl Acad Sci U S A. 2007;104(21):8749–8754. doi: 10.1073/pnas.0702854104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Welsh J.L., Wagner B.A., van't Erve T.J., et al. Pharmacological ascorbate with gemcitabine for the control of metastatic and node-positive pancreatic cancer (PACMAN): results from a phase I clinical trial. Cancer Chemother. Pharmacol. 2013;71(3):765–775. doi: 10.1007/s00280-013-2070-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Allen B.G., Bodeker K.L., Smith M.C., et al. First-in-Human phase I clinical trial of pharmacologic ascorbate combined with radiation and temozolomide for newly diagnosed glioblastoma. Clin. Cancer Res. 2019;25(22):6590–6597. doi: 10.1158/1078-0432.CCR-19-0594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Furqan M., Abu-Hejleh T., Stephens L.M., et al. Pharmacological ascorbate improves the response to platinum-based chemotherapy in advanced stage non-small cell lung cancer. Redox Biol. 2022;53 doi: 10.1016/j.redox.2022.102318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schoenfeld J.D., Sibenaller Z.A., Mapuskar K.A., et al. O2(-) and H2O2-mediated disruption of Fe metabolism causes the differential susceptibility of NSCLC and GBM cancer cells to pharmacological ascorbate. Cancer Cell. 2017;31(4):487–500 e8. doi: 10.1016/j.ccell.2017.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Alexander M.S., Wilkes J.G., Schroeder S.R., et al. Pharmacological ascorbate reduces radiation-induced normal tissue toxicity and enhances tumor radiosensitization in pancreatic cancer. Cancer Res. 2018;78(24):6838–6851. doi: 10.1158/0008-5472.CAN-18-1680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Du J., Martin S.M., Levine M., et al. Mechanisms of ascorbate-induced cytotoxicity in pancreatic cancer. Clin. Cancer Res. 2010;16(2):509–520. doi: 10.1158/1078-0432.CCR-09-1713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Du J., Carroll R.S., Steers G.J., et al. Catalase modulates the radio-sensitization of pancreatic cancer cells by pharmacological ascorbate. Antioxidants. 2021;10(4) doi: 10.3390/antiox10040614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Olney K.E., Du J., van 't Erve T.J., et al. Inhibitors of hydroperoxide metabolism enhance ascorbate-induced cytotoxicity. Free Radic. Res. 2013;47(3):154–163. doi: 10.3109/10715762.2012.755263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Doskey C.M., Buranasudja V., Wagner B.A., et al. Tumor cells have decreased ability to metabolize H2O2: implications for pharmacological ascorbate in cancer therapy. Redox Biol. 2016;10:274–284. doi: 10.1016/j.redox.2016.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Callaghan C.M., Abukhiran I.M., Masaadeh A., et al. Manipulation of redox metabolism using pharmacologic ascorbate opens a therapeutic window for radio-sensitization by ATM inhibitors in colorectal cancer. Int. J. Radiat. Oncol. Biol. Phys. 2023;115(4):933–944. doi: 10.1016/j.ijrobp.2022.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Padayatty S.J., Sun A.Y., Chen Q., et al. Vitamin C: intravenous use by complementary and alternative medicine practitioners and adverse effects. PLoS One. 2010;5(7) doi: 10.1371/journal.pone.0011414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.U.S. Food and Drug Administration (FDA). McGuff pharmaceuticals Inc. 12/28/10: Warning letter. https://web.archive.org/web/20110106224234/http://www.fda.gov/ICECI/EnforcementActions/WarningLetters/ucm238251.htm (04 January; date last accessed).
- 21.Monti D.A., Mitchell E., Bazzan A.J., et al. Phase I evaluation of intravenous ascorbic acid in combination with gemcitabine and erlotinib in patients with metastatic pancreatic cancer. PLoS One. 2012;7(1) doi: 10.1371/journal.pone.0029794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jennison C., Turnbull B.W. Statistical approaches to interim monitoring of medical trials: a review and commentary. Stat. Sci. 1990;5(3):299–317. [Google Scholar]
- 23.Chang M. John Wiley; Hoboken, N.J.: 2008. Classical and Adaptive Clinical Trial Designs Using ExpDesign Studio [trademark Symbol] [Google Scholar]
- 24.Saville B.R., Connor J.T., Ayers G.D., et al. The utility of Bayesian predictive probabilities for interim monitoring of clinical trials. Clin. Trials. 2014;11(4):485–493. doi: 10.1177/1740774514531352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Eisenhauer E.A., Therasse P., Bogaerts J., et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1) European Journal of Cancer. 2009;45(2):228–247. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 26.Cullen JJ. A Phase 2 Trial of High-dose Ascorbate for Pancreatic Cancer (PACMAN 2.1). ClinicalTrials.gov identifier: NCT02905578. https://clinicaltrials.gov/study/NCT02905578 (23 October 2023; date last accessed).
- 27.National Cancer Institute (NCI). Cancer Therapy Evaluation Program (CTEP): Common Terminology Criteria for Adverse Events (CTCAE). https://ctep.cancer.gov/protocoldevelopment/electronic_applications/ctc.htm (14 November; date last accessed).
- 28.de Haes J., Curran D., Young T., et al. Quality of life evaluation in oncological clinical trials - the EORTC model. The EORTC Quality of Life Study Group. Eur. J. Cancer. 2000;36(7):821–825. doi: 10.1016/s0959-8049(00)00007-1. [DOI] [PubMed] [Google Scholar]
- 29.European Organisation for Research and Treatment of Cancer (EORTC). EORTC QLQ-C30 scoring manual. https://www.eortc.org/app/uploads/sites/2/2018/02/SCmanual.pdf. [DOI] [PubMed]
- 30.Kim C.G., Sohn J., Chon H., et al. Incidence of febrile neutropenia in Korean female breast cancer patients receiving preoperative or postoperative doxorubicin/cyclophosphamide followed by docetaxel chemotherapy. J Breast Cancer. 2016;19(1):76–82. doi: 10.4048/jbc.2016.19.1.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Von Hoff D.D., Ervin T., Arena F.P., et al. Increased survival in pancreatic cancer with nab-paclitaxel plus gemcitabine. N. Engl. J. Med. 2013;369(18):1691–1703. doi: 10.1056/NEJMoa1304369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Svirbely J.L., Szent-Gyorgyi A. The chemical nature of vitamin C. Biochem. J. 1932;26(3):865–870. doi: 10.1042/bj0260865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cameron E., Campbell A. Innovation vs. quality control: an ‘unpublishable’ clinical trial of supplemental ascorbate in incurable cancer. Med. Hypotheses. 1991;36:185–189. doi: 10.1016/0306-9877(91)90127-k. [DOI] [PubMed] [Google Scholar]
- 34.Cameron E., Pauling L. Supplemental ascorbate in the supportive treatment of cancer: prolongation of survival times in terminal human cancer. Proc Natl Acad Sci U S A. 1976;73(10):3685–3689. doi: 10.1073/pnas.73.10.3685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cameron E., Pauling L. Supplemental ascorbate in the supportive treatment of cancer: reevaluation of prolongation of survival times in terminal human cancer. Proc Natl Acad Sci U S A. 1978;75(9):4538–4542. doi: 10.1073/pnas.75.9.4538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Creagan E.T., Moertel C.G., O'Fallon J.R., et al. Failure of high-dose vitamin C (ascorbic acid) therapy to benefit patients with advanced cancer. A controlled trial. N. Engl. J. Med. 1979;301(13):687–690. doi: 10.1056/NEJM197909273011303. [DOI] [PubMed] [Google Scholar]
- 37.Moertel C.G., Fleming T.R., Creagan E.T., et al. High-dose vitamin C versus placebo in the treatment of patients with advanced cancer who have had no prior chemotherapy. A randomized double-blind comparison. N. Engl. J. Med. 1985;312(3):137–141. doi: 10.1056/NEJM198501173120301. [DOI] [PubMed] [Google Scholar]
- 38.Levine M., Conry-Cantilena C., Wang Y., Welch R.W., Washko P.W., Dhariwal K.R., Park J.B., Lazarev A., Graumlich J.F., King J., Cantilena L.R. Vitamin C Pharmacokinetics in healthy volunteers: evidence for a recommended dietary allowance. Proc. Natl. Acad. Sci. U.S.A. 1996;93:3704–3709. doi: 10.1073/pnas.93.8.3704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Padayatty S., Sun H., Wang Y., Riordan H.D., Hewitt S.M., Katz A., Wesley R.A., Levine M. Vitamin C pharmacokinetics: implications for oral and intravenous use. Ann. Intern. Med. 2004;140:533–537. doi: 10.7326/0003-4819-140-7-200404060-00010. [DOI] [PubMed] [Google Scholar]
- 40.Burris H.A., 3rd, Moore M.J., Andersen J., et al. Improvements in survival and clinical benefit with gemcitabine as first-line therapy for patients with advanced pancreas cancer: a randomized trial. J. Clin. Oncol. 1997;15(6):2403–2413. doi: 10.1200/JCO.1997.15.6.2403. [DOI] [PubMed] [Google Scholar]
- 41.Conroy T., Desseigne F., Ychou M., et al. FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N. Engl. J. Med. 2011;364(19):1817–1825. doi: 10.1056/NEJMoa1011923. [DOI] [PubMed] [Google Scholar]
- 42.Yun J., Mullarky E., Lu C., et al. Vitamin C selectively kills KRAS and BRAF mutant colorectal cancer cells by targeting GAPDH. Science. 2015;350(6266):1391–1396. doi: 10.1126/science.aaa5004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ma Y., Chapman J., Levine M., et al. High-dose parenteral ascorbate enhanced chemosensitivity of ovarian cancer and reduced toxicity of chemotherapy. Sci. Transl. Med. 2014;6(222) doi: 10.1126/scitranslmed.3007154. [DOI] [PubMed] [Google Scholar]
- 44.Polireddy K., Dong R., Reed G., et al. High dose parenteral ascorbate inhibited pancreatic cancer growth and metastasis: mechanisms and a phase I/IIa study. Sci. Rep. 2017;7(1) doi: 10.1038/s41598-017-17568-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Klein I., Lehmann H.C. Pathomechanisms of paclitaxel-induced peripheral neuropathy. Toxics. 2021;9(10) doi: 10.3390/toxics9100229. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data generated in this study are not publicly available due to protect our patient participants’ privacy and confidentiality. Data are available upon reasonable request from the corresponding author and execution of an appropriate data usage agreement. Data are available only from those participants who opted into data sharing.
The protocol, informed consent, and statistical analysis plan are immediately available.
Data will be made available on request.