Abstract
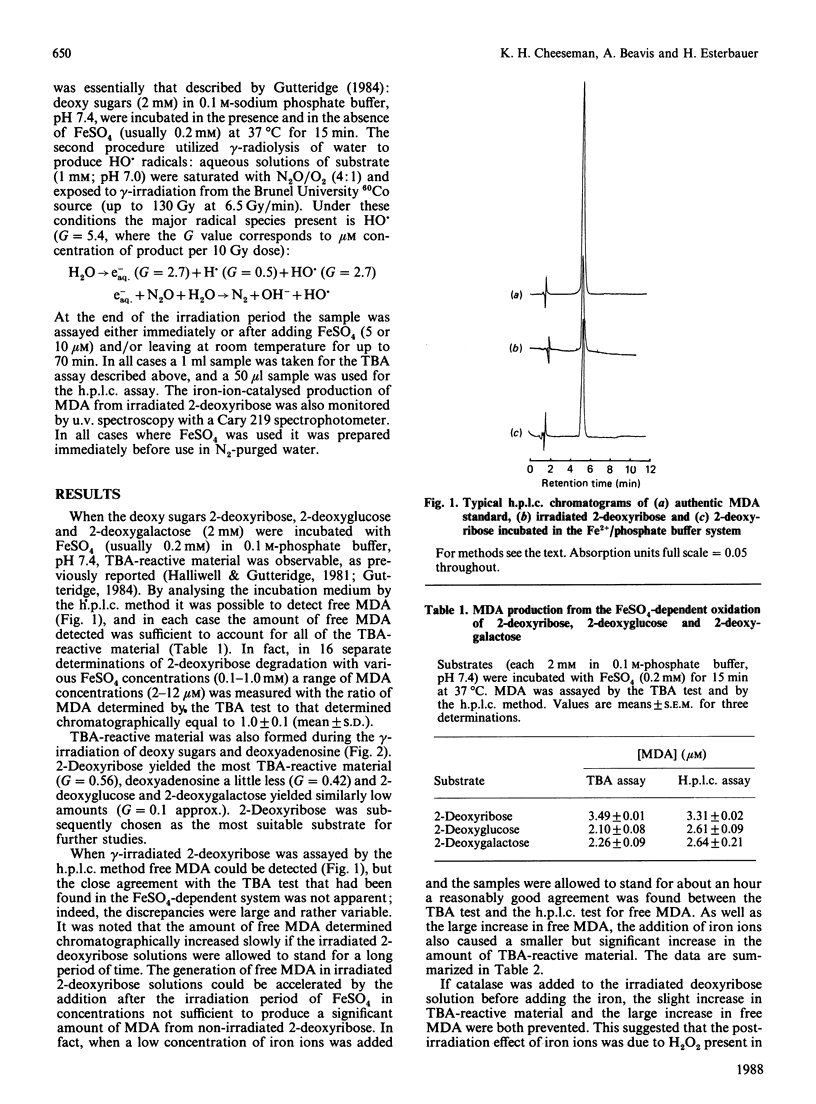
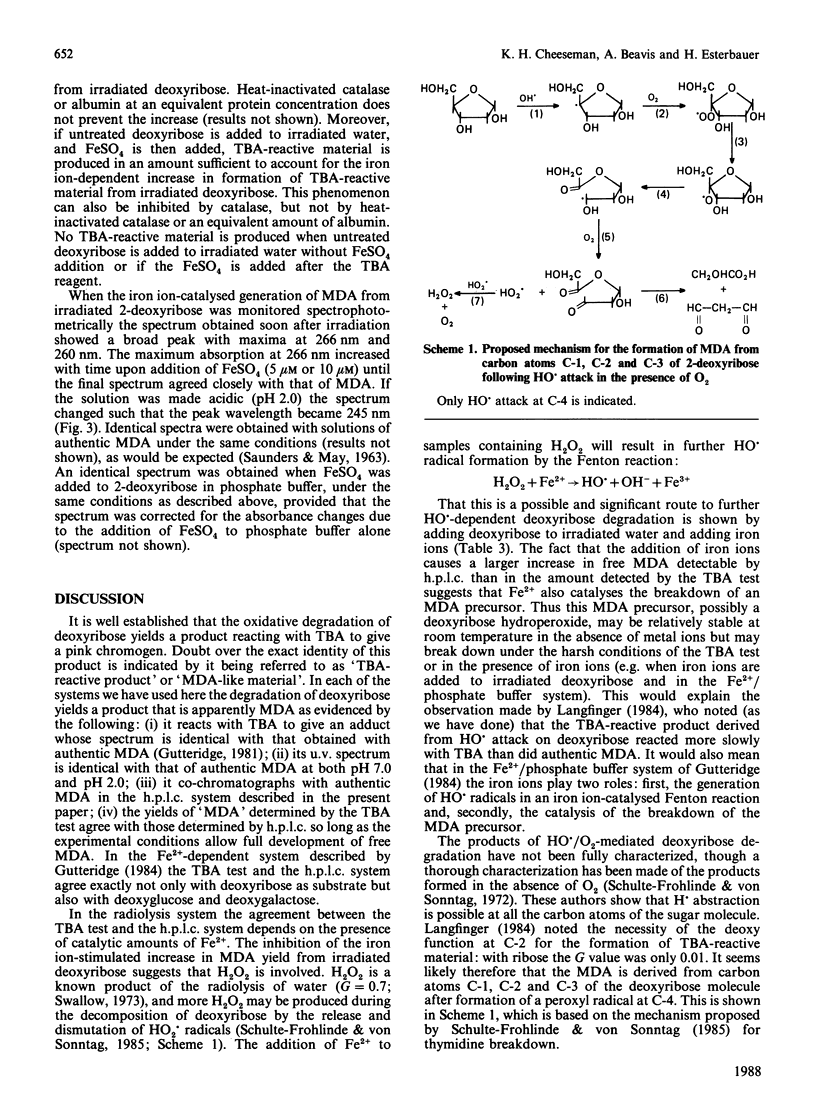
The degradation of 2-deoxyribose to thiobarbituric acid-reactive material was investigated with two hydroxyl-radical-generating systems: (i) a defined gamma-radiolysis method and (ii) incubation with FeSO4 in phosphate buffer. In each case the thiobarbituric acid-reactive material can be accounted for by malondialdehyde, as measured by an h.p.l.c. method for free malondialdehyde. In the radiolysis system there is a large post-irradiation increase in free malondialdehyde if iron ions are added to the samples. It is proposed that this is due to iron ions catalysing the formation of hydroxyl radicals from radiolytically generated H2O2 as well as stimulating the breakdown of an intermediate deoxyribose degradation product. A mechanism for the formation of malondialdehyde during deoxyribose degradation is proposed.
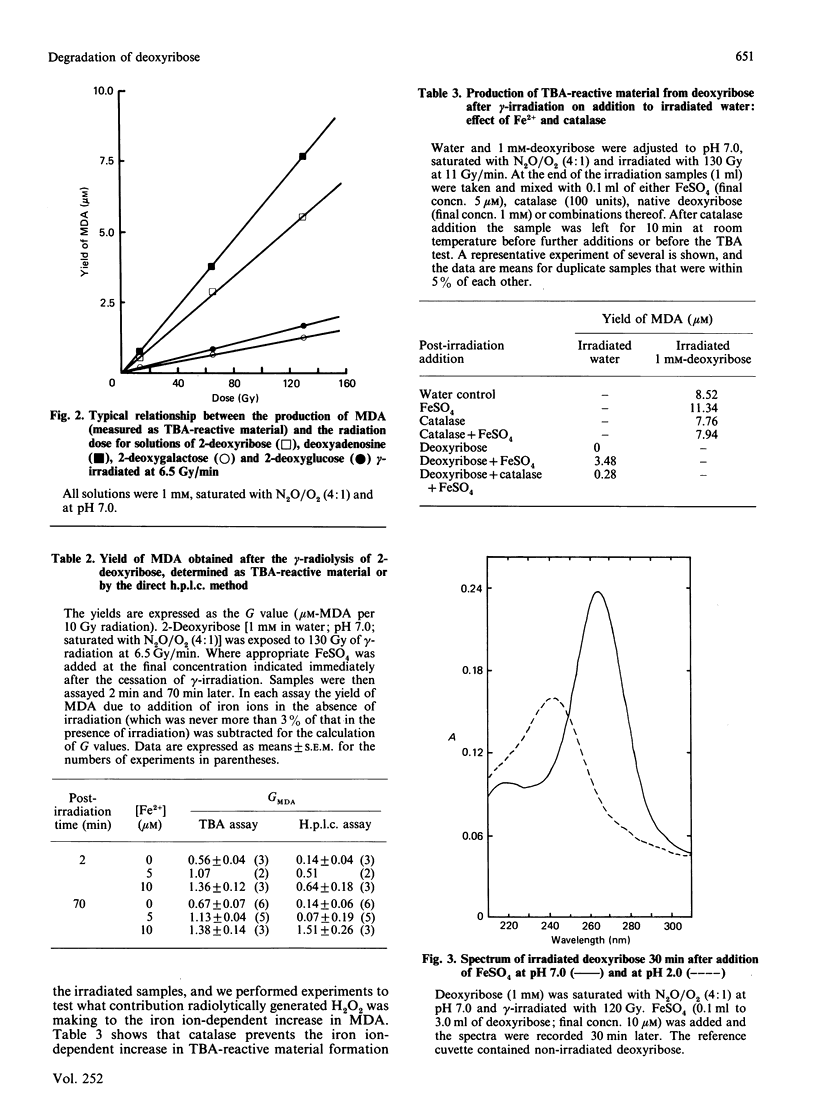
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Burger R. M., Berkowitz A. R., Peisach J., Horwitz S. B. Origin of malondialdehyde from DNA degraded by Fe(II) x bleomycin. J Biol Chem. 1980 Dec 25;255(24):11832–11838. [PubMed] [Google Scholar]
- Esterbauer H., Lang J., Zadravec S., Slater T. F. Detection of malonaldehyde by high-performance liquid chromatography. Methods Enzymol. 1984;105:319–328. doi: 10.1016/s0076-6879(84)05041-2. [DOI] [PubMed] [Google Scholar]
- Giloni L., Takeshita M., Johnson F., Iden C., Grollman A. P. Bleomycin-induced strand-scission of DNA. Mechanism of deoxyribose cleavage. J Biol Chem. 1981 Aug 25;256(16):8608–8615. [PubMed] [Google Scholar]
- Gutteridge J. M. Identification of malondialdehyde as the TBA-reactant formed by bleomycin-iron free radical damage to DNA. FEBS Lett. 1979 Sep 15;105(2):278–282. doi: 10.1016/0014-5793(79)80629-8. [DOI] [PubMed] [Google Scholar]
- Gutteridge J. M., Quinlan G. J. Free radical damage to deoxyribose by anthracycline, aureolic acid and aminoquinone antitumour antibiotics. An essential requirement for iron, semiquinones and hydrogen peroxide. Biochem Pharmacol. 1985 Dec 1;34(23):4099–4103. doi: 10.1016/0006-2952(85)90200-x. [DOI] [PubMed] [Google Scholar]
- Gutteridge J. M., Quinlan G. J., Wilkins S. Mitomycin C-induced deoxyribose degradation inhibited by superoxide dismutase. A reaction involving iron, hydroxyl and semiquinone radicals. FEBS Lett. 1984 Feb 13;167(1):37–41. doi: 10.1016/0014-5793(84)80828-5. [DOI] [PubMed] [Google Scholar]
- Gutteridge J. M. Reactivity of hydroxyl and hydroxyl-like radicals discriminated by release of thiobarbituric acid-reactive material from deoxy sugars, nucleosides and benzoate. Biochem J. 1984 Dec 15;224(3):761–767. doi: 10.1042/bj2240761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutteridge J. M. Thiobarbituric acid-reactivity following iron-dependent free-radical damage to amino acids and carbohydrates. FEBS Lett. 1981 Jun 15;128(2):343–346. doi: 10.1016/0014-5793(81)80113-5. [DOI] [PubMed] [Google Scholar]
- Halliwell B., Gutteridge J. M. Formation of thiobarbituric-acid-reactive substance from deoxyribose in the presence of iron salts: the role of superoxide and hydroxyl radicals. FEBS Lett. 1981 Jun 15;128(2):347–352. doi: 10.1016/0014-5793(81)80114-7. [DOI] [PubMed] [Google Scholar]
- Slater T. F. Overview of methods used for detecting lipid peroxidation. Methods Enzymol. 1984;105:283–293. doi: 10.1016/s0076-6879(84)05036-9. [DOI] [PubMed] [Google Scholar]
- Slater T. F., Sawyer B. C. The stimulatory effects of carbon tetrachloride and other halogenoalkanes on peroxidative reactions in rat liver fractions in vitro. General features of the systems used. Biochem J. 1971 Aug;123(5):805–814. doi: 10.1042/bj1230805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WARAVDEKAR V. S., SASLAW L. D. A sensitive colorimetric method for the estimation of 2-deoxy sugars with the use of the malonaldehyde-thiobarbituric acid reaction. J Biol Chem. 1959 Aug;234(8):1945–1950. [PubMed] [Google Scholar]
- van Berkel T. J., Dekker C. J., Kruijt J. K., van Eijk H. G. The interaction in vivo of transferrin and asialotransferrin with liver cells. Biochem J. 1987 May 1;243(3):715–722. doi: 10.1042/bj2430715. [DOI] [PMC free article] [PubMed] [Google Scholar]


