Abstract
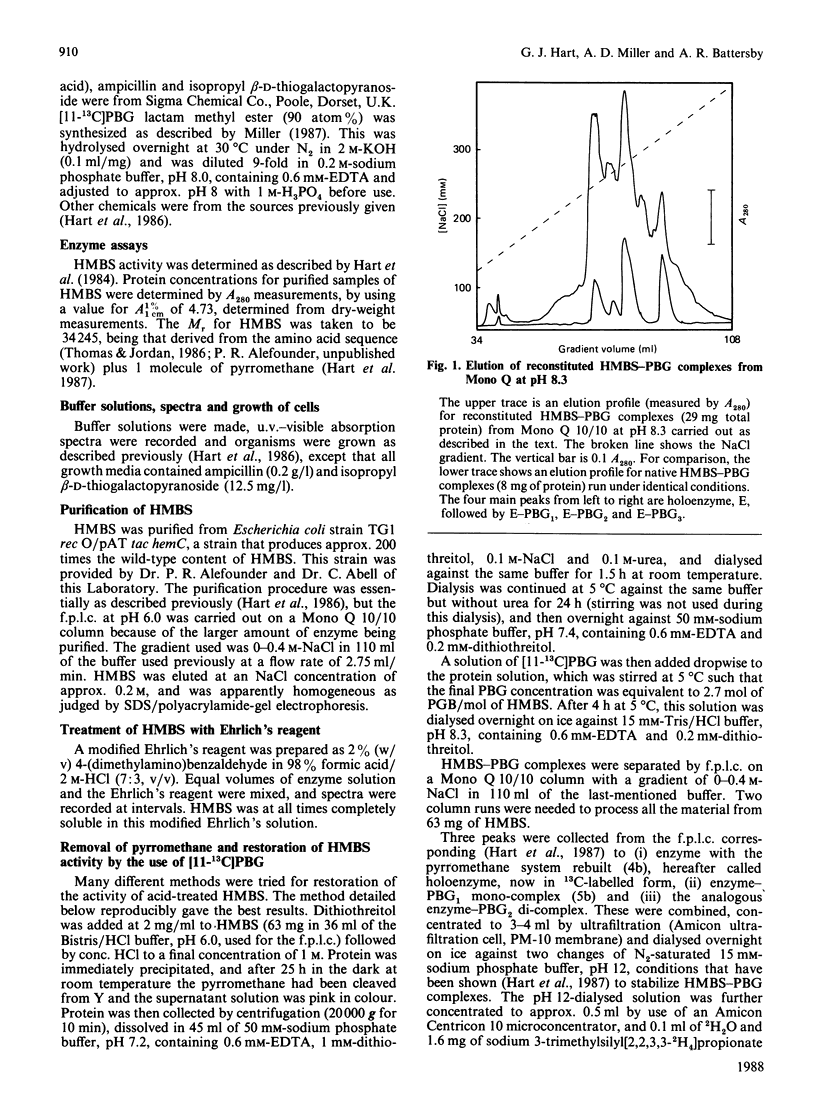
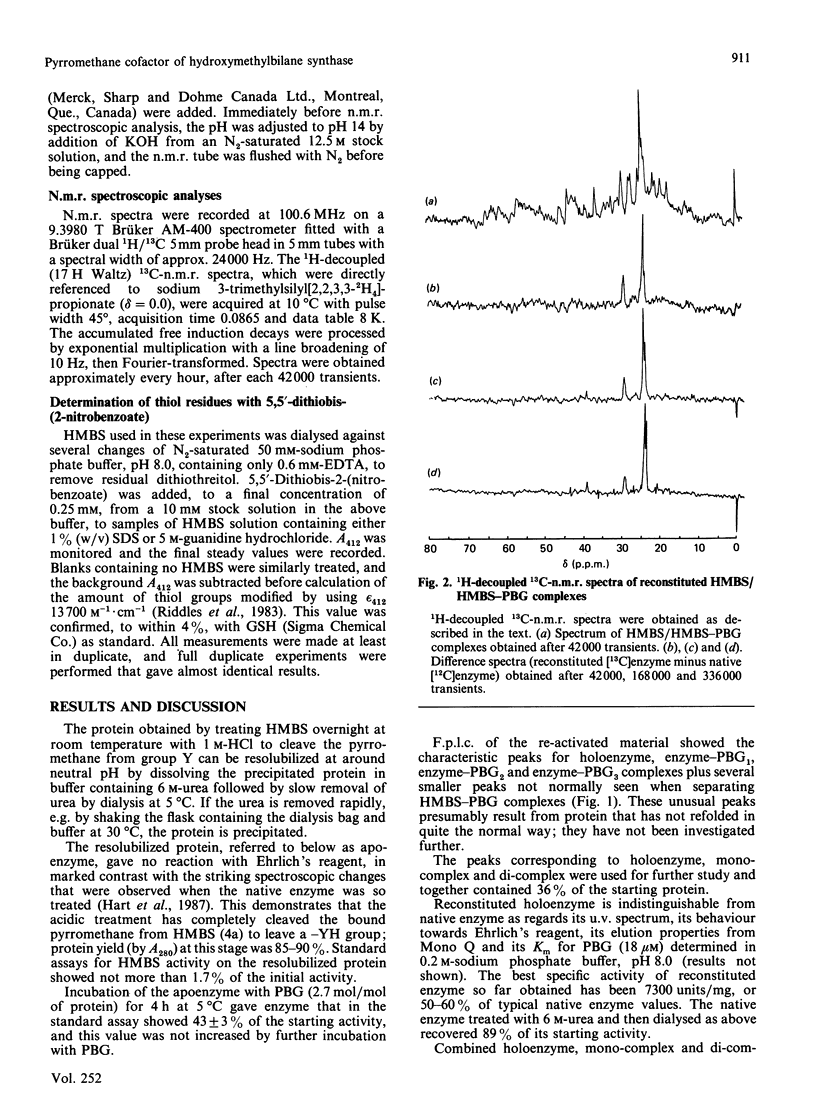
Hydroxymethylbilane synthase (porphobilinogen deaminase) from Escherichia coli uses a novel pyrromethane cofactor to bind the growing pyrrolic chain for hydroxymethylbilane biosynthesis [Hart, Miller, Leeper & Battersby (1987) J. Chem. Soc. Chem. Commun. 1762-1765]. We show that this cofactor is bound to the protein through the sulphur atom of a cysteine residue.
Full text
PDF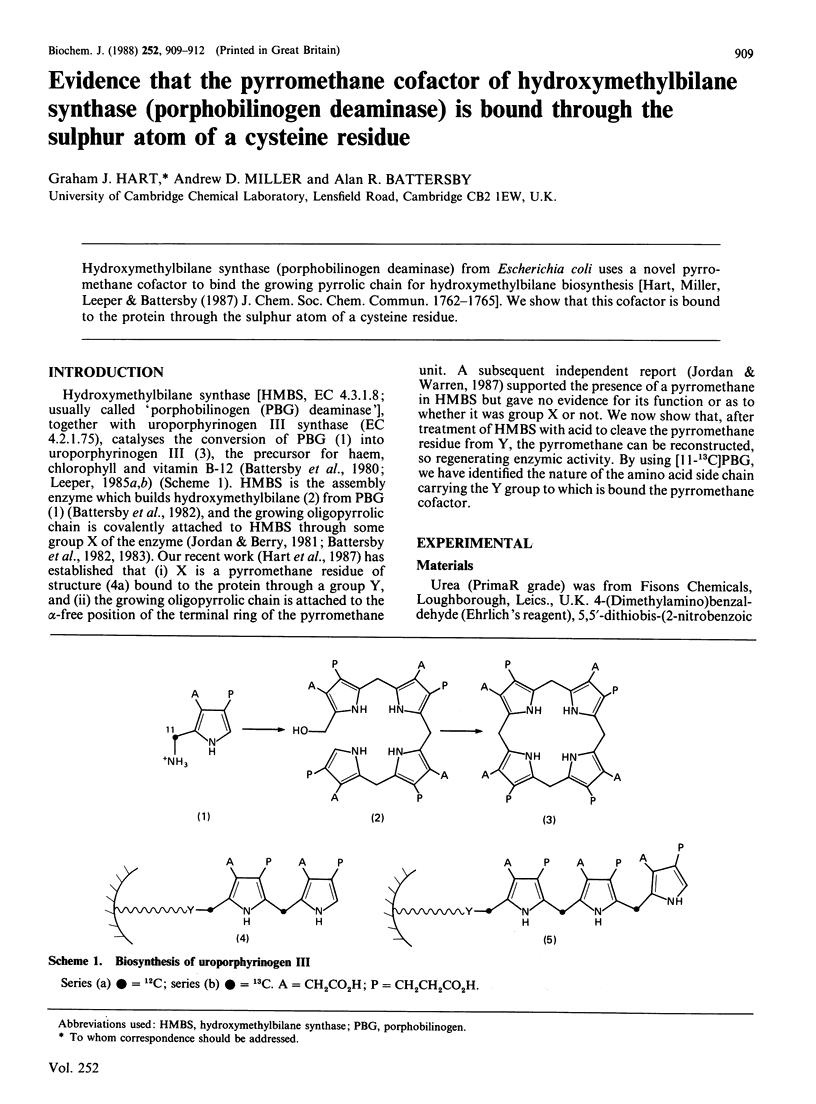

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Battersby A. R., Fookes C. J., Matcham G. W., McDonald E. Biosynthesis of the pigments of life: formation of the macrocycle. Nature. 1980 May 1;285(5759):17–21. doi: 10.1038/285017a0. [DOI] [PubMed] [Google Scholar]
- Hart G. J., Abell C., Battersby A. R. Purification, N-terminal amino acid sequence and properties of hydroxymethylbilane synthase (porphobilinogen deaminase) from Escherichia coli. Biochem J. 1986 Nov 15;240(1):273–276. doi: 10.1042/bj2400273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart G. J., Leeper F. J., Battersby A. R. Modification of hydroxymethylbilane synthase (porphobilinogen deaminase) by pyridoxal 5'-phosphate. Demonstration of an essential lysine residue. Biochem J. 1984 Aug 15;222(1):93–102. doi: 10.1042/bj2220093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jordan P. M., Berry A. Mechanism of action of porphobilinogen deaminase. The participation of stable enzyme substrate covalent intermediates between porphobilinogen and the porphobilinogen deaminase from Rhodopseudomonas spheroides. Biochem J. 1981 Apr 1;195(1):177–181. doi: 10.1042/bj1950177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jordan P. M., Warren M. J. Evidence for a dipyrromethane cofactor at the catalytic site of E. coli porphobilinogen deaminase. FEBS Lett. 1987 Dec 10;225(1-2):87–92. doi: 10.1016/0014-5793(87)81136-5. [DOI] [PubMed] [Google Scholar]
- Leeper F. J. The biosynthesis of porphyrins, chlorophylls, and vitamin B12. Nat Prod Rep. 1985 Feb;2(1):19–47. doi: 10.52054/FVVO.16.2.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leeper F. J. The biosynthesis of porphyrins, chlorophylls, and vitamin B12. Nat Prod Rep. 1985 Dec;2(6):561–580. doi: 10.1039/np9850200561. [DOI] [PubMed] [Google Scholar]
- Riddles P. W., Blakeley R. L., Zerner B. Reassessment of Ellman's reagent. Methods Enzymol. 1983;91:49–60. doi: 10.1016/s0076-6879(83)91010-8. [DOI] [PubMed] [Google Scholar]
- Thomas S. D., Jordan P. M. Nucleotide sequence of the hemC locus encoding porphobilinogen deaminase of Escherichia coli K12. Nucleic Acids Res. 1986 Aug 11;14(15):6215–6226. doi: 10.1093/nar/14.15.6215. [DOI] [PMC free article] [PubMed] [Google Scholar]


