Abstract
We describe here the neurovirulence properties of a herpes simplex virus type 1 γ34.5 second-site suppressor mutant. γ34.5 mutants are nonneurovirulent in animals and fail to grow in a variety of cultured cells due to a block at the level of protein synthesis. Extragenic suppressors with restored capacity to replicate in cells that normally do not support the growth of the parental γ34.5 deletion mutant have been isolated. Although the suppressor virus reacquires the ability to grow in nonpermissive cultured cells, it remains severely attenuated in mice and is indistinguishable from the mutant γ34.5 parent virus at the doses investigated. Repairing the γ34.5 mutation in the suppressor mutant restores neurovirulence to wild-type levels. These studies illustrate that (i) the protein synthesis and neurovirulence defects observed in γ34.5 mutant viruses can be genetically separated by an extragenic mutation at another site in the viral chromosome; (ii) the extragenic suppressor mutation does not affect neurovirulence; and (iii) the attenuated γ34.5 mutant, which replicates poorly in many cell types, can be modified by genetic selection to generate a nonpathogenic variant that regains the ability to grow robustly in a nonpermissive glioblastoma cell line. As this γ34.5 second-site suppressor variant is attenuated and replicates vigorously in neoplastic cells, it may have potential as a replication-competent, viral antitumor agent.
A pathogen's ability to be virulent in its host is governed by specific determinants encoded in its genome. Attenuated isolates have lost their virulence by specific changes that affect these loci. These attenuated viruses often grow less robustly in cultured cells or display a host range phenotype and can thus only sustain a productive infection in particular cell lines. The γ34.5 gene carried by herpes simplex virus type 1 (HSV-1) is critical for the virus to grow in a diverse assortment of cell types, including neuronal cells. γ34.5 mutants are nonneurovirulent upon intracranial injection into mice and can be safely administered to monkeys (3, 9, 22, 28). Furthermore, these mutants fail to grow in a variety of cultured human cells, as viral DNA replication triggers the premature cessation of protein synthesis (10). The block in protein synthesis coincides with the accumulation of phosphorylated eIF2α, a substrate for the cellular PKR kinase and a critical translation initiation factor that is inactivated by phosphorylation (8). The γ34.5 gene product functions, in part, by binding the cellular protein phosphatase 1α (PP1α) and targeting PP1α activity to inactive, phosphorylated eIF2α. This maintains steady-state pools of unphosphorylated, active eIF2 in HSV-1-infected cells (15).
Recently, we described the isolation of HSV-1 γ34.5 variants that have reacquired the ability to grow in nonpermissive cells that fail to support the replication of the parental γ34.5 deletion mutant (30). As these viruses lacked all γ34.5 coding sequences, they are thus second-site suppressor mutants (5, 30). All of these variants contain rearrangements within a 595-bp region where the unique short component of the viral genome joins the short terminal repeats (TRs) (Fig. 1). As a direct result of these dominant mutations, multiple mRNA species, including several novel mRNAs of unknown function, are affected (31). Notably, the Us11 mRNA, which normally accumulates late in infection, is overproduced at immediate-early times. Moreover, expression of the Us11 RNA binding protein as an immediate-early gene product is necessary and sufficient to rescue the growth defect of γ34.5 mutants in nonpermissive cells (31). The Us11 polypeptide prevents activation of the cellular PKR kinase and is therefore a second HSV-1-encoded function dedicated to precluding the accumulation of phosphorylated eIF2α (6, 31). While the temporal deregulation of Us11 expression is critical for the suppressor mutant to overcome the block to replication in cultured cells, the effect of this specific, novel mutation on neurovirulence in a wild-type genetic background remains to be determined.
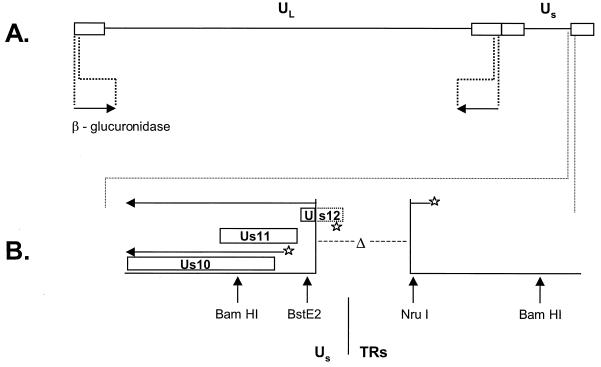
FIG. 1.
Structures of the mutant viruses utilized in this study. The locations of the γ34.5 gene and the SUP locus on the viral genome are shown. Solid lines, unique-long (UL) region and unique short (US) region; rectangles, repetitive regions. (A) In the γ34.5 deletion mutant virus used in this study (SPBg5e), both copies of the viral γ34.5 gene were replaced with a gene that encodes β-glucuronidase (arrows) (30). (B) The SUP1 virus was derived from SPBg5e by sequential passage in nonpermissive cells (30) and contains two mutations: (i) both copies of the γ34.5 gene have been replaced with the β-glucuronidase gene, as described for panel A; (ii) a 583-bp deletion (Δ) between the BstEII and NruI sites that spans the junction region where the viral Us segment joins the TR component. The region at the Us-TR junction is referred to as the SUP locus. Deletions affecting the SUP locus are necessary and sufficient to enable Δ34.5 mutants to replicate in nonpermissive cells. The Us10, Us11, and Us12 open reading frames are shown. Broken rectangle, segment of the Us12 open reading frame that is removed by the deletion; horizontal arrows, two RNAs that are synthesized from the mutant SUP locus; stars, promoter elements that direct the synthesis of these RNAs. Note that the SUP deletion removes the endogenous late Us11 promoter and a large segment of the Us12 open reading frame, including the ATG codon. This allows the transcript initiating from the immediate-early Us12 promoter in the TRs to direct the synthesis of the Us11 protein. Accumulation of Us11 at immediate-early times allows the SUP mutants to sustain protein synthesis and thus replicate in nonpermissive cells that do not support the growth of Δ34.5 mutants. The 34.5RΔSUP virus has the 583-bp deletion between the BstEII and NruI sites and two wild-type copies of the γ34.5 genes at their natural locations shown in panel A.
We report here that although these γ34.5 second-site suppressor mutants have regained their ability to sustain protein synthesis and replicate efficiently in nonpermissive cultured cells, they remain severely attenuated in mice and are indistinguishable from the parental γ34.5 deletion virus at the doses examined. This illustrates that the protein synthesis defect of the γ34.5 mutant can be sufficiently corrected to substantially augment viral replication in culture without restoring virulence, thus genetically separating the translational control and neurovirulence phenotypes observed in γ34.5 mutant viruses. It further demonstrates that it is formally possible to modify the host range of an attenuated virus by genetic selection such that it replicates efficiently in a nonpermissive, neoplastic cell line and yet remains nonpathogenic in animals. In addition, we have repaired the mutant γ34.5 allele in the suppressor virus to isolate the dominant suppressor mutation in a wild-type genetic background. This virus displays neurovirulence properties similar to those of its wild-type counterpart. Thus, the multiple mRNAs altered by the suppressor mutation do not affect neurovirulence.
MATERIALS AND METHODS
Cells and viruses.
Vero cells and U373 cells were from the American Type Culture Collection and were propagated as described previously (31). The Patton strain of HSV-1 was used throughout this work. Recombinant virus SPBg5e lacks both copies of the γ34.5 gene and contains the β-glucuronidase gene at both γ34.5 loci (30). The SUP1 suppressor is isogenic to the SPBg5e virus except that it contains a 583-bp deletion between nucleotides 145416 and 145999 (corresponding to the nucleotide numbers in strain 17; GenBank accession no. X14112).
Animals.
BALB/c mice were purchased from Charles River Laboratories. Prior to injection, dilutions of wild-type HSV-1 (Patton strain), γ34.5 deletion mutant SPBg5e, or suppressor variant SUP1 were prepared in Dulbecco modified Eagle medium–1% fetal bovine serum.
To assay for neurovirulence, groups of five female BALB/c mice (21 days old) were injected intracranially with 30 μl of diluted virus as described previously (9). Six-week-old strain 129 Ev/Sv mice, bred in the Washington University School of Medicine biosafety level 2 animal facility, were inoculated intracerebrally with 20 μl of diluted virus. Survival of the injected animals was monitored over a 21-day period. Female nude mice, each weighing 14 to 16 g (Goodwin Institute), were injected via an intraperitoneal (i.p.) route with 0.25 ml of virus diluted in Dulbecco modified Eagle medium–10% fetal bovine serum. Survival of the injected animals was monitored over a 21-day period.
Isolation of 34.5RΔSUP.
Viral DNA of genotype Δ34.5ΔSUP was cotransfected along with a plasmid containing a wild-type HSV-1 (Patton strain) BamHI SP fragment into permissive Vero cells by the Ca phosphate technique as described previously (30). Once plaques appeared, a cell-free lysate was prepared by freeze-thawing, and dilutions were used to infect nonpermissive U373 cells. Following the appearance of cytopathic effect, a cell-free lysate was prepared by freeze-thawing. Individual isolates were subsequently obtained through two rounds of plaque purification in Vero cells. Viral DNA was isolated as described previously (31). The physical structure of selected genomic regions was determined by Southern analysis as described previously (31).
Analysis of viral protein synthesis.
High-multiplicity infections, metabolic labeling, and gel electrophoresis were performed as described by Mohr and Gluzman (30).
RESULTS
The suppressor virus replicates in cultured glioblastoma cells and is attenuated in mice.
To illustrate the growth properties of the γ34.5 mutant and the suppressor mutant viruses in cultured cells, 3 × 106 U373 human glioblastoma cells were infected with 100 PFU (multiplicity of infection [MOI] = 0.3 × 10−4) of either γ34.5 deletion mutant SPBg5e, the SUP1 suppressor, or wild-type HSV-1 (Patton strain). At 3 and 5 days postinfection, lysates were prepared by freeze-thawing and titrated in permissive monkey kidney cells (Vero). Table 1 demonstrates that the SUP1 suppressor and wild-type both grow to titers approximately 105- to 106-fold greater than those of the γ34.5 deletion mutant at 3 days postinfection and 104- to 105-fold greater at 5 days postinfection. Thus, a nonpermissive glioblastoma cell line that does not support efficient growth of a γ34.5 mutant allows the γ34.5 second-site suppressor virus to replicate robustly.
TABLE 1.
Replication of the suppressor virus and the γ34.5 deletion mutant virus in human glioblastoma cellsa
| Virus | Titer (PFU/ml) for expt:
|
|||
|---|---|---|---|---|
| 1 at day:
|
2 at day:
|
|||
| 3 | 5 | 3 | 5 | |
| WT | 8 × 106 | 2.7 × 106 | 6 × 105 | 3.6 × 107 |
| SUP1 | 7.4 × 106 | 2.2 × 106 | 1.2 × 106 | 4.9 × 106 |
| Δ34.5 | 5 | 32 | 17.5 | 125 |
| 34.5RΔSUP | 2.5 × 106 | 9 × 106 | 1 × 107 | 4.7 × 107 |
Duplicate plates (experiments 1 and 2) containing 3 × 106 U373 cells were infected with 100 PFU of either wild-type HSV-1 (WT), the suppressor virus (SUP1), the γ34.5 deletion mutant (Δ34.5), or a recombinant that contains the suppressor mutation in a wild-type genetic background (34.5RΔSUP). After 3 or 5 days, lysates were prepared and titrated in permissive African green monkey kidney (Vero) cells. Values reflect the averages of duplicate titers from two independent dilutions. In experiment 1, only three points were available for Δ34.5 (day 5) and two points were available for WT (day 5). In experiment 2, only two points were available for Δ34.5 (day 5) and SUP1 (day 5).
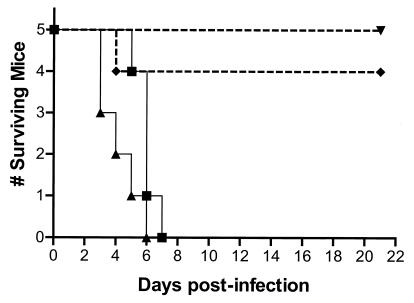
γ34.5 gene mutants display two phenotypes. First, they grow poorly in a variety of cultured cells due to the premature cessation of protein synthesis (10). Second, they exhibit dramatically reduced neurovirulence in mice (3, 9, 22). Although the suppressor virus replicates in cultured cells that are nonpermissive for the growth of γ34.5 mutants, the neurovirulence properties of this mutant have never been characterized. To evaluate the neurovirulence of the suppressor mutant in animals, groups of five female BALB/c mice were injected intracranially with dilutions of either γ34.5 deletion mutant SPBg5e, the SUP1 suppressor, or the wild-type virus. The injected mice were monitored for survival over the next 21 days. Figure 2 demonstrates that all of the mice that received either 300 or 3,000 PFU of wild-type HSV-1 died by 7 days postinfection. Only one mouse died in the group injected with 3 × 105 PFU of γ34.5 deletion mutant SPBg5e, and this death occurred on day 4. This is in accord with previous studies that demonstrated that γ34.5 mutant viruses are radically attenuated in their neurovirulence properties (3, 9, 22). Among the mice injected with the SUP1 suppressor virus, no deaths occurred in the group that received 6 × 104 PFU and a single death occurred in the group injected with 6 × 105 PFU (P = 0.031 for wild-type at 300 PFU versus SUP1 at 6 × 105 PFU by log rank test). Thus, the suppressor virus, which reacquired the ability to grow in nonpermissive cells in culture, remains severely attenuated for neurovirulence and is indistinguishable from the parental γ34.5 deletion mutant following intracerebral injection of 106 PFU, the largest dose examined in our study (S. Ward, I. Mohr, and D. Leib, unpublished observations).
FIG. 2.
The SUP1 suppressor virus is neuroattenuated in immunocompetent animals. BALB/c mice were injected intracranially with various amounts of either wild-type (WT), SPBg5e, or SUP1 virus as described in the text. The survival of the injected animals is shown over a 21-day period. ▪, WT at 300 PFU; ▴, WT at 3,000 PFU; ▾, SUP1 at 6 × 104 PFU; ♦, SUP1 at 6 × 105 PFU or SPBg5e at 3 × 105 PFU.
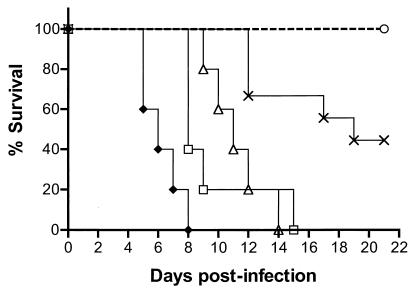
The virulence properties of the SUP1 virus were next analyzed in immunocompromised mice. Following an i.p. injection with diluted virus, the animals were observed for a total of 21 days and their survival was monitored (Fig. 3). One hundred percent of the animals that received either 106, 105, or 104 PFU of the wild-type HSV-1 virus were killed by the virus. Groups which received the higher doses were killed more rapidly than those which received the lower doses. For example, animals which received 106 PFU were all killed between day 5 and day 8, while those which received 104 PFU succumbed between day 9 and day 14. Fifty-five percent of the animals injected with 103 PFU of the wild-type HSV-1 virus died between days 12 to 19 in the study time period. The SUP1 virus was indistinguishable from the γ34.5 mutant parent SPBg5e in this assay, as 100% of the mice survived i.p. administration of 106 PFU (Fig. 3; P = 0.015 for wild-type at 103 PFU versus SUP1 at 106 PFU by log rank test) and 2 × 107 PFU (unpublished observations). Thus, the suppressor virus remains as attenuated as the γ34.5 mutant parent virus in immunocompromised animals at the doses examined.
FIG. 3.
The SUP1 suppressor virus is attenuated in immunocompromised animals. Nude mice were injected i.p. with various amounts of either wild-type (WT), SPBg5e, or SUP1 as described in the text. The survival of the injected animals is shown over a 21-day period. ♦, WT at 106 PFU (n = 5); □, WT at 105 PFU (n = 5); ▵, WT at 104 PFU (n = 5); ×, WT at 103 PFU (n = 9); ○, SUP1 (n = 8) or SPBg5e (n = 8) at 106 PFU.
Isolation and characterization of a recombinant virus that contains the dominant suppressor mutation in a wild-type genetic background.
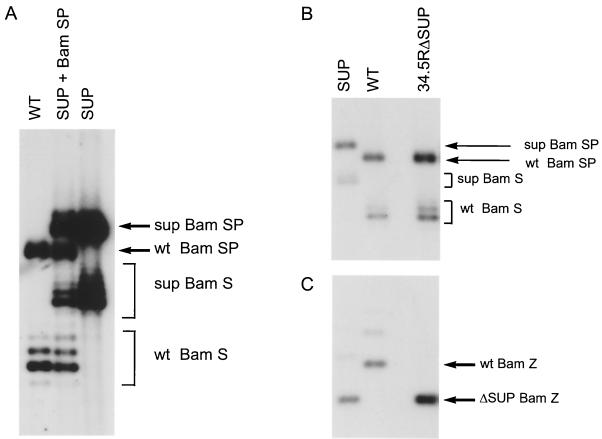
The suppressor mutation lies immediately before the Us-TR junction, removing most of the Us12 open reading frame. Although larger deletions spanning this region have been generated without affecting neurovirulence, deletions that correspond exactly to the SUP mutation have not been produced in a wild-type background (21, 32). However, as the SUP mutations are dominant in trans (31), it remains formally possible that the SUP mutation could affect virulence. This could involve the overexpression of Us11 as an immediate-early protein or alterations to other novel transcripts of unknown function that traverse the SUP locus (31). To evaluate the contribution of the dominant suppressor allele to neurovirulence, the γ34.5 mutation was repaired. The HSV-1 BamHI SP fragment was cotransfected along with viral DNA from the suppressor mutant (genotype Δ34.5ΔSUP) into permissive Vero cells. This fragment contains the wild-type γ34.5 gene flanked by sequences to foster homologous recombination within the endogenous γ34.5 loci. A control transfection was performed in the absence of plasmid DNA. Following the appearance of plaques, cell-free lysates were prepared by freeze-thawing. The lysate prepared from the transfection performed with SUP viral DNA plus the SP fragment contains a mixed viral population that is overwhelmingly composed of viruses having the Δ34.5ΔSUP genotype. However, a small fraction of the viruses in this lysate have undergone a recombination event that replaces the β-glucuronidase gene resident at both γ34.5 loci in the suppressor virus with a wild-type γ34.5 gene. As the repaired recombinant has only a single mutation at the SUP locus, we reasoned that it might have a competitive advantage over the suppressor virus that has a dominant suppressor mutation and that lacks both copies of the γ34.5 gene. Thus, a virus with a repaired γ34.5 gene would be enriched in the population if cell-free lysates from transfected Vero cells were grown in nonpermissive U373 cells. Southern analysis of the population selected on U373 cells demonstrates that it is dramatically enriched for recombinants that contain wild-type BamHI S and SP fragments (Fig. 4A). BamHI S termini are naturally heterogeneous due to variations in a repetitive sequence component. This enriched population greatly facilitated isolation of recombinant viruses that contain the SUP mutation in a wild-type γ34.5 genetic background. We refer to this virus as γ34.5RΔSUP. The physical structures of the γ34.5 loci and the SUP locus were verified by Southern analysis of the purified isolate. Figure 4B demonstrates that the γ34.5RΔSUP virus contains a wild-type copy of the γ34.5 gene in the BamHI S and SP fragments. The slower mobility of the S and SP fragments in the suppressor mutant reflects the presence of the larger β-glucuronidase gene at these loci. γ34.5RΔSUP also retains the faster-migrating BamHI Z fragment characteristic of the suppressor virus (genotype Δ34.5ΔSUP) as documented in Fig. 4C.
FIG. 4.
Enrichment for recombinant viruses with repaired γ34.5 alleles and physical analysis of the purified recombinant genomes. (A) Vero cells were transfected with HSV-1 viral DNA (genotype Δ34.5ΔSUP) and the wild-type (WT) BamHI SP fragment. Cell-free lysates from the transfection plate were passaged once in nonpermissive U373 cells as described in the text. Prior to plaque purification, the population was used to infect Vero cells at high multiplicity and viral DNA was isolated. This DNA, along with DNA from wild-type HSV-1 and SUP1, was digested with BamHI, fractionated by electrophoresis on 1% agarose gels, transferred to a nylon membrane, and hybridized to the 32P-labeled BamHI-BstXI segment (nucleotides 123459 to 124679) from the BamHI S fragment. This probe identifies sequences in the authentic γ34.5 loci. Heterogeneity at the genomic BamHI S and BamHI SP loci is due to natural variations in a repetitive sequence component. The slower-migrating SP and S fragments observed in the SUP virus reflect the fact that all γ34.5 coding sequences have been replaced with sequences encoding β-glucuronidase. (B) Following two rounds of plaque purification on Vero cells, viral DNA was isolated from a 34.5RΔSUP isolate. DNA from 34.5RΔSUP, SUP1 (genotype Δ34.5ΔSUP), and the wild-type HSV-1 Patton strain was digested, fractionated, and hybridized as described for panel A. (C) Same as in panel B except that the membrane was hybridized to a 32P-labeled BamHI-BstEII probe (nucleotides 144875 to 145316) from the BamHI Z fragment. This probe detects sequences near the Us-TR junction in the SUP locus. Slower-migrating forms of the BamHI Z fragment are due to natural variations in a repetitive sequence component.
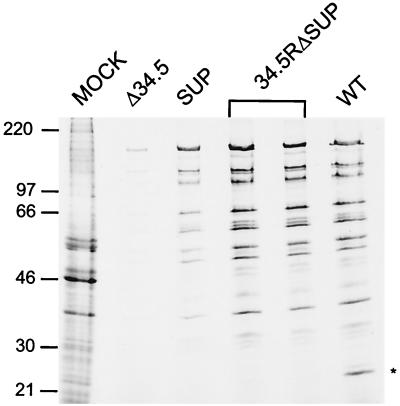
Comparing the growth properties of 34.5RΔSUP to the SUP suppressor mutant (genotype Δ34.5ΔSUP) following infection of U373 cells at a low MOI revealed that 34.5RΔSUP displayed an 8- to 10-fold growth advantage in experiment 2 at both 3 and 5 days postinfection and a 4-fold growth advantage in experiment 1 at 5 days postinfection (Table 1). Earlier studies in our laboratory have demonstrated that, while the suppressor virus directs substantially more late viral protein synthesis than the parental Δ34.5 mutant, it does not completely restore protein synthesis to wild-type levels (30). To evaluate the ability of the 34.5RΔSUP virus to synthesize late viral proteins, replicate cultures of U373 cells were mock infected or infected with either Δ34.5, SUP, 34.5RΔSUP, or wild-type virus. At late times postinfection, cultures were labeled with [35S]-labeled amino acids, detergent lysates were prepared, and the isolated proteins were fractionated on sodium dodecyl sulfate-polyacrylamide gels. The autoradiogram in Fig. 5 demonstrates that the suppressor mutant directs substantially more protein synthesis than the Δ34.5 parent virus and that 34.5RΔSUP directs greater levels of viral protein synthesis at late times postinfection than the SUP (genotype Δ34.5ΔSUP) parent virus. To quantitate this difference, an equal volume from each lysate was precipitated with trichloroacetic acid and the amount of 35S incorporated into protein during the 1-h pulse-labeling interval was measured. While lysates prepared from SUP-infected cells contained 4-fold more labeled protein than Δ34.5 lysates, 34.5RΔSUP lysates accumulated 2.4-fold more newly synthesized polypeptides than lysates derived from cells infected with the SUP parent (genotype Δ34.5ΔSUP). The amount of labeled protein in 34.5RΔSUP lysates differed from that in wild-type lysates by approximately 3%. Thus, repairing the γ34.5 mutation in the background of the dominant SUP allele restores the rate of late viral protein synthesis to wild-type levels. This suggests that cells infected with the suppressor virus may contain elevated levels of phosphorylated eIF2α compared to cells infected with 34.5RΔSUP. While the suppressor virus expresses Us11 as an abundant immediate-early protein, both copies of the γ34.5 gene have been deleted. 34.5RΔSUP, however, expresses both the γ34.5 gene product and the Us11 protein. Thus, the expression of multiple gene products that can regulate the accumulation of phosphorylated eIF2α by the 34.5RΔSUP and wild-type viruses may lead to enhanced rates of protein synthesis.
FIG. 5.
Analysis of late viral protein synthesis. U373 cells were mock infected or infected at an MOI of 5 with either Δ34.5, SUP (genotype Δ34.5ΔSUP), 34.5RΔSUP (in duplicate), or wild-type (WT) virus. At late times postinfection, the cultures were labeled for 1 h with 35S-labeled amino acids, and total protein was subsequently isolated and fractionated on a sodium dodecyl sulfate–12.5% polyacrylamide gel. The fixed, dried gel was exposed to Kodak XAR film. The sizes of molecular mass standards (in kilodaltons) appear on the left. Asterisk, mobility of the Us11 polypeptide, an abundant late protein in cells infected with WT virus. As the suppressor mutation causes Us11 to be expressed with immediate-early kinetics in cells infected with the SUP or 34.5RΔSUP viruses, its rate of synthesis is markedly reduced and it is not effectively labeled at late times postinfection.
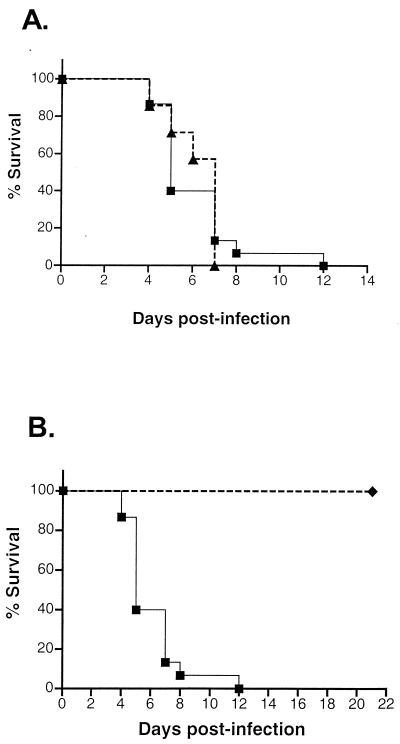
To assess if the suppressor mutation contributes to the attenuation of the SUP1 virus, mice were injected intracerebrally with 103 PFU of 34.5RΔSUP. Figure 6A demonstrates that 100% of the animals injected with 34.5RΔSUP died by 7 days postinjection. Ninety percent of the animals injected with an equivalent amount of the wild-type HSV-1 Patton virus succumbed by day 7 (P > 0.92 by log rank test), and 100% succumbed by day 12 (Fig. 6A). In comparison, 100% of the animals injected with the suppressor parent virus (Δ34.5ΔSUP) survived (P < 0.0001 by log rank test) after being monitored for 21 days postinfection (Fig. 6B). Thus, the dominant suppressor mutation does not appear to contribute to the attenuation phenotype of the suppressor virus. The mutation in the γ34.5 gene is therefore completely responsible for the attenuated phenotype of the suppressor virus.
FIG. 6.
Repairing the γ34.5 allele in the suppressor virus is sufficient to restore neurovirulence. Mice were injected intracranially with 103 PFU of either wild-type HSV-1 (Patton strain), 34.5RΔSUP, or the SUP1 suppressor (genotype Δ34.5ΔSUP), and their survival was monitored over time. (A) Wild-type, n = 15; 34.5RΔSUP, n = 7. (B) Wild-type, n = 15; SUP, n = 10. Data were pooled from at least two experiments. ▪, wild type; ▴, 34.5RΔSUP; ♦, SUP.
DISCUSSION
In this report, we characterized the virulence properties of an HSV-1 γ34.5 variant that contains an extragenic suppressor mutation. Although this virus replicates to greater levels than the γ34.5 parent virus in cultured glioblastoma cells, it remains severely attenuated and is indistinguishable from the γ34.5 mutant following intracranial injection of 106 PFU into immunocompetent mice or i.p. administration of 2 × 107 PFU to immunocompromised mice. This demonstrates that the inability of γ34.5 mutants to sustain protein synthesis at late times postinfection can be genetically separated from their attenuated neurovirulence properties. These two phenotypes could reflect independent, separable functions intrinsic to the γ34.5 polypeptide. As the suppressor virus is capable of sustained protein synthesis in nonpermissive cells, there appear to be ancillary functions necessary to restore neurovirulence to γ34.5 mutants. The carboxy-terminal 64 amino acids encoded by the HSV-1 γ34.5 gene have extensive homology with the carboxy terminus of the products of the murine myD116 cDNA and of the rodent GADD34 gene (11). Furthermore, a recombinant virus harboring a fusion transgene that encodes the amino-terminal 205 amino acids of the γ34.5 protein joined to the carboxyl-terminal 133 amino acids of myD116 enables γ34.5 mutants to sustain protein synthesis in nonpermissive SK-N-SH cells (14). However, the γ34.5 amino terminus does not restore full neurovirulence when fused to the myD116 carboxy-terminal region, suggesting either that the bona fide, intact γ34.5 protein is required or that the hybrid protein assumes a structure which does not restore full neurovirulence (1). In addition, while viruses with deletions within the amino-terminal domain of the γ34.5 protein are neuroattenuated, a mutant virus that produces a truncated γ34.5 polypeptide consisting of only the amino-terminal portion of the protein is neuroattenuated as well (2). There may thus be specific characteristics of the authentic γ34.5 carboxyl-terminal domain that are required for neurovirulence. Finally, although the suppressor mutation is dominant in trans, we have demonstrated that it does not appear to affect neurovirulence. The attenuated phenotype of the suppressor mutant appears to be governed by the mutant γ34.5 allele, as repairing the γ34.5 mutation restores neurovirulence to wild-type levels.
Interestingly, the 34.5RΔSUP virus, in which the γ34.5 mutation was repaired, has a competitive growth advantage over the parent suppressor virus, which lacks both copies of the γ34.5 gene and which contains a dominant suppressor allele. In addition, 34.5RΔSUP replicates to greater levels than the suppressor mutant following low-multiplicity infection of U373 cells and synthesizes late viral proteins at an elevated rate compared to the suppressor parent virus. While the 34.5RΔSUP virus expresses both γ34.5 and Us11 genes, the suppressor virus, which lacks the γ34.5 gene, only produces the Us11 polypeptide. Thus, the expression of multiple gene products that coordinately act to prevent the accumulation of phosphorylated eIF2α by the 34.5RΔSUP and wild-type viruses could lead to enhanced rates of protein synthesis. The 2.4-fold differential in the rate of protein synthesis between the suppressor and the 34.5RΔSUP virus might account for the competitive growth advantage of 34.5RΔSUP and may contribute to its neurovirulence.
Cellular factors also play a prominent role in determining virulence, as recent studies demonstrate that γ34.5 mutants are virulent in mice that lack the receptors for type I interferons and in mice that do not produce functional PKR (19, 20). Although the absence of the PKR gene product is required to restore neurovirulence to γ34.5 mutants, it is striking that the suppressor mutant, which precludes PKR activation and the accumulation of phosphorylated eIF2α via immediate-early Us11 expression, remains neuroattenuated in wild-type mice. Further structure-function analysis on the region(s) of the γ34.5 protein involved in neurovirulence is necessary to understand this observation.
Attenuated, replication-competent HSVs are also potentially useful as therapeutic antineoplastic agents (reviewed in references 23 and 26). Several groups have demonstrated that mice inoculated intracranially with either syngeneic or xenogeneic glioma cells exhibit enhanced survival if the glioma implants are challenged by HSV infection (1, 4, 7, 17, 18, 24, 27, 28, 29, 35). While animals in initial experiments succumbed to encephalitis due to extensive lytic growth of the virus, later studies were thwarted by viruses that were overattenuated due to mutation of the γ34.5 gene, causing the animals to die from regrowth of the tumor. Recent work has focused on trying to improve the ability of γ34.5 mutants to destroy tumor cells, either by expressing ectopic transgenes or attempting to correct for their limited replicative potential, as a means of increasing the number of surviving animals (1, 12, 33). Our studies demonstrate that it is possible to select for and isolate γ34.5 variants capable of enhanced growth in a human tumor cell line. Unlike some γ34.5 mutants further engineered in attempts to enhance their replicative ability in neoplastic cells, the γ34.5 second-site suppressor mutant virus is completely devoid of all γ34.5-related genetic material. The suppressor mutant overcomes the PKR-imposed restriction to viral replication by expressing Us11, a distinct HSV-1-encoded gene product that can regulate eIF2α phosphorylation, as an immediate-early protein. As the suppressor virus retains the attenuated neurovirulence properties of the γ34.5 mutant and efficiently replicates in and kills glioblastoma cells in vitro, it may be an excellent candidate for use as an antineoplastic agent. Additionally, the SUP mutation inactivates the Us12 gene (13, 30). The protein product of the Us12 gene, α47, binds to the cellular TAP molecule and effectively blocks viral antigen presentation to cytotoxic T cells (16, 37). SUP-infected tumor cells in an immunocompetent host would thus display increased amounts of viral antigens on the surfaces of infected cells. This may be a highly desirable property for an antineoplastic agent, as immune cells recruited into the vicinity of the lesion may also participate in tumor regression (36). Alternatively, it may be necessary to restore an immunomodulatory function to the virus in order to prevent the host immune response from limiting viral spread within the tumor. While two different γ34.5 mutant viruses have been evaluated for toxicity in humans, their efficacy in treating glioblastoma remains to be determined (25, 34). As the γ34.5 second-site suppressor mutant is attenuated and exhibits enhanced growth in tumor cells, it might form the next generation of prototypes from which a therapeutic, viral antineoplastic agent may eventually emerge.
ACKNOWLEDGMENTS
We thank Michael Botchan, Fenyong Liu, and Jeremy Poppers for critical reading of the manuscript and the reviewers for constructive, helpful comments. In particular, we thank one reviewer for suggesting the potential relationship between the rate of protein synthesis and neurovirulence that led to the experiment presented in Fig. 5.
Funds from the Department of Microbiology, the Kaplan Cancer Center, and a grant from the National Institutes of Health supported I.M. The honors research program at NYU School of Medicine and a fellowship from the New York Academy of Medicine supported D.S., in part. D.L. was supported, in part, by a grant from the National Institutes of Health (EY 09083) and a Robert E. McCormick Scholarship from Research to Prevent Blindness.
REFERENCES
- 1.Andreansky S, He B, Gillespie G Y, Soroceanu L, Markert J, Chou J, Roizman B, Whitley R J. The application of genetically engineered herpes simplex viruses to the treatment of experimental brain tumors. Proc Natl Acad Sci USA. 1996;93:11313–11318. doi: 10.1073/pnas.93.21.11313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Andreansky S, Soroceanu L, Flotte E R, Chou J, Markert J M, Gillespie G Y, Roizman B, Whitely R J. Evaluation of genetically engineered herpes simplex viruses as oncolytic agents for human malignant brain tumors. Cancer Res. 1997;57:1502–1509. [PubMed] [Google Scholar]
- 3.Bolovan C A, Sawtell N M, Thompson R L. ICP34.5 mutants of herpes simplex virus type 1 strain 17syn+ are attenuated for neurovirulence in mice and for replication in confluent primary mouse embryo cell cultures. J Virol. 1994;68:48–55. doi: 10.1128/jvi.68.1.48-55.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Boviatsis E J, Scharf M J, Chase M, Harrington K, Kowall N W, Breakefield X O, Chiocca E A. Antitumor activity and reporter gene transfer into rat brain neoplasms inoculated with herpes simplex virus vectors defective in thymidine kinase or ribonucleotide reductase. Gene Ther. 1994;1:323–331. [PubMed] [Google Scholar]
- 5.Cassady K, Gross M, Roizman B. The second-site mutation in the herpes simplex virus recombinants lacking the γ34.5 genes precludes the shutoff of protein synthesis blocking the phosphorylation of eIF2α. J Virol. 1998;72:7005–7011. doi: 10.1128/jvi.72.9.7005-7011.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cassady K, Gross M, Roizman B. The herpes simplex virus Us11 protein effectively compensates for the γ34.5 gene if present before activation of the protein kinase R by precluding its phosphorylation and that of the alpha subunit of eukaryotic translation initiation factor 2. J Virol. 1998;72:8620–8626. doi: 10.1128/jvi.72.11.8620-8626.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chambers R, Gillespie G Y, Soroceanu L, Adreansky S, Chatterjee S, Chou J, Roizman B, Whitely R J. Comparison of genetically engineered herpes simplex viruses for the treatment of brain tumors in a scid mouse model of human malignant glioma. Proc Natl Acad Aci USA. 1995;92:1411–1415. doi: 10.1073/pnas.92.5.1411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chou J, Chen J J, Gross M, Roizman B. Association of Mr 90,000 phosphoprotein with protein kinase PKR in cells exhibiting enhanced phosphorylation of translation initiation factor eIF2α and premature shutoff of protein synthesis after infection with γ34.5- mutants of herpes simplex virus 1. Proc Natl Acad Sci USA. 1995;92:10516–10520. doi: 10.1073/pnas.92.23.10516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chou J, Kern E R, Whitely R J, Roizman B. Mapping of herpes simplex virus-1 neurovirulence to γ34.5, a gene nonessential for growth in culture. Science. 1990;250:1262–1266. doi: 10.1126/science.2173860. [DOI] [PubMed] [Google Scholar]
- 10.Chou J, Roizman B. The γ34.5 gene of herpes simplex virus 1 precludes neuroblastoma cells from triggering total shutoff of protein synthesis characteristic of programmed cell death in neuronal cells. Proc Natl Acad Sci USA. 1992;89:3266–3270. doi: 10.1073/pnas.89.8.3266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chou J, Roizman B. The herpes simplex virus 1 γ34.5 gene function which blocks the response to infection maps to the homologous domain of the gene expressed during growth arrest and DNA damage. Proc Natl Acad Sci USA. 1994;91:5247–5251. doi: 10.1073/pnas.91.12.5247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chung R Y, Saeki Y, Chiocca E A. B-myb promoter retargeting of herpes simplex virus γ34.5 gene-mediated virulence toward tumor and cycling cells. J Virol. 1999;73:7556–7564. doi: 10.1128/jvi.73.9.7556-7564.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.He B, Chou J, Brandimarti R, Mohr I, Gluzman Y, Roizman B. Suppression of the phenotype of γ134.5− herpes simplex virus type 1: failure of activated RNA-dependent protein kinase to shut off protein synthesis is associated with a deletion in the domain of the α47 gene. J Virol. 1997;71:6049–6054. doi: 10.1128/jvi.71.8.6049-6054.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.He B, Chou J, Liebermann D A, Hoffman B, Roizman B. The carboxyl terminus of the murine MyD116 gene substitutes for the corresponding domain of the γ34.5 gene of herpes simplex virus to preclude the premature shutoff of total protein synthesis in infected human cells. J Virol. 1996;70:84–90. doi: 10.1128/jvi.70.1.84-90.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.He B, Gross M, Roizman B. The γ34.5 protein of herpes simplex virus 1 complexes with protein phosphatase 1 alpha to dephosphorylate the alpha subunit of the eukaryotic translation initiation factor 2 and preclude the shutoff of protein synthesis by double-stranded RNA-activated protein kinase. Proc Natl Acad Sci USA. 1997;94:843–848. doi: 10.1073/pnas.94.3.843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hill A, Jugovic P, York I, Russ G, Bennink J, Yewdell J, Ploegh H, Johnson D. Herpes simplex virus turns off the TAP to evade host immunity. Nature. 1995;375:411–415. doi: 10.1038/375411a0. [DOI] [PubMed] [Google Scholar]
- 17.Jia W G J, McDermott M, Goldie J, Cynander M, Tan J, Tufaro F. Selective destruction of gliomas in immunocompromised rats by thymidine kinase defective herpes simplex virus type-1. J Natl Cancer Inst. 1994;86:1209–1215. doi: 10.1093/jnci/86.16.1209. [DOI] [PubMed] [Google Scholar]
- 18.Kaplitt M G, Tjuvajev J G, Leib D A, Berk J, Pettigrew K D, Posner J B, Pfaff D W, Rabkin S D, Blasberg R G. Mutant herpes simplex virus induced regression of tumors growing in immunocompetent cells. J Neuro-Oncol. 1994;19:137–147. doi: 10.1007/BF01306455. [DOI] [PubMed] [Google Scholar]
- 19.Leib D A, Harrison T E, Laslo K M, Machalek M A, Moorman N J, Virgin H W. Interferons regulate the phenotype of wild-type and mutant herpes simplex viruses in vivo. J Exp Med. 1999;189:663–672. doi: 10.1084/jem.189.4.663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Leib D A, Machalek M A, Williams B R G, Silverman R H, Virgin H W. Specific phenotypic restoration of an attenuated virus by knockout of a host resistance gene. Proc Natl Acad Sci USA. 2000;97:6097–6101. doi: 10.1073/pnas.100415697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Longnecker R, Roizman B. Generation of an inverting herpes simplex virus type 1 mutant lacking the L-S junction a sequences, an origin of DNA synthesis, and several genes including those specifying glycoprotein E and the α47 gene. J Virol. 1986;58:583–591. doi: 10.1128/jvi.58.2.583-591.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.MacLean A R, Ul-Fareed M, Robertson L, Harland J, Brown S M. Herpes simplex virus type 1 deletion variants 1714 and 1716 pinpoint neurovirulence-related sequences in Glasgow strain 17+ between immediate early gene 1 and the ‘a’ sequence. J Gen Virol. 1991;72:631. doi: 10.1099/0022-1317-72-3-631. [DOI] [PubMed] [Google Scholar]
- 23.Markert J M, Gillespie G Y, Weichselbaum R R, Roizman B, Whitely R J. Genetically engineered HSV in the treatment of glioma: a review. Rev Med Virol. 2000;10:17–30. doi: 10.1002/(sici)1099-1654(200001/02)10:1<17::aid-rmv258>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 24.Markert J M, Malick A, Coen D M, Martuza R L. Reduction and elimination of encephalitis in an experimental glioma therapy model with attenuated herpes simplex virus mutants that retain susceptibility to acyclovir. Neurosurgery. 1993;32:597–603. doi: 10.1227/00006123-199304000-00016. [DOI] [PubMed] [Google Scholar]
- 25.Markert J M, Medlock M D, Rabkin S D, Gillespie G Y, Todo T, Hunter W D, Palmer C A, Feigenbaum F, Tornatore C, Tufaro F, Martuza R L. Conditionally replicating herpes simplex virus mutant G207 for the treatment of malignant glioma: results of a phase 1 trial. Gene Ther. 2000;7:867–874. doi: 10.1038/sj.gt.3301205. [DOI] [PubMed] [Google Scholar]
- 26.Martuza R. Conditionally replicating herpes vectors for cancer therapy. J Clin Investig. 2000;105:841–846. doi: 10.1172/JCI9744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Martuza R L, Malick A, Markert J M, Ruffner K L, Coen D M. Experimental therapy of human glioma by means of a genetically engineered virus mutant. Science. 1991;252:854–856. doi: 10.1126/science.1851332. [DOI] [PubMed] [Google Scholar]
- 28.Mineta T, Rabkin S D, Martuza R L. Treatment of malignant gliomas using gancyclovir-hypersensitive ribonucleotide reductase deficient herpes simplex viral mutants. Cancer Res. 1994;54:3963–3966. [PubMed] [Google Scholar]
- 29.Mineta T, Rabkin S D, Yazaki T, Hunter W D, Martuza R L. Attenuated multi-mutated herpes simplex virus-1 for the treatment of malignant gliomas. Nat Med. 1995;1:938–943. doi: 10.1038/nm0995-938. [DOI] [PubMed] [Google Scholar]
- 30.Mohr I, Gluzman Y. A herpesvirus genetic element which affects translation in the absence of the viral GADD34 function. EMBO J. 1996;15:4759–4766. [PMC free article] [PubMed] [Google Scholar]
- 31.Mulvey M, Poppers J, Mohr I. A herpesvirus RNA-binding, ribosome-associated protein confers a growth advantage upon mutants deficient in a GADD34-related function. J Virol. 1999;73:3375–3385. doi: 10.1128/jvi.73.4.3375-3385.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nishiyama Y, Kurachi R, Daikoku T, Umene K. The US9,10,11,12 genes of herpes simplex type 1 are of no importance for its neurovirulence and latency in mice. Virology. 1993;194:419–423. doi: 10.1006/viro.1993.1279. [DOI] [PubMed] [Google Scholar]
- 33.Parker J N, Gillespie G Y, Love C E, Randall S, Whitely R J, Markert J M. Engineered herpes simplex virus expressing IL-12 in the treatment of experimental murine brain tumors. Proc Natl Acad Sci USA. 2000;97:2208–2213. doi: 10.1073/pnas.040557897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rampling R, Cruickshank G, Papanastassiou V, Nicoll J, Hadley D, Brennan D, Petty R, MacLean A, Harland J, McKie E, Mabbs R, Brown M. Toxicity evaluation of replication-competent herpes simplex virus (ICP 34.5 null mutant 1716) in patients with recurrent malignant glioma. Gene Ther. 2000;7:859–866. doi: 10.1038/sj.gt.3301184. [DOI] [PubMed] [Google Scholar]
- 35.Randazzo B P, Kesari S, Gesser R M, Alsop D, Ford J, Brown S M, Maclean A, Fraser N W. Treatment of experimental intracranial murine melanoma with a neuroattenuated herpes simplex virus mutant. Virology. 1995;211:94–101. doi: 10.1006/viro.1995.1382. [DOI] [PubMed] [Google Scholar]
- 36.Toda M, Rabkin S D, Kojima H, Martuza R L. Herpes simplex virus as an in situ cancer vaccine for the induction of specific anti-tumor immunity. Hum Gene Ther. 1999;10:385–393. doi: 10.1089/10430349950018832. [DOI] [PubMed] [Google Scholar]
- 37.York I A, Roop C, Andrews D W, Riddell S R, Graham F L, Johnson D C. A cytosolic herpes simplex virus protein inhibits antigen presentation to CD8+ lymphocytes. Cell. 1994;77:525–535. doi: 10.1016/0092-8674(94)90215-1. [DOI] [PubMed] [Google Scholar]