Abstract
Osteoarthritis and sports injuries often lead to cartilage defects. How to promote its repair and rebuild the smooth cartilage surface has been a hot spot of research in recent years. Kartogenin (KGN), a small molecule discovered in recent years, has been shown to promote the proliferation and chondrogenic differentiation of mesenchymal stem cells (MSCs). As more and more studies have been conducted on KGN, its mechanism of action has been gradually revealed. However, KGN is insoluble in water and therefore easily removed by body fluids. In order to address such issues, a number of systems for efficient intra-articular delivery of KGN have been developed. In addition, due to the complex pathology of cartilage repair, KGN is often used in combination with other drugs to target different stages. In addition, with the rapid development of tissue engineering, scholars have combined KGN with various scaffolds by physical or chemical methods. In this paper, we firstly introduce the general properties of KGN followed by a review of the latest advances in the intra-articular delivery modes of KGN. Finally, we discuss the prospects for the application of KGN in cartilage regeneration, which is aimed at providing a new idea and target for the treatment of cartilage defects.
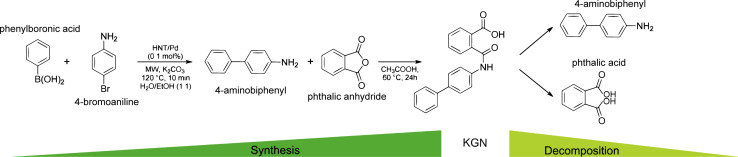
Synthesis and decomposition of KGN molecule.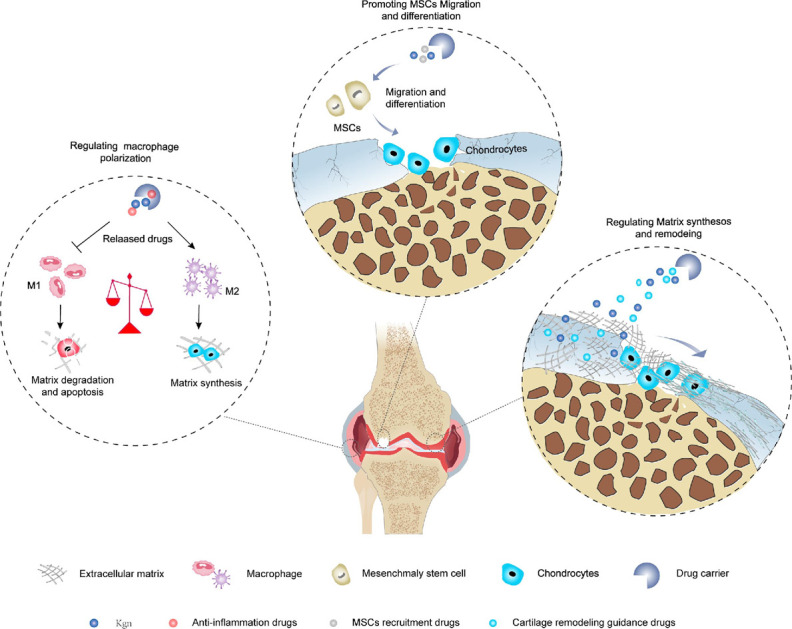
1. Introduction
Cartilage defects is a common orthopaedic disease. Most often caused by osteoarthritis (OA) and sports injuries, it is characterized by joint pain, swelling, and limited movement, causing a great burden to society.1 A variety of pathological mechanisms are involved after cartilage defects, including degradation of extracellular matrix, insufficient matrix regeneration, chondrocyte apoptosis and hypertrophy, but the absence of vascularization and innervation, low cell mobility and low amounts of precursor cells result in extremely limited self-healing capacity of articular cartilage.2 Over the past decades, various methods like intra-articular hyaluronic acid injections, microfracture and chondroplasty have been proposed to promote cartilage regeneration, but none of them can restore the structure and function of natural hyaline cartilage.3 In addition, although chondrocyte- or cartilage-tissue-based approaches such as autologous/allogeneic osteochondral transplantation, autologous chondrocyte implantation (ACI) and matrix-induced autologous chondrocyte implantation (MACI) have shown promising clinical efficacy, their clinical applications are limited by their associated shortcomings such as high donor-area morbidity, loss of hyaline chondrogenic phenotypes and poor integration with the surrounding cartilage. When pharmacologic and surgical treatment strategies fail, the disease progresses to end-stage OA, at which point arthroplasty may become the only certain and unavoidable option. However, currently available prostheses have a limited lifespan and do not meet the needs of younger, more active patients. These harsh realities provide new opportunities for the development of tissue engineering.
Since the 1990s, advances in tissue engineering techniques have expanded the selection of regenerative treatment of cartilage defects. In order to repair cartilage defects, tissue engineering techniques consisting of scaffolds, seed cells and signaling factors have been developed.4 Through a full research on the structure of natural hyaline cartilage as well as continuous exploration of the composition and structural design of scaffolds, human have been able to mimic the complex structure of native hyaline cartilage tissues. More importantly, the scaffold design has been gradually improved by the combination of different types of seed cells and signaling factors, and the interaction between the scaffolds and the native tissues.5 However, despite remarkable achievements have been made in the field of regenerative medicine for cartilage defects in the past decades, the regeneration of cartilage defects is still challenging due to the spatial diversity of cartilage tissue in composition, structure and function and the complexity of cartilage repair pathology.
Mesenchymal stem cells (MSCs) have multi-directional differentiation potential, good immunosuppressive and immunomodulatory ability, which have gradually replaced the chondrocytes as the most common cell source for cartilage defects repair in cellular and tissue engineering applications.6 Promoting the directional differentiation of MSCs to regenerate articular cartilage has become a research hotspot, and MSCs isolated from bone marrow (BMSCs), adipose tissue (ADMSCs), umbilical cord (UCMSCs), and synovial fluid (SFMSCs) have shown considerable application prospects in cartilage repair.7 Transforming growth factor beta (TGF-β), a widely used chondrogenic factor, can enhance MSCs-based articular cartilage repair. However, its half-life in vivo is very short with only a few minutes to a few hours, meaning that multiple injections may be required, increasing the physical and financial burden on the patient. In addition, high doses of TGF-β may lead to osteophyte formation, synovitis, synovial fibrosis, joint swelling and damage to healthy cartilage. Moreover, TGF-β is prone to denaturation during storage and use, causing immune responses in vivo.8 The bone morphogenetic proteins (BMPs) family contains 20 polypeptide members (BMP-1–18, BMP-3b and BMP-8b), which play roles in cartilage and bone development. Several BMPs including BMP-2, -4, -6, -7, -13, and -14 were found to enhance COL II and aggrecan (ACAN) synthesis by chondrocytes in vitro and to stimulate chondrogenic differentiation of MSCs in vivo. However, BMPs may lead to bone formation during ectopic implantation, suggesting that regulation and modulation of BMPs is also necessary to achieve optimal cartilage tissue engineering strategies.9 Insulin-like growth factor 1 (IGF-1), a single-type polypeptide with amino acid sequence similarity to insulin, can maintain cartilage homeostasis by balancing the synthesis and degradation of proteoglycan in chondrocytes, and thus promoting the survival and proliferation of chondrocytes. However, under inflammatory conditions in OA, IGF-1 levels and chondrocyte responsiveness to IGF-1 will decline progressively, thereby resulting in the inability of IGF-1 to maintain structural and functional integrity.10
Kartogenin (KGN) is a small molecule screened in 2012 by Johnson et al. from more than 22 000 structurally diverse heterocyclic pharmacophore molecules and found to promote new chondrogenesis, reduction of chondrolysis and joint weight-bearing capacity in a dose-dependent manner through animal studies.11 Due to its superior physicochemical properties, KGN has been considered as a chondrogenic and chondroprotective drug and has been extensively studied in the field of biologic treatment of cartilage defects.12 With the increasing number of studies, the mechanism of action of KGN has been gradually revealed. In addition, a large number of studies have utilized the carboxyl group, hydrogen bond donor site and hydrogen bond acceptor group on the structure of KGN to apply it to various carriers in tissue engineering by physical binding or chemical cross-linking, which not only overcomes the drawbacks of KGN, such as its high degree of hydrophobicity and short retention period, but also brings a new way for the tissue engineering treatment of cartilage defects.13 To optimize the therapeutic effects of KGN, biomedical engineering technologies such as microfluidics, 3D printing and emerging smart materials can offer various innovative pathways. These technologies can enhance KGN's bioavailability, targeting and its effectiveness in damaged cartilage tissue.14–16 However, there is a lack of a unified international overview of KGN for the treatment of cartilage defects, especially in terms of mechanism of action, and there is no comprehensive understanding of how KGN is delivered. In this review, we first provide an overview of KGN, followed by a review of the efficient intra-articular delivery of KGN including the new advances that have been made in recent years on the cartilage tissue engineering, and finally we discussed and looked forward to its application prospects in the future.
2. Overview of KGN
2.1. Physical and chemical properties
KGN is a small heterocyclic drug compound with a molecular weight of 317.3 g mol−1. With an EC50 value of 100 nM, it has been widely used in the study of cartilage regeneration as an inducer of MSCs differentiation into chondrocytes in recent years.17 Due to the favored chondrogenic differentiation-inducing ability of KGN, Hayek et al.18 have called it a “game changer in regenerative medicine”. KGN has superior physicochemical properties. Firstly, it is dose-dependent, non-cytotoxic and has a long half-life, secondly, it is stable and can be stored and transported at room temperature. In addition, this substance is also easier to prepared by fusing phenylboronic acid with 4-iodoaniline or 4-bromoaniline, and can be hydrolyzed by amide bond-breaking substances such as amidases and peptidases, releasing their products 4-aminobiphenyl (4-ABP) and phthalic acid (PA).19 Studies have shown that 4-ABP is a stronger inducer of chondrogenic differentiation than KGN.17Fig. 1 illustrates the pathways of its synthesis and decomposition.20 However, KGN has a certain hydrophobicity, which may lead to its low solubility in the body, affecting its bioavailability and drug delivery efficiency. It may also cause nonspecific distribution of the drug in the body, weakening its targeted action in specific tissues. Through appropriate chemical modifications, these limitations can be overcome, further enhancing the application potential of KGN in cartilage repair. The KGN molecule contains hydroxyl groups (–OH), which are common reactive sites. These can react with acid compounds to form ester derivatives, and through esterification, KGN can be linked to other drug carriers or chemical groups to adjust its release rate or stability. Kang et al.21 modified the terminal hydroxyl group using 3-hydroxypropionic acid to produce carboxylated KGN (COOH-KGN), which was then covalently bonded to the hydrophilic polyethylene glycol (PEG), improving water solubility and biological stability of KGN, with in vitro results showing sustained release for over five days. In addition, the carboxyl group (–COOH) on the KGN molecule is also an important site for chemical modification, Through the condensation reaction between the carboxyl group and the amine group, an amide bond can be generated, which in turn can be attached to other molecules or ligands. Chen et al.22 prepared CHI–KGN conjugates using the amide reaction between the carboxyl group of KGN and the amino group of CHI, which improved KGN's water solubility and achieved a sustained-release effect. Furthermore, the KGN molecule contains an aromatic ring structure. In the future, the lipid solubility and water solubility of KGN can be modulated by introducing hydrophilic or hydrophobic groups on the aromatic ring, which is essential for improving the bioavailability or targeting properties of KGN.
Fig. 1. Synthesis and decomposition of KGN molecule. This figure has been adapted from ref. 20 with permission from ACS Publications, Copyright © 2019.
2.2. Mechanism of action of KGN in promoting cartilage repair
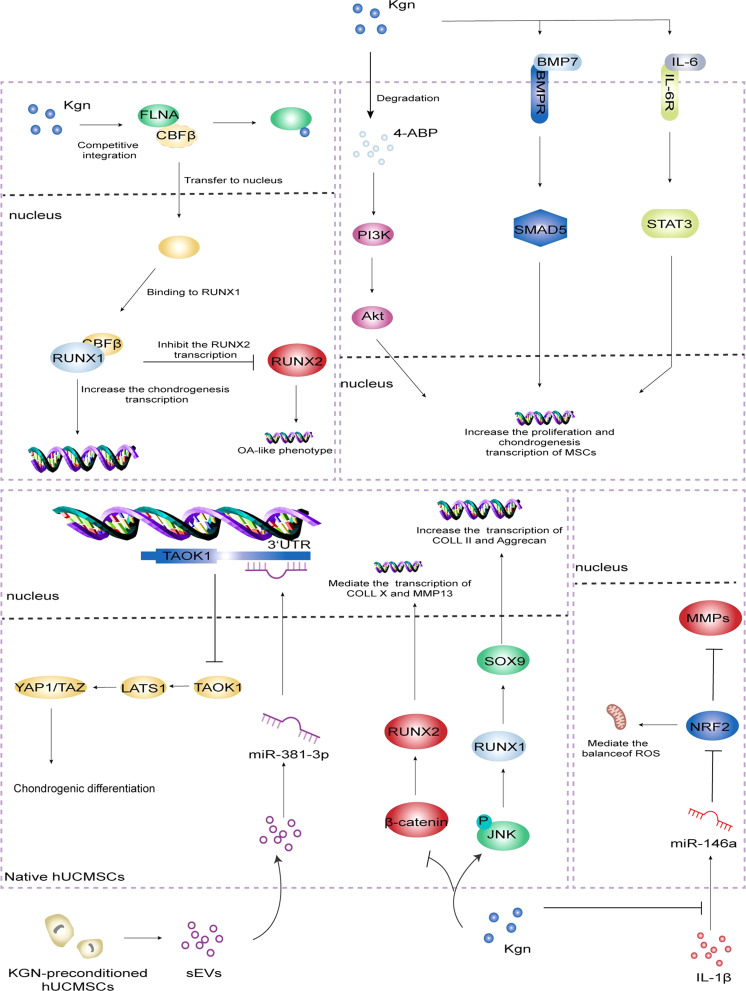
Filamin A/CBFβ/RUNX1 was identified as the classical pathway for KGN to promote cartilage repair. Filamin A (FLNA) is an actin-binding protein. Normally, FLNA binds to CBFβ to form a complex that is conserved in the cytoplasm.23 In addition, RUNX2 is a key factor in osteogenesis and chondrocyte hypertrophy, and its knockout can reduce osteogenesis, inhibit chondrocyte hypertrophy and cartilage calcification, and even delay the progression of OA. CBFβ–RUNX1 dimer can inhibit the transcription of RUNX2, keeping it at a low level and further enhancing the chondrogenesis. On the other hand, KGN can upregulated the levels of COLII, ACAN, and MMPs inhibitors, suggesting that KGN not only maintains the chondrocyte phenotype, but also protects the cartilage matrix from degradation.12,24
Subsequent studies identified additional cellular signaling pathways and receptors involved in KGN-induced chondrogenic differentiation, divided into two major categories: activating and inhibiting. The PI3K-Akt pathway is involved in the regulation of chondrocyte proliferation and differentiation, and is a key regulator of terminal chondrocyte differentiation in embryonic and adult chondrogenesis.25 Zhang et al.17 found that 4-ABP, the degradation product of KGN, can activate PI3K-Akt pathway, promote MSCs proliferation and chondrogenic differentiation, RSK-3 is its potential target, functional regulation of RSK-3 may become an effective strategy to promote cartilage regeneration. Numerous studies have shown that this pathway is important in maintaining the integrity of cartilage tissue because of its direct regulation of cell cycle progression and resistance to oxidative stress. However, compared to other pathways, PI3K-Akt is more concerned with cell proliferation and survival, but less relevant to the regulation of inflammatory responses.26–28 The BMP signaling pathway plays a key regulatory role in chondrogenesis and osteogenic homeostasis. This pathway mediates signaling through a classical route dependent on SMAD transcription factors and regulates the differentiation of MSCs in cartilage formation. Upon activation of the receptor by BMP-7, Smad5 is phosphorylated and translocated to the nucleus, where it regulates the expression of genes involved in chondrogenesis.29 This pathway plays a key role in regulating the differentiation of MSCs into chondrocytes while enhancing extracellular matrix (ECM) production.30,31 Zhou et al.32 used the lentiviral overexpression vector carrying BMP-7 to transfect MSCs and found that BMP-7 could further upregulate the expression of its downstream Smad5 and other four cartilage phenotype genes. The results demonstrated that KGN promoted the chondrogenic differentiation of MSCs through activation of BMP-7/Smad5 signaling pathway. Compared to the PI3K-Akt pathway, the BMP-7/Smad5 pathway is more focused on inducing chondrogenic-specific differentiation of stem cells than on their survival or proliferation. Traditionally, IL-6 has been exclusively associated with arthritis, bone and muscle loss, and other chronic inflammatory diseases, but Stat3, a key gene activated by IL-6, is critical for proliferation, survival, maturation, and regeneration of cartilage-forming cells in joints and growth plates, it plays a complex dual role in cartilage repair through its interaction with the Stat3 signaling pathway.33–35 Liu et al.36 found that KGN could activate Stat3 by binding to gp130, a receptor for IL-6, which in turn upregulated chondrocyte gene expression and played a positive role in damage repair during the late inflammatory phase. Unlike the above pathways, the IL-6/Stat3 pathway has a bidirectional regulatory capacity, involving both inflammation regulation and chondrogenesis, and exhibits complex biological functions.
The Hippo signaling pathway consists of a conserved group of kinases which can inhibit cell growth.37–39 Jing et al.40 isolated small extracellular vesicles (sEVs) from KGN-pretreated MSCs and re-internalized them, and the level of miR-381-3p in the cells was significantly increased. It can target the 3′ untranslated region of TAOK1 to inhibit Hippo signaling pathway and promote chondrogenic differentiation of MSCs. Activation of JNK leads to phosphorylation of c-Jun, which results in the decreased proteoglycan synthesis and increased generation of matrix metalloproteinase 13 (MMP-13).41 Similarly, conditional activation of the β-catenin gene in chondrocytes leads to their OA-like phenotype.42 Jing et al.43 found that pretreatment of human umbilical cord mesenchymal stem cells (hUCMSCs) with KGN could place hUCMSCs in the pre-cartilage stage and promote chondrogenesis by enhancing JNK phosphorylation and inhibiting β-catenin. Compared to other pathways, its function is more focused on the inhibition of cell proliferation and balancing the rhythm of tissue regeneration. Reportedly, miR-146a is substantially upregulated in OA and is associated with the clinicopathological features of patients as a potential regulator of joint health.44–47 Hou et al.48 found that IL-1β significantly upregulated miR-146a, and the overexpression of miR-146a could downregulate the protein level of nuclear factor erythroid 2-related factor 2 (NRF2) and upregulate the expression of MMPs. KGN can downregulate IL-1β-stimulated reactive oxygen species in chondrocytes by activating NRF2. The strong inhibitory effect on miR-146a overexpression in IL-1β-stimulated chondrocytes proved that KGN could effectively prevente the cartilage degeneration and alleviate the progression of OA through the miR-146a/NRF2 axis. KGN-regulated activation of NRF2 not only improves antioxidant capacity but also enhances chondrocyte viability and tissue repair potential through anti-inflammatory effects. The mechanisms covered above are summarized in Fig. 2.
Fig. 2. Mechanism of action of KGN in promoting cartilage repair.
In a word, KGN plays a role in promoting cartilage repair through multiple signaling pathways, each of which functions differently in cell differentiation, proliferation, anti-apoptosis, anti-inflammation and anti-oxidation. Future studies may optimize the cartilage repair effect of KGN by jointly regulating these pathways to promote cartilage regeneration more effectively.
2.3. Long-term efficacy of KGN in vivo
The long-term effects of kartogenin (KGN) in cartilage repair have been the focus of attention in the field of regenerative medicine. In vivo, the long-term effects of KGN are mainly characterized by the stability and functionality of the regenerated cartilage, as well as its sustained role in delaying cartilage degeneration and functional recovery.49,50 KGN works in vivo by promoting the differentiation of MSCs to chondrocytes, generating hyaline cartilage that resembles natural cartilage tissue rather than fibrocartilage. This hyaline cartilage production is essential for long-term joint stability.51,52 Regenerated cartilage is not only structurally close to natural cartilage, but its functional performance has also been recognized. Xu et al.53 demonstrated that KGN, when combined with a particulate delivery system, effectively restored the biomechanical properties of damaged joints and maintained the prolonged functionality of regenerated cartilage, and demonstrated good repair and functional outcomes at 12 weeks postoperatively. This functional restoration of regenerated cartilage significantly reduces pain due to cartilage degeneration, improves joint mobility, and reduces the occurrence of degenerative changes.
However, based on the current level of research, there is still a lack of specific long-term follow-up data on the long-term effects of KGN in cartilage repair, especially for functional validation beyond one year. Most studies have focused on short-term observations (usually a few months), and more clinical trials and long-term follow-up studies, especially in humans, are needed to obtain clear conclusions about the long-term functionality and stability of KGN in cartilage repair.
3. Delivery strategies
Local management of cartilage defects by intra-articular injection is a traditional method, however, when injected into the joint cavity, the KGN has a short retention and is easily cleared by blood and lymph. Therefore, KGN has been loaded into different drug carriers to increase its solubility and prolong the residence time. Notably, due to the complexity of the pathological process of cartilage regeneration, the combination of KGN with other drugs which target different stages of repair can improve the results, meanwhile, also provides new strategies to deliver KGN. In addition, the rapid innovation of scaffold technologies of tissue engineering in recent years has provided a whole new way for intra-articular delivery of KGN.
3.1. Individual delivery of KGN
3.1.1. Polymer microsphere
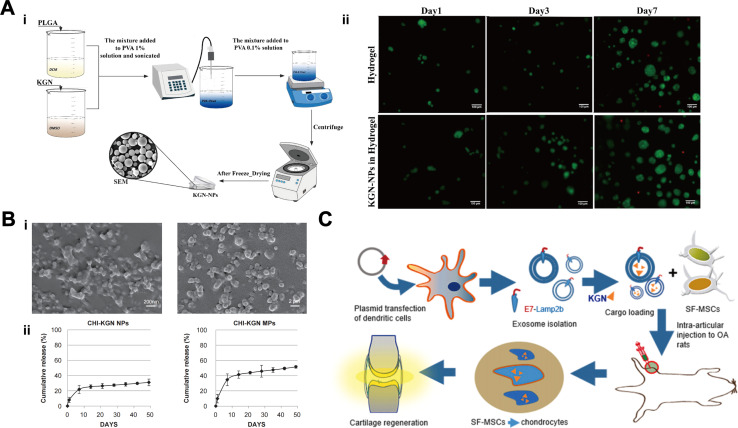
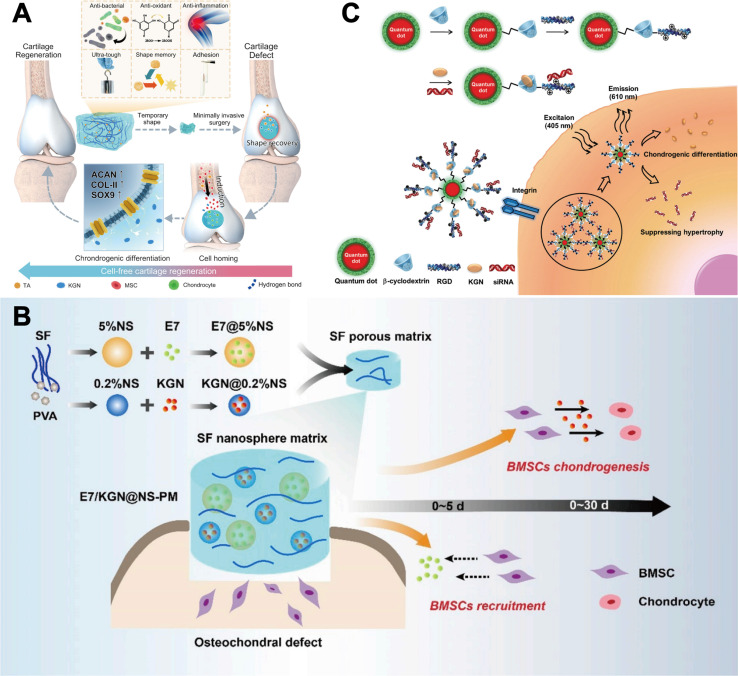
Micron (1–1000 μm) or nanoscale (1–1000 nm) particles can be loaded with high concentrations of drugs, are protective and slow-release, and are widely used as delivery agents.54 These polymers have been used either directly for intra-articular injections or co-located with other therapeutic cells and/or bioactive molecules on polymer scaffolds for repairing cartilage defects. As an outstanding representative, poly(lactic-co-glycolic acid) (PLGA) with good biocompatibility, vesicle- and film-forming properties, and non-toxicity is widely used to fabricate degradable nanoparticles (NPs) or microspheres (MPs) to control drug release. The amino group of PLGA can covalently bind to the carboxyl group of KGN, thereby enhancing the permeability and retention of KGN and promoting its therapeutic effect.55 Its preparation is also relatively simple, usually prepared by solvent evaporation or spray drying, and KGN is uniformly encapsulated in it, moreover, the degradation time can be adjusted according to the application scenario by adjusting the ratio of lactic acid to glycolic acid, and the adjustment interval is between several weeks and several months.56–58Fig. 3A illustrates the preparation of KGN-PLGA NPs.59 Zhao et al.62 encapsulated KGN into PLGA NPs and then showing the effect of controlled and sustained drug release, KGN released about 75% at 30 days postoperatively which further promoted the chondrogenic differentiation of BMSCs. Chitosan (CS) is derived from chitin and has excellent biological properties such as good biocompatibility, biodegradability, and non-toxicity, which makes it a promising carrier for drug delivery. On account of its water-soluble capacity,63 chemically-linked CS-KGN polymer can enhance the solubility and biocompatibility of KGN drug, thereby enhancing its efficacy. Kang et al.60 prepared CS-KGN-NPs and CS-KGN-MPs and found that the release of KGN from NPs and MPs reached approximately 30% and 50%, respectively, at 50 days postoperatively (Fig. 3B), both resulted in the expression of higher levels of cartilage markers in BMSCs than KGN alone. The fluorescence images showed that CS-KGN-MPs existed in rats for more than 3 weeks and significantly reduced the degree of cartilage degeneration. Bai et al.64 fabricated injectable KGN-loaded PLGA microspheres by microfluidics and used the EDC/NHS reaction to anchor CS on the surface of the microspheres to form microsphere complexes. Based on the charge-bound cell adhesion capacity of CS, this new microsphere can be loaded with a large number of MSCs (>1 × 104/mm−3), and it could significantly enhance the expression of cartilage repair-related genes and chondrogenic differentiation of MSCs. More importantly, PLGA-CS@KGN resulted in a significant reduction in the volume of osteochondritis dissecans, which alleviated the progression of OA in rats obviously.
Fig. 3. Delivery of KGN via polymer microspheres. (A) Preparation of PLGA-KGN NPs (i). Hydrogels after addition of KGN showed no cytotoxicity (ii). This figure has been adapted from ref. 59 with permission from Elsevier, Copyright © 2021. (B) Scanning electron micrographs (SEM) of CS-KGN NPs and CS-KGN MPs (i). Release curves of KGN in CS-KGN NPs and CS-KGN MPs (ii). This figure has been adapted from ref. 60 with permission from Elsevier, Copyright © 2014. (C) Exosome-encapsulated KGN for cartilage repair. This figure has been adapted from ref. 61 with permission from Elsevier, Copyright © 2021.
The monitoring of tissue regeneration is particularly important, but the vast majority of materials do not allow direct observation of the regeneration process of cartilage. Fluorescent visualization microspheres offer a novel option for KGN delivery, allowing monitoring of therapeutic effects. Yao et al.65 constructed nanomaterials capable of fluorescence visualization by chemically grafting poly(ethylene glycol) (PEG), KGN, hydrogenated soybean phosphatidylcholine (HSPC) and fluorescein (PPKHF) via “click chemistry” using mercapto polyhedral oligosilsesquioxane (POSS-SH) as a nanoplatform. Then PPKHF was homogeneously mixed with methacrylate hyaluronic acid to prepare hydrogel microspheres (MHS@PPKHF).This material exhibited excellent hydration lubrication properties, more importantly, the changes of fluorescence signals in the joint can be observed by the in vivo imaging system (IVIS). Skipping the cumbersome staining and provides a more convenient and quicker means to observe the progression of cartilage repair. Similarly, polyamide-amine (PAMAM) is a class of dendritic macromolecules with monodisperse and controllable topology. It can be easily functionalized by core modification or surface modification to encapsulate various biomolecules and deliver them to specific regions in an efficient manner with few side effects.66 Hu et al.67 fabricated injectable PAMAM nanocarriers modified by polyethylene glycol (PEG) and prepared PEG-PAMAM-KGN (PPK) and KGN-PEG-PAMAM (KPP) by binding KGN to the surface and terminal groups of PAMAM respectively. Results revealed that fluorescein-labeled PAMAM could persist in the joint of rats for at least 21 days and the intensity of nuclear localization of CBFβ was significantly increased by KPP injection as well as the expression of chondrogenic markers. It is shown that loaded KGN on the end groups of PAMAM allows the maximization of its efficacy.
3.1.2. Exosomes
As a natural nano-carrier, exosomes (EXO) can regulate the repair and regeneration process of damaged sites by affecting biological functions such as cell proliferation, migration, and differentiation. It has low immunogenicity, no cytotoxicity and targeted delivery ability, which has advantages over synthetic nano-carriers. Exosomes are usually isolated from the culture medium by ultracentrifugation or immunoprecipitation techniques, and its degradation is usually fast, within a few days to a week in the body.68,69 Engineered exosomes have significantly improved the efficacy and precision of drug delivery, and are currently being widely used in targeted therapy for various diseases, including tumors, inflammatory diseases, and degenerative diseases.70 Encapsulation of KGN into EXO for delivery is a novel approach in recent years. Xu et al.61 designed a new MSCs-targeted drug delivery platform based on EXO, which can effectively deliver KGN into MSCs and promote their chondrogenic differentiation, KGN released about 75% in 7 days, thereby enhancing the repair of cartilage defects in OA (Fig. 3C). Delivery of KGN by MSCs-targeting exosomes (E7-EXO) optimizes the biological efficacy of MSCs and is a new kind of stem cell therapy. The strength of this study lies in the use of EXO to encapsulate intact KGN without chemical covalent cross-linking, achieving good biocompatibility and delivery efficiency for drug delivery.
Furthermore, it has been suggested that KGN acts indirectly by using EXO secreted by KGN-pretreated MSCs. Shao et al.71 divided rabbit chondrocytes into three groups: EXO group, KGN-EXO group (EXO secreted by KGN-preconditioned MSCs) and control group (no intervention). Results showed the EXO secreted by KGN-preconditioned MSCs has the best cartilage repair effect in vitro and in vivo. In addition, Liu et al.72 found that EXO derived from KGN-preconditioned BMSCs exhibited a notably enhanced cartilage matrix formation and notably reduced matrix degradation than EXO derived from BMSCs directly. Similarly, Jing et al.40 proved that miR-381-abundant small extracellular vesicles derived from kartogenin-preconditioned MSCs promote chondrogenesis of MSCs by targeting TAOK1. Moreover, the optimal concentration of KGN acting on MSCs-EXO for cartilage repair has been determined. Xie et al.73 induced ADMSCs by KGN at concentrations of 100 nM, 500 nM, 1 μM, 5 μM, and 10 μM, respectively, and found that ADMSCs-EXO induced by 5 μM of KGN could promote the proliferation, cloning, migration and chondrogenic differentiation of ADMSCs maximally, inhibit apoptosis, and significantly increase the expression levels of chondrogenesis-related genes (ACAN, Col II and SOX9) and decreased the expression levels of chondrolysis-related genes such as MMP-3. In summary, KGN combined with EXO can be used as a potential therapeutic approach in the future.
Therefore, the drug release characteristics of the different delivery systems have their own advantages, with polymeric microspheres being suitable for therapeutic regimens that require long-lasting drug release, whereas exosomes support rapid drug release and are suitable for short-term high-efficacy drug action.
3.2. Co-delivery of KGN
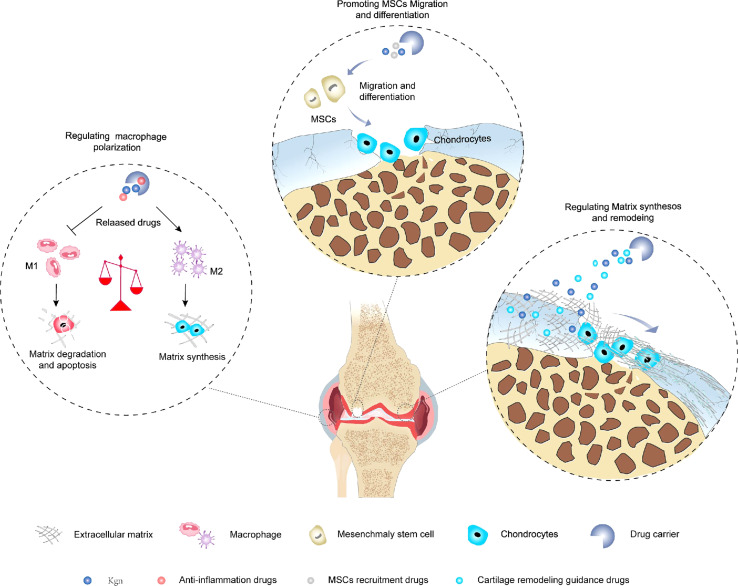
There are five major phases involved in cartilage regeneration: inflammation, catabolism, cell recruitment, anabolism and tissue remodeling.74 The inflammatory phase and the catabolic phase are evident in the first few days after injury. After 7–14 days, anabolic activities, including chondrogenic differentiation and matrix deposition, are activated and gradually become dominant. 4 weeks later, new cartilage is formed, however, tissue remodeling is still required before cartilage can mature, and the outcome of this process determines the type of regenerated tissue. Therefore, additional drugs are needed to target different pathologic processes in order to improve the microenvironment in which KGN acts. In addition, due to the poor regeneration performance of cartilage, it is necessary to improve the level of anabolism to promote the repair of cartilage defects.75 In recent years, many scholars have combined anti-inflammatory strategies, cell recruitment strategies, and cartilage remodeling strategies with KGN-mediated enhanced synthesis of cartilage, respectively (Fig. 4), showed a favorable effect on hyaline cartilage regeneration.
Fig. 4. Combination of anti-inflammatory strategies, cell recruitment strategies, and cartilage remodeling strategies with KGN-mediated cartilage-enhanced synthesis strategies.
3.2.1. KGN combined with inflammation/catabolism inhibition
Inhibition of inflammation and cartilage catabolism combined with enhancement of cartilage anabolism is a promising strategy for the treatment of cartilage defects.76 In general, drugs targeted to inhibit inflammation and catabolism need to be released rapidly in the short term to suppress the severe inflammatory response and catabolic activity in the early phase, whereas sustained chondrogenic differentiation induction and cartilage matrix deposition are required in the middle to late phase of the injury. Kang et al.77 synthesized heat-responsive nanospheres based on chitosan oligosaccharide conjugate with multiple F127 grafted carboxyl groups. They loaded KGN and diclofenac (DCF) onto the surface and core of the nanospheres, respectively, where immediate release of DCF and sustained release of KGN could be achieved by temperature changes. This nanospheres can inhibit the progression of OA in rats and the efficacy can be further enhanced by cold treatment. Yang et al.78 designed a multiple hydrogen-bond crosslinked hydrogel loaded with tannic acid (TA) and KGN via the polymerization reaction. Ta can reduce the inflammatory response and modulate in situ oxidative stress, creating a microenvironment conducive to the healing process. In addition, sequential release of TA and KGN could promote the migration of BMSCs into the hydrogel scaffold and induce their chondrogenic differentiation, thus leading to full-thickness cartilage regeneration in vivo (Fig. 5A). Curcumin is a highly pleiotropic molecule with potent antioxidant, anticancer, and anti-inflammatory properties, and Csaki et al.81 demonstrated that curcumin can eliminate apoptosis and inflammation induced by IL-1β. In one study, Asgari et al.82 encapsulated KGN-loaded PLGA microspheres into MSCs aggregates and delivered them in vivo via curcumin-containing GelMA injectable hydrogels. Results in vitro proved that curcumin alleviated the inflammatory environment at the defect site and enhanced the effect of KGN in promoting the expression of more cartilage markers. Results in vivo proved that optimal cartilage regeneration was observed in the treatment group receiving KGN and curcumin. Thus, we learned that anti-inflammatory drugs can inhibit catabolism, improve the action environment of KGN, then enhance the repair effect of KGN, which provides a new idea for the treatment of OA.
Fig. 5. KGN combined with different repair strategies. (A) KGN combined with TA-mediated anti-inflammatory strategy. This figure has been adapted from ref. 78 with permission from Nature, Copyright © 2023. (B) KGN combined with E7-mediated BMSCs recruitment strategy. This figure has been adapted from ref. 79 with permission from KeAi, Copyright © 2020. (C) KGN combined with siRNA targeting silencing of RUNX2. This figure has been adapted from ref. 80 with permission from Wiley, Copyright © 2016.
3.2.2. KGN combined with endogenous MSCs recruitment
When sufficient numbers of endogenous stem cells accumulate in cartilage defects, they differentiate into functional chondrocytes and deposit additional ECM, which is essential for the formation of new cartilage.83–85 However, endogenous repair of cartilage defects is usually limited by insufficient number of cells in the early stage and incomplete cell differentiation in the late stage, and sequential presentation of inducible molecules to coordinate the tissue repair cascade reaction will further enhance the repair outcomes.86 Zhang et al.79 incorporated the tunable drug-loaded silk fibroin (SF) nanospheres into the SF porous matrix to obtain a whole-filament derived sequential delivery system. By varying the initial SF/poly (vinyl alcohol) (PVA) concentration of the nanospheres, this specially designed system provided an rapid release of E7 (a BMSCs affinity peptide) initially to recruit BMSCs at an early stage, and a relatively slow and sustained release of KGN to induce chondrogenic differentiation of BMSCs, thereby enhancing cartilage regeneration (Fig. 5B). Similarly, Dai et al.87 reported an injectable hydrogel microspheres with sequential drug release properties called Col-Apt@KGN MPs, where Apt19S (a synthetic nucleic acid) was rapidly released to recruit MSCs within 6 days while KGN was slowly released through degradation of PLGA microspheres for 33 days. The hydrogel could facilitated the adhesion, proliferation and chondrogenic differentiation of MSCs and effectively promote the recruitment of endogenous MSCs, then enhance the secretion of cartilage-specific extracellular matrix mediated by KGN, finally realize the reconstruction of a rabbit model of full-layer cartilage defects. This combinatorial strategy avoids the disadvantage of insufficient cell number during the repair process and is believed to have great application prospects in the future.
3.2.3. KGN combined with organizational remodeling guidance
During the healing process of hyaline cartilage, the direction of cartilage remodeling can easily deviate from hyaline cartilage to bone tissue or fibrocartilage due to a variety of influences such as osteogenic factors and vascular endothelial growth factor (VEGF).88 For example, although microfracture can relieve the symptoms of OA temporarily, most of the newly formed cartilage is fibrocartilage, this type of cartilage has poor mechanical properties and is prone to degradation compared with hyaline cartilage, leading to poor efficacy.89 Therefore, accurate guidance of hyaline cartilage remodeling is very important for qualified cartilage regeneration. Runt-related transcription factor 2 (RUNX2) is an osteogenic transcription factor that also mediates chondrocyte hypertrophy and chondrogenic induction of MSCs.90 Previous studies have shown that silencing RUNX2 results in enhanced chondrogenesis and reduced hypertrophy of regenerating cartilage.91 RNA interference is a powerful technique to silence specific target genes. Xu et al.80 developed a multifunctional nanocarrier modified with β-cyclodextrin (β-CD) and Arg-Gly-Asp (RGD) peptide, which was able to carry KGN in a hydrophobic pocket affixed to β-CD, meanwhile, the RGD peptide combined with siRNA targeting silencing of RUNX2 through electrostatic interactions (Fig. 5C). Results indicated that KGN combined with siRNA not only enhanced the chondrogenesis and development of new cartilage, but also effectively suppressed the expression of genes for cartilage hypertrophy and calcification. In addition, anti-angiogenic strategies have been used in conjunction with chondrogenic strategies to produce a more stable hyaline cartilage phenotype. Murphy et al.92 co-delivered BMP2 and VEGFR1 via hydrogel into an adult mouse model of OA, and found that the combination of the two resulted in the differentiation of stem cells recruited by microfracture into more hyaline cartilage than fibrocartilage. This regimen provides a new direction for the treatment of cartilage defects, but there are no reports on the combination of KGN with antiangiogenic drugs. In addition, although several attempts have been made by scholars and produced promising results, the rational timing and sequence of drug release in this combination strategy remains unclear and needs to be explored in the future.
3.3. Tissue engineering scaffolds delivery of KGN
In tissue engineering, more and more studies have been conducted on KGN in cartilage defects repair. Scaffolds, growth factors and cells are the three main elements of cartilage tissue engineering. Scaffolds act as ECM to provide three-dimensional(3D) structural support for cell adhesion and proliferation. In recent years, a variety of scaffolds have been designed for cartilage regeneration, including chemically or physically cross-linked hydrogels and natural or synthetic porous materials.
3.3.1. Hydrogel
Hydrogels are polymeric materials with hydrophilic structural features that facilitate chondrogenic differentiation and cartilage regeneration of MSCs induced by biologically active molecules because they provide a 3D microenvironment with high water content. Currently, hydrogels are being used as therapeutic cell and drug carriers for the repair of articular cartilage.93 These hydrogels exhibit soft consistency, low interfacial tension and high biocompatibility, closely resembling natural tissues. Their ability to be customized for site-specific and sustained drug delivery has enabled them to be widely used in regenerative medicine. PLGA has been used in the fabrication of hydrogels in addition to the microspheres described above. Similarly, PEG has been widely used in the design of biomaterials due to its good cytocompatibility, non-immunogenicity and easy modification properties. Li et al.94 synthesized a temperature-sensitive PLGA–PEG–PLGA hydrogel for the repair of cartilage defects by combined piggybacking of KGN and BMSCs, in which KGN was physically encapsulated and could be cumulatively released by about 42.2% for 196 h. In vivo results revealed that the combination of Gel/KGN/BMSCs showed the best repair effects. Similarly, Wang et al.95 applied a PLGA–PEG–PLGA temperature-sensitive hydrogel equipped with KGN to a rabbit knee anterior cruciate ligament transection (ACLT) model and found that the KGN group had the best cartilage repair and the least joint inflammation after 3 weeks. In addition, Lee et al.96 combined MSCs, KGN and RGD-coated layered double hydroxide in a PEG-poly(l-alanine)-poly (l-aspartic acid) block hydrogel to form a novel 2D/3D nanocomplexed system, which was gel-forming at 37 °C, with energy storage modulus up to 750–820 Pa, and could release KGN continuously for more than 21 days.
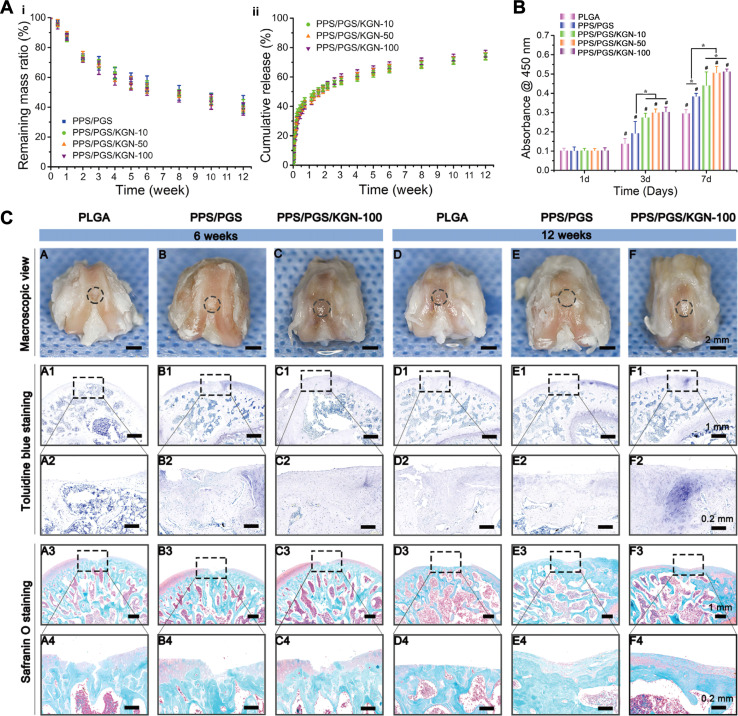
β-Cyclodextrin (β-CD) is a product of starch generated by acidolytic cyclization. It can encapsulate drug molecules and mainly used to increase drug stability and prevent drug from oxidation and decomposition.97 In addition, studies have shown that it enhances drug loading doses and modulates drug release.98 Yuan et al.98 loaded hydrophobic KGN into the hydrophobic cavity of aldehyde-modified β-CD (β-CD-CHO, OCD) via host–guest interactions, and then fixed the KGN loaded OCD onto CS-derivated hydrogel through Schiff base reaction. Achieved a sustained release effect of KGN and complete cartilage regeneration was obtained. Xu et al.99 prepared a photocrosslinked hydrogel composed of acrylyl β-CD and methylacrylyl gelatin by host–guest interactions. KGN was attached to a part of the hydrophobic cavity of β-CD and could be released at a stable and constant rate for 28 days (Fig. 6A). In recent years, due to the weak mechanical properties of natural β-CD, Fallahi et al.103 used β-CD grafted alginate (Alg-β-CD) and combined it with multiple physically crosslinked polyglycosylamined amines. Supramolecular interactions, including electrostatic forces, host–guest interactions, and temperature-dependent hydrophobic interactions improved the mechanical properties of the hydrogels, enabling a shear modulus in excess of 40 kPa. Eventually, the sustained release of KGN was achieved by combining the cavity of β-CD with the hydrogel network.
Fig. 6. KGN combined with hydrogels. (A) Cumulative release of KGN encapsulated in the GelMA and HGM (host–guest macromer) hydrogels. This figure has been adapted from ref. 99 with permission from Elsevier, Copyright © 2019. (B) KGN crosslinked with PN (polyurethane nanoparticles) (i) to achieve a sustained release effect (ii). This figure has been adapted from ref. 100 with permission from Taylor & Francis, Copyright © 2018. (C) Cumulative release of KGN in drug nano box decorated HAMA hydrogels with two different concentrations of β-CD-AOI2 (HA2CD2 and HA2CD4) compared with the control group (non-decorated HAMA hydrogel). This figure has been adapted from ref. 101 with permission from Elsevier, Copyright © 2020. (D) Schematic of the preparation of monitorable hydrogels encapsulating KGN. This figure has been adapted from ref. 102 with permission from Wiley, Copyright © 2023.
Although CS has advantages such as excellent biocompatibility and degradability, it has poor solubility, weak mechanical strength and difficult processability. Sodium alginate (SA) and oxidised sodium alginate (OSA) derivatives are often incorporated into CS-based systems due to their great accessibility and non-immunogenicity.104 Li et al.105 fabricated CS-KGN/OSA composite hydrogels by an efficient Schiff base reaction between the aldehyde group of OSA and the amine group of CS-KGN. The hydrogel can deliver KGN locally and sustainably for a long time. Co-culture of this hydrogel with BMSCs resulted in a significant up-regulation of cartilage-specific gene expression, as well as an increase in DNA levels and ACAN content, thus validating the durability of cartilage formation and its great potential application in the clinic. In addition, Dehghan-Baniani et al.106 enhanced the shear modulus of CS-based hydrogels to 78 ± 5 kPa by chemically modifying it using N-(β-maleimidopropyloxy) succinimide ester (BMPS). In addition, it could continuously release KGN for about 40 days, thus eliminating the need for multiple injections, and its great mechanical properties could promote chondrogenic differentiation of ADMSCs.
To further enhance therapeutic efficacy, especially for localized applications, nanoparticles are increasingly being combined with hydrogels to form composite biomaterial systems for controlled drug delivery.107 Zare et al.108 prepared KGN-PLGA NPs and loaded them into alginate sulphate hydrogels, which exhibited linear and sustained release of KGN over a period of 30 days with desirable initial burst reduction. In addition, it is well known that experiments on large animal are a bridge to translate the animal results into clinical applications. Yan et al.109 randomised 48 minipigs into 3 treatment groups: the KGN- PLGA – Hyaluronic Acid (HA) group, the HA group and the control group (untreated). Results showed that NPs combined hydrogel maximally promoted hyaline cartilage and subchondral bone tissue repair in a model of osteochondral defects at a 12 month follow-up. In another study, Fan et al.100,110 achieved a loading efficiency of 14% by grafting KGN onto polyurethane (PN) NPs via an EDC/NHS condensation reaction, KGN achieves a slow-release effect, approximately 20% released in 30 days, allowing the scheme to protect articular cartilage and inhibit the progression of OA (Fig. 6B).
The remarkable difference in cell type and matrix composition between cartilage and subchondral bone makes it challenging to simultaneously regenerate both parts. The composition, structure and function of single-phase scaffolds differ greatly from the complex osteochondral organization, which greatly limits their therapeutic effectiveness. Hence, the biphasic scaffold, consisting of two different phases designed to repair cartilage and bone tissues respectively, is a superior strategy to tackle the aforementioned challenges and achieve successful osteochondral repair.111 Silk proteins (SF), the main components of silkworm cocoons, have superior mechanical properties and biocompatibility, but the formation of SF into hydrogels by β-folding is too slow and under strict criteria, limiting their use in biomedical applications.112 In 2018, researchers discovered for the first time that the introduction of a double bond on the SF molecule via glycidyl methacrylate not only improves the water solubility of SF, but also makes it possible to form light-curing hydrogels, expanding the applications of SF.113 Berberine (BBR), which is the main active ingredient of Rhizoma Coptidis in exerting its antimicrobial effect, which can induce osteogenic differentiation of MSCs and inhibit the progression of OA.114 Jiang et al.115 prepared a bilayer methacrylate sericin protein hydrogel (SilMA) by layered photocuring technology and loaded SF microsphere-encapsulated KGN and BBR in the cartilage layer and subchondral bone layer respectively, which ultimately led to the long-term regulation of chondrogenic and osteogenic differentiation of BMSCs. Furthermore, this bilayered composite hydrogel could exert its efficacy in an inflammatory microenvironment and achieve satisfactory cartilage and subchondral bone regeneration 8 weeks after implantation. These materials are readily available, biocompatible, and can be efficiently integrated by simple processes, thus have great potential for clinical applications in osteochondral regeneration. Similarly, Liu et al.101 designed a biphasic hydrogel and loaded KGN and melatonin through β-CD in the cartilage and subchondral bone layers respectively. The in vitro release curves (Fig. 6C) show that the drug nanobox-decorated CRH (cartilage-regenerating hydrogel) groups, including HA2CD2 and HA2CD4, had an initial KGN release of ∼42% and ∼25%, respectively, within the first 10 h, while the control group reached ∼83% drug release within the same timeframe. The HA2CD2 group continuously released from ∼42% to ∼88% of KGN within about 24 days. However, HA2CD4 released ∼55% (from ∼25% to ∼80%) of the drug within about 28 days. This indicated that increasing β-CD-AOI2 would lead to a delay in drug release.
In addition, with the development of imaging techniques, there has been a growing interest in tissue repair processes. Magnetic Resonance Imaging (MRI) is widely used to assess morphological changes in biomaterial degradation and neotissue reconstruction due to its safety and non-invasiveness.116 It offers significant advantages in evaluating various regeneration strategies with ideal soft tissue resolution and depth of penetration. For example, Hong et al.117 reported a chitosan-modified Fe3O4-CS/KGN nanoprobe which not only can be used to discriminate the location of defects, but also has an important role in promoting the differentiation of ADSCs into chondrocytes. Similarly, Yang et al.118 grafted KGN onto the surface of modified ultrasmall superparamagnetic iron-oxide (USPIO), achieved both contrast enhancement on MRI and promotion of chondrogenic differentiation of BMSCs. In addition, synthetic melanin nanoparticles (SMNP) have excellent biocompatibility and coordinated separation of paramagnetic metal centres.119 Chen et al.102 loaded KGN into hydrogels after grafting to SMNP. SMNP-KGN/Gel showed good mechanical properties, thermal stability and contrast enhancement on MRI, and the degradation of hydrogel and cartilage regeneration process could be monitored simultaneously by MRI within 12 weeks. Demonstrated that this approach can be used for non-invasive imaging in cartilage regenerative medicine to guide precise treatment (Fig. 6D).
Therefore, as a tissue engineering scaffold, hydrogel has a 3D structure that can accommodate cells for cartilage repair, and the materials enhanced with mechanical properties better meets the needs of cartilage tissue engineering, moreover, the combination of microspheres and hydrogel can exert the advantages of both simultaneously. In addition, biphasic hydrogel scaffolds provide a new option for the repair of osteochondral defects. However, many natural hydrogel materials degrade too rapidly and may be difficult to achieve sustained drug release.120 In addition, hydrogels typically have low mechanical strength to withstand the stresses of a highly loaded joint site, so hydrogels may be more suitable for early intervention in cartilage injuries or in scenarios that require injectable therapies, and are especially superior in treatments that do not require long-term mechanical support but do require sustained release of the drug.
3.3.2. Porous scaffold
Porous scaffolds are the most commonly used type of scaffolds in tissue engineering, which tend to have a tougher texture than hydrogels, create a better 3D environment for cell survival and differentiation, and have favorable drug-loading capacity and enable customized release profiles. Currently, technologies such as 3D printing, salt leaching and freeze-drying have been applied to fabricate porous scaffolds, which have shown promising restorative effects.121
Application of porous scaffolds alone for drug delivery is a traditional approach. Chen et al.55 synthesized a poly urea scaffold with side-chain amino groups (PEEUUN) and grafted KGN onto the PEEUUN scaffolds (PEEUUN–KGN). Results showed that the PEEUUN–KGN scaffolds have a degradable 3D structure, interconnected pores, good elasticity and excellent cytocompatibility. Meanwhile, KGN could be released stably and continuously, thus promoting the differentiation of UCMSCs into chondrocytes and ultimately cartilage regeneration. In reality, surgical treatment of cartilage defects in OA patients is quite difficult due to the narrow space and irregularity of the defect area, often requiring the scaffolding material to have a certain shape changing ability to accommodate different shapes of the defects. Xuan et al.122 designed porous scaffolds constructed by hybridization of the bioelastomers poly (sebacate glyceride) (PGS) and poly (1,3-propanediol glyceride) (PPS). The crystallized PPS chain acted as reversible switching phases to fix the shape temporarily, allowing the user to customize the permanent shape of the scaffold according to the defect. In addition, they covalently combined KGN with the free hydroxyl group in PGS, increased KGN content did not affect the biotoxicity of the scaffolds and KGN obtained a sustained release of up to 3 months due to the suitable degradation properties of the scaffold. Outcomes in vivo indicated that chondrogenic KGN-releasing scaffolds enhanced the repair ability of the cartilage defects (Fig. 7).
Fig. 7. KGN combined with porous scaffold. (A) In vitro degradation (i) and KGN release results (ii) of shape-memory scaffolds with different KGN contents. (B) CCK-8 assay of BMSCs on scaffolds with different KGN contents. (C) KGN enhanced cartilage repair in vivo. This figure has been adapted from ref. 122 with permission from Elsevier, Copyright © 2020.
Similar to hydrogels, the binding of NPs to porous scaffolds can likewise improve the therapeutic efficacy of KGN. Sun et al.123 encapsulated KGN into PLGA NPs and mount them on COL/CHI/HA porous scaffolds. By adjusting the size of the NPs and the content of hyaluronic acid sodium (HAS), the cumulative release rate of KGN can be more than 90% within 21 days. In another study, PLGA-KGN NPs and TGF-β1 NPs were mounted on a bilayered porous scaffold with COL/CHI/HAS in the surface layer and COL/CHI/SF in the transitional layer, which also provided excellent repair of cartilage defects.124 Hong et al.125 explored a novel combination therapy using poly-l-lysine/KGN(L–K) NPs and PLGA/methacrylate hyaluronic acid (PLHA) composite scaffolds. PLHA has an appropriate stiffness close to hyaline cartilage and promotes proliferation and chondrogenic differentiation of ADSCs. Experiments of in vivo showed that the combination of NPs and scaffolds resulted in the smoothest regenerated cartilage tissues.
Combining KGN-loaded hydrogels with porous scaffolds is a desirable option due to the poor mechanical properties of natural hydrogels and the realistic need to promote cartilage and subchondral bone regeneration simultaneously.121 Zhang et al.126 developed a multifunctional biphasic scaffold consisting of KGN-loaded GelMA as the cartilage layer and HA-coated polycaprolactone (PCL/HA) as the subchondral bone layer with the addition of tannins (TA) and E7 peptide, SEM demonstrated the bilayer structure and contact surfaces. The TA/E7 endowed the scaffold with the ability of pro-migration of cells and resistance to oxidative stress, which enabled the scaffold better to promote chondral and subchondral bone repair simultaneously. Similarly, Liu et al.127 used KGN-containing hyaluronic acid hydrogel as the cartilage layer and alan phosphate-containing hydroxyapatite (Hap) as the subchondral bone layer. Compared with drug-free scaffolds, the chondrogenic differentiation and osteogenic differentiation of MSCs in drug-containing scaffolds were significantly enhanced, and the simultaneous reconstruction of cartilage and subchondral bone was achieved.
The above studies suggest that porous scaffolds have great potential as delivery carriers for KGN in cartilage regeneration. Implantation alone, in combination with NPs or hydrogels are all viable application methods. Incorporation of KGN into the scaffolds could enhance cartilage formation and promote cartilage repair. However, they are more complex to prepare and implant, increasing the cost and invasiveness to the patient, and may be more suitable for long-term repair scenarios, especially cartilage regeneration that requires stable mechanical support in weight-bearing joints.
4. Summary and future perspectives
In conclusion, KGN is a chondrogenic drug with excellent physicochemical properties and can delay and treat cartilage defects through various mechanisms. A variety of methods have been developed for delivering KGN and each of them has its unique advantages. Microspheres-based drug delivery can achieve a sustained release and enhance the longevity of action of KGN. Exosomes-based drug delivery is a novel approach for intra-articular application of KGN, which can promote cartilage generation through direct encapsulation and indirect intervention. In addition, targeting different stages in cartilage repair by combining KGN with other drugs can improve the microenvironment for the action of KGN, or generate hyaline cartilage of better quality. In addition, physical binding or chemical cross-linking of KGN on a variety of scaffolds has created more ideal conditions for the treatment of cartilage defects by tissue engineering. When combined with biomaterials that modify their physicochemical and surface properties, KGN exhibits better performance, resulting in enhanced synergistic effects. Over the past decade, we have witnessed significant advances in the biological functions and applications of KGN, expanding our understanding and advancing its therapeutic potential.
Although the potential of KGN in cartilage repair has been widely recognized, its clinical application still faces several challenges, including metabolic pathways, long-term safety, and potential interactions with other drugs. In order to increase the value of clinical applications of KGN, it is important to develop strategies to address these issues and to promote future developments through collaboration between chemists and clinicians. First, to date, the metabolic pathway of KGN in the body is not completely clear, and if it is rapidly metabolized in the liver and excreted through the kidneys as most drugs are, its bioavailability, as well as its stability and therapeutic efficacy in the body, will inevitably be compromised, and the rapid metabolism of KGN may limit the duration of its action in the cartilage. Seconds, the safety of KGN as a novel cartilage repair drug for long-term use has not been fully clarified. Currently, there are limited long-term toxicological studies on KGN, and potential side effects, especially in chronic use, such as effects on liver or kidney function, have not been adequately studied. Furthermore, the potential drug interactions of KGN have not been fully investigated, especially when multiple medications (e.g., anti-inflammatory drugs, pain relievers) are used to treat arthritis or other concomitant conditions, KGN may interact with these medications, affecting their efficacy or triggering adverse reactions. For example, non-steroidal anti-inflammatory drugs (NSAIDs) may reduce the efficacy of KGN by competitively inhibiting the same metabolic pathways. To address these challenges, chemists play an important role in the optimization of KGN and the development of delivery systems. First, chemists can help develop more rational dosing regimens and reduce possible side effects by studying the metabolic pathways and pharmacokinetic properties of KGN in the body. Seconds, chemists should conduct rigorous clinical trials in conjunction with clinicians to progressively assess the safety of KGN and closely monitor liver and kidney function and other potential toxic reactions. Furthermore, before using KGN in the clinic, drug interaction studies should be carried out in vitro and in animal models to identify possible interaction risks, as well as to explore the synergistic effects of KGN with other pro-cartilage repair drugs.
Overall, the clinical application of KGN is promising, but a series of challenges still need to be overcome in practical application. Through the joint efforts of chemists and clinicians to develop effective solutions to these problems, KGN is expected to become one of the core drugs for cartilage repair in the future.
Data availability
Data sharing is not applicable to this article as no new data were created or analyzed in this study.
Author contributions
Xuemiao Liu and Pengfei Liu: writing – original draft preparation, collecting information, methodology. Han Li: collecting information, methodology. Ying Cen, Guichun Jiang and Weiguo Zhang: conceptualization, collecting information, revising draft. Kang Tian and Xing Wang: revising draft, and finalizing the manuscript.
Conflicts of interest
The author declares no conflict of interest, financial or otherwise.
Acknowledgments
This research was funded by National Natural Science Foundation of China (52373162, 51973226 and 81601901), Natural Science Foundation of Liaoning China (2019-MS-079), Peak Climbing Program Dalian (2022DF012) and Dalian Science and Technology Innovation Fund (2023JJ13SN051).
References
- Moradi M. Parvizpour F. Arabpour Z. Zargarzadeh N. Nazari M. Rashnavadi H. Sefat F. Dehghani S. Latifi M. Jafarian A. Curr. Stem Cell Res. Ther. 2024;19:653–661. doi: 10.2174/1574888X18666230418121122. [DOI] [PubMed] [Google Scholar]
- Jang S. Lee K. Ju J. H. Int. J. Mol. Sci. 2021;22:2619. doi: 10.3390/ijms22052619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia Z. Wang S. Liang Y. Liu Q. Am. J. Transl. Res. 2019;11:2056–2069. [PMC free article] [PubMed] [Google Scholar]
- Chen C.-H. Kao H.-H. Lee Y.-C. Chen J.-P. Pharmaceuticals. 2023;16:1293. doi: 10.3390/ph16091293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graceffa V. Vinatier C. Guicheux J. Stoddart M. Alini M. Zeugolis D. I. Biomaterials. 2019;192:199–225. doi: 10.1016/j.biomaterials.2018.11.014. [DOI] [PubMed] [Google Scholar]
- Fernández-Garza L. E. Barrera-Barrera S. A. Barrera-Saldaña H. A. Pharmaceuticals. 2023;16:1334. doi: 10.3390/ph16091334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prakash N. Kim J. Jeon J. Kim S. Arai Y. Bello A. B. Park H. Lee S. H. Biomater. Res. 2023;27:31. doi: 10.1186/s40824-023-00371-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du X. Cai L. Xie J. Zhou X. Bone Res. 2023;11:2. doi: 10.1038/s41413-022-00239-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng X. B. Zhang Y. Wang Y. Q. He Q. Yu Q. J. Cell. Biochem. 2019;120:5570–5582. doi: 10.1002/jcb.27841. [DOI] [PubMed] [Google Scholar]
- Józefiak A. Larska M. Pomorska-Mól M. Ruszkowski J. J. Viruses. 2021;13:v13081488. doi: 10.3390/v13081488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson K. Zhu S. Tremblay M. S. Payette J. N. Wang J. Bouchez L. C. Meeusen S. Althage A. Cho C. Y. Wu X. Schultz P. G. Science. 2012;336:717–721. doi: 10.1126/science.1215157. [DOI] [PubMed] [Google Scholar]
- Cai J. Y. Zhang L. Chen J. Chen S. Y. Curr. Med. Sci. 2019;39:16–20. doi: 10.1007/s11596-019-1994-6. [DOI] [PubMed] [Google Scholar]
- Almeida B. Wang Y. Shukla A. Ann. Biomed. Eng. 2020;48:2090–2102. doi: 10.1007/s10439-019-02430-x. [DOI] [PubMed] [Google Scholar]
- Zhu Y. Kong B. Liu R. Zhao Y. Smart Med. 2022;1:e20220006. doi: 10.1002/SMMD.20220006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lei Y. Zhang Q. Kuang G. Wang X. Fan Q. Ye F. Smart Med. 2022;1:e20220014. doi: 10.1002/SMMD.20220014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dana K. P. Tate M. N. Jon P. V. H. Aesthetic Plast. Res. 2014;1:43–50. [Google Scholar]
- Zhang S. Hu P. Liu T. Li Z. Huang Y. Liao J. Hamid M. R. Wen L. Wang T. Mo C. Alini M. Grad S. Wang T. Chen D. Zhou G. Theranostics. 2019;9:7108–7121. doi: 10.7150/thno.38182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayek A. Kerstetter-Fogle A. E. Sachlos E. Bollenbach T. Regen. Med. 2012;7:475. [PubMed] [Google Scholar]
- Chen P. Liao X. Drug Deliv. 2023;30:2254519. doi: 10.1080/10717544.2023.2254519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massaro M. Buscemi G. Arista L. Biddeci G. Cavallaro G. D'Anna F. Di Blasi F. Ferrante A. Lazzara G. Rizzo C. Spinelli G. Ullrich T. Riela S. ACS Med. Chem. Lett. 2019;10:419–424. doi: 10.1021/acsmedchemlett.8b00465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang M.-L. Jeong S.-Y. Im G.-I. Tissue Eng. 2017;23:630–639. doi: 10.1089/ten.tea.2016.0524. [DOI] [PubMed] [Google Scholar]
- Chen Y.-R. Yan X. Yuan F.-Z. Lin L. Wang S.-J. Ye J. Zhang J.-Y. Yang M. Wu D.-C. Wang X. Yu J.-K. Adv. Sci. 2022;9:2105571. doi: 10.1002/advs.202105571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fong E. L. Chan C. K. Goodman S. B. Biomaterials. 2011;32:395–409. doi: 10.1016/j.biomaterials.2010.08.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei J. Shimazu J. Makinistoglu M. P. Maurizi A. Kajimura D. Zong H. Takarada T. Lezaki T. Pessin J. E. Hinoi E. Karsenty G. Cell. 2015;161:1576–1591. doi: 10.1016/j.cell.2015.05.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kita K. Kimura T. Nakamura N. Yoshikawa H. Nakano T. Genes Cells. 2008;13:839–850. doi: 10.1111/j.1365-2443.2008.01209.x. [DOI] [PubMed] [Google Scholar]
- Xu K. He Y. Moqbel S. A. A. Zhou X. Wu L. Bao J. Int. J. Biol. Macromol. 2021;175:351–360. doi: 10.1016/j.ijbiomac.2021.02.029. [DOI] [PubMed] [Google Scholar]
- Sun K. Luo J. Guo J. Yao X. Jing X. Guo F. Osteoarthritis Cartilage. 2020;28:400–409. doi: 10.1016/j.joca.2020.02.027. [DOI] [PubMed] [Google Scholar]
- Li G. Liu S. Chen Y. Zhao J. Xu H. Weng J. Yu F. Xiong A. Udduttula A. Wang D. Liu P. Chen Y. Zeng H. Nat. Commun. 2023;14:3159. doi: 10.1038/s41467-023-38597-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang C. Jiang C. Jin J. Lei P. Cai Y. Wang Y. Mater. Today Bio. 2023;23:100819. doi: 10.1016/j.mtbio.2023.100819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abula K. Muneta T. Miyatake K. Yamada J. Matsukura Y. Inoue M. Sekiya I. Graf D. Economides A. N. Rosen V. Tsuji K. FEBS Lett. 2015;589:1240–1248. doi: 10.1016/j.febslet.2015.04.004. [DOI] [PubMed] [Google Scholar]
- Lee P. T. Li W. J. J. Cell. Biochem. 2017;118:172–181. doi: 10.1002/jcb.25623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Q. Zhang J. H. Yuan S. Shao J. H. Cai Z. Y. Chen S. Cao J. Wu H. S. Qian Q. R. Med. Sci. Mon. 2019;25:4960–4967. doi: 10.12659/MSM.916696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu N. Q. Lin Y. Li L. Lu J. Geng D. Zhang J. Jashashvili T. Buser Z. Magallanes J. Tassey J. Shkhyan R. Sarkar A. Lopez N. Lee S. Lee Y. Wang L. Petrigliano F. A. Van Handel B. Lyons K. Evseenko D. Commun. Biol. 2022;5:64. doi: 10.1038/s42003-021-02944-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang T. Chen T. Qiu J. Gao W. Qiu X. Zhu Y. Wang X. Chen Y. Zhou H. Deng Z. Li P. Xu C. Peng Y. Liang A. Su P. Gao B. Huang D. Cell Death Dis. 2021;12:886. doi: 10.1038/s41419-021-04170-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamagata K. Nakayamada S. Zhang T. Zhang X. Tanaka Y. Clin. Exp. Rheumatol. 2020;38:670–679. [PubMed] [Google Scholar]
- Liu T. Li X. Wang T. Chen X. Zhang S. Liao J. Wang W. Zou X. Zhou G. Biochem. Biophys. Res. Commun. 2020;532:385–392. doi: 10.1016/j.bbrc.2020.08.059. [DOI] [PubMed] [Google Scholar]
- Sun K. Guo J. Guo Z. Hou L. Liu H. Hou Y. He J. Guo F. Ye Y. Ageing Res. Rev. 2023;90:102015. doi: 10.1016/j.arr.2023.102015. [DOI] [PubMed] [Google Scholar]
- Li M. Zhang F. J. Bai R. J. J. Inflamm. Res. 2024;17:1105–1120. doi: 10.2147/JIR.S444758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang S. Zhang B. Liao Z. Chen Y. Guo W. Wu J. Liu H. Weng R. Su D. Chen G. Zhang Z. Li C. Long J. Xiao Y. Ma Y. Zhou T. Xu C. Su P. Mol. Ther. 2024;32:1461–1478. doi: 10.1016/j.ymthe.2024.02.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jing H. Zhang X. Luo K. Luo Q. Yin M. Wang W. Zhu Z. Zheng J. He X. Biomaterials. 2020;231:119682. doi: 10.1016/j.biomaterials.2019.119682. [DOI] [PubMed] [Google Scholar]
- Ge H. X. Zou F. M. Li Y. Liu A. M. Tu M. J. Recept. Signal Transduct. Res. 2017;37:431–436. doi: 10.1080/10799893.2017.1360353. [DOI] [PubMed] [Google Scholar]
- Wu Q. Zhu M. Rosier R. N. Zuscik M. J. O'Keefe R. J. Chen D. Ann. N. Y. Acad. Sci. 2010;1192:344–350. doi: 10.1111/j.1749-6632.2009.05212.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jing H. Zhang X. Gao M. Luo K. Fu W. Yin M. Wang W. Zhu Z. Zheng J. He X. Faseb. J. 2019;33:5641–5653. doi: 10.1096/fj.201802137RRR. [DOI] [PubMed] [Google Scholar]
- Wang H. Zhang Y. Zhang C. Zhao Y. Shu J. Tang X. Front. Immunol. 2024;15:1361606. doi: 10.3389/fimmu.2024.1361606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skrzypa M. Szala D. Gablo N. Czech J. Pajak J. Kopanska M. Trzeciak M. Gargasz K. Snela S. Zawlik I. Pol. Przegl. Chir. 2019;91:1–5. doi: 10.5604/01.3001.0013.0135. [DOI] [PubMed] [Google Scholar]
- Miranda-Duarte A. Borgonio-Cuadra V. M. González-Huerta N. C. Rojas-Toledo E. X. Ahumada-Pérez J. F. Morales-Hernández E. Pérez-Hernández N. Rodríguez-Pérez J. M. Vargas-Alarcón G. Mol. Biol. Rep. 2021;48:1549–1557. doi: 10.1007/s11033-021-06207-1. [DOI] [PubMed] [Google Scholar]
- Liu J. N. Lu S. Fu C. M. J. Orthop. Surg. Res. 2022;17:148. doi: 10.1186/s13018-022-02989-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou M. Zhang Y. Zhou X. Liu T. Yang H. Chen X. He F. Zhu X. Cell Death Dis. 2021;12:483. doi: 10.1038/s41419-021-03765-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y. Zhao X. Zhang R. Huang Y. Li Y. Shan M. Zhong X. Xing Y. Wang M. Zhang Y. Zhao Y. Front. Bioeng. Biotechnol. 2020;8:600103. doi: 10.3389/fbioe.2020.600103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurgul S. J. Moreira A. Xiao Y. Varma S. N. Liu C. Costa P. F. Williams G. R. Polymers. 2023;15:1275. doi: 10.3390/polym15051275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elder S. Roberson J. G. Warren J. Lawson R. Young D. Stokes S. Ross M. K. Molecules. 2022;27:3739. doi: 10.3390/molecules27123739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W. Chen R. Xu X. Zhu L. Liu Y. Yu X. Tang G. Front. Pharmacol. 2022;13:922032. doi: 10.3389/fphar.2022.922032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu X. Shi D. Shen Y. Xu Z. Dai J. Chen D. Teng H. Jiang Q. Arthritis Res. Ther. 2015;17:20. doi: 10.1186/s13075-015-0537-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang T. Xie L. Zhang R. T. Tian W. D. Chin. J. Dent. Res. 2022;25:29–36. doi: 10.3290/j.cjdr.b2752709. [DOI] [PubMed] [Google Scholar]
- Chen C. Huang K. Zhu J. Bi Y. Wang L. Jiang J. Zhu T. Yan X. Zhao J. J. Mater. Chem. B. 2020;8:4106–4121. doi: 10.1039/c9tb02754h. [DOI] [PubMed] [Google Scholar]
- Su Y. Zhang B. Sun R. Liu W. Zhu Q. Zhang X. Wang R. Chen C. Drug Deliv. 2021;28:1397–1418. doi: 10.1080/10717544.2021.1938756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rocha C. V. Gonçalves V. da Silva M. C. Bañobre-López M. Gallo J. Int. J. Mol. Sci. 2022;23:2034. doi: 10.3390/ijms23042034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo X. Zuo X. Zhou Z. Gu Y. Zheng H. Wang X. Wang G. Xu C. Wang F. Int. J. Mol. Sci. 2023;24:4333. doi: 10.3390/ijms24054333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zare P. Pezeshki-Modaress M. Davachi S. M. Zare P. Yazdian F. Simorgh S. Ghanbari H. Rashedi H. Bagher Z. Carbohydr. Polym. 2021;266:118123. doi: 10.1016/j.carbpol.2021.118123. [DOI] [PubMed] [Google Scholar]
- Zhou Q. Cai Y. Jiang Y. Lin X. Int. J. Biol. Sci. 2020;16:1811–1820. doi: 10.7150/ijbs.41637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu X. Liang Y. Li X. Ouyang K. Wang M. Cao T. Li W. Liu J. Xiong J. Li B. Xia J. Wang D. Duan L. Biomaterials. 2021;269:120539. doi: 10.1016/j.biomaterials.2020.120539. [DOI] [PubMed] [Google Scholar]
- Zhao Y. Zhao X. Zhang R. Huang Y. Li Y. Shan M. Zhong X. Xing Y. Wang M. Zhang Y. Zhao Y. Front. Bioeng. Biotechnol. 2020;8:600103. doi: 10.3389/fbioe.2020.600103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almajidi Y. Q. Gupta J. Sheri F. S. Zabibah R. S. Faisal A. Ruzibayev A. Adil M. Saadh M. J. Jawad M. J. Alsaikhan F. Narmani A. Farhood B. Int. J. Biol. Macromol. 2023;253:127278. doi: 10.1016/j.ijbiomac.2023.127278. [DOI] [PubMed] [Google Scholar]
- Bai L. Han Q. Han Z. Zhang X. Zhao J. Ruan H. Wang J. Lin F. Cui W. Yang X. Hao Y. Adv. Healthc. Mater. 2024;13:e2302327. doi: 10.1002/adhm.202302327. [DOI] [PubMed] [Google Scholar]
- Yao Y. Wei G. Deng L. Cui W. Adv. Sci. 2023;10:e2207438. doi: 10.1002/advs.202207438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wróblewska M. Winnicka K. Int. J. Mol. Sci. 2015;16:20277–20289. doi: 10.3390/ijms160920277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu Q. Ding B. Yan X. Peng L. Duan J. Yang S. Cheng L. Chen D. Nanomedicine. 2017;13:2189–2198. doi: 10.1016/j.nano.2017.05.011. [DOI] [PubMed] [Google Scholar]
- Zhang H. Huang J. Alahdal M. Biomed. Pharmacother. 2023;168:115715. doi: 10.1016/j.biopha.2023.115715. [DOI] [PubMed] [Google Scholar]
- Zou Z. Li H. Xu G. Hu Y. Zhang W. Tian K. Int. J. Nanomed. 2023;18:4751–4778. doi: 10.2147/IJN.S417422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang M. L. Ko J. Y. Kim J. E. Im G. I. Biomaterials. 2014;35:9984–9994. doi: 10.1016/j.biomaterials.2014.08.042. [DOI] [PubMed] [Google Scholar]
- Shao J. Zhu J. Chen Y. Fu Q. Li L. Ding Z. Wu J. Han Y. Li H. Qian Q. Zhou Y. Stem Cell. Int. 2021;2021:6624874. doi: 10.1155/2021/6624874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu C. Li Y. Yang Z. Zhou Z. Lou Z. Zhang Q. Nanomedicine. 2020;15:273–288. doi: 10.2217/nnm-2019-0208. [DOI] [PubMed] [Google Scholar]
- Xie A. Xue J. Wang Y. Yang C. Xu M. Jiang Y. Dis. Markers. 2022;2022:6943630. doi: 10.1155/2022/6943630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson D. D. Chubinskaya S. Guilak F. Martin J. A. Oegema T. R. Olson S. A. Buckwalter J. A. J. Orthop. Res. 2011;29:802–809. doi: 10.1002/jor.21359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao T. Li X. Li H. Deng H. Li J. Yang Z. He S. Jiang S. Sui X. Guo Q. Liu S. Acta Pharm. Sin. B. 2023;13:4127–4148. doi: 10.1016/j.apsb.2022.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhijie M. Song W. He D. Zhang X. He Y. Li H. Adv. Funct. Mater. 2022;32:2113380. [Google Scholar]
- Kang M. L. Kim J. E. Im G. I. Acta Biomater. 2016;39:65–78. doi: 10.1016/j.actbio.2016.05.005. [DOI] [PubMed] [Google Scholar]
- Yang Y. Zhao X. Wang S. Zhang Y. Yang A. Cheng Y. Chen X. Nat. Commun. 2023;14:7771. doi: 10.1038/s41467-023-43334-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W. Ling C. Zhang A. Liu H. Jiang Y. Li X. Sheng R. Yao Q. Chen J. Bioact. Mater. 2020;5:832–843. doi: 10.1016/j.bioactmat.2020.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J. Li J. Lin S. Wu T. Huang H. Zhang K. Sun Y. Yeung K. W. K. Li G. Bian L. Adv. Funct. Mater. 2016;26:2463–2472. [Google Scholar]
- Csaki C. Mobasheri A. Shakibaei M. Arthritis Res. Ther. 2009;11:R165. doi: 10.1186/ar2850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asgari N. Bagheri F. Eslaminejad M. B. Ghanian M. H. Sayahpour F. A. Ghafari A. M. Stem Cell Res. Ther. 2020;11:289. doi: 10.1186/s13287-020-01797-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W. Ouyang H. Dass C. R. Xu J. Bone Res. 2016;4:15040. doi: 10.1038/boneres.2015.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Z. Li H. Yuan Z. Fu L. Jiang S. Gao C. Wang F. Zha K. Tian G. Sun Z. Huang B. Wei F. Cao F. Sui X. Peng J. Lu S. Guo W. Liu S. Guo Q. Acta Biomater. 2020;114:31–52. doi: 10.1016/j.actbio.2020.07.008. [DOI] [PubMed] [Google Scholar]
- Pacelli S. Basu S. Whitlow J. Chakravarti A. Acosta F. Varshney A. Modaresi S. Berkland C. Paul A. Adv. Drug Deliv. Rev. 2017;120:50–70. doi: 10.1016/j.addr.2017.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen X. Zhang Y. Gu Y. Xu Y. Liu Y. Li B. Chen L. Biomaterials. 2016;106:205–216. doi: 10.1016/j.biomaterials.2016.08.023. [DOI] [PubMed] [Google Scholar]
- Dai W. Liu Q. Li S. Gao Y. Feng C. Guo L. Xiao Y. Lin H. Fan Y. Zhang X. J. Mater. Chem. B. 2023;11:4050–4064. doi: 10.1039/d3tb00105a. [DOI] [PubMed] [Google Scholar]
- Zhang W. Chen J. Zhang S. Ouyang H. W. Arthritis Res. Ther. 2012;14:221. doi: 10.1186/ar4025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orth P. Gao L. Madry H. Knee Surg. Sports Traumatol. Arthrosc. 2020;28:670–706. doi: 10.1007/s00167-019-05359-9. [DOI] [PubMed] [Google Scholar]
- Ding M. Lu Y. Abbassi S. Li F. Li X. Song Y. Geoffroy V. Im H. J. Zheng Q. J. Cell. Physiol. 2012;227:3446–3456. doi: 10.1002/jcp.24045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeon S. Y. Park J. S. Yang H. N. Lim H. J. Yi S. W. Park H. Park K. H. Biomaterials. 2014;35:8236–8248. doi: 10.1016/j.biomaterials.2014.05.092. [DOI] [PubMed] [Google Scholar]
- Murphy M. P. Koepke L. S. Lopez M. T. Tong X. Ambrosi T. H. Gulati G. S. Marecic O. Wang Y. Ransom R. C. Hoover M. Y. Steininger H. Zhao L. Walkiewicz M. P. Quarto N. Levi B. Wan D. C. Weissman I. L. Goodman S. B. Yang F. Longaker M. T. Chan C. K. F. Nat. Med. 2020;26:1583–1592. doi: 10.1038/s41591-020-1013-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao Y. Zhang X. Zhou H. Pharmaceutics. 2023;15:2405. doi: 10.3390/pharmaceutics15102405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X. Ding J. Zhang Z. Yang M. Yu J. Wang J. Chang F. Chen X. ACS Appl. Mater. Interfaces. 2016;8:5148–5159. doi: 10.1021/acsami.5b12212. [DOI] [PubMed] [Google Scholar]
- Wang S. J. Qin J. Z. Zhang T. E. Xia C. Front. Chem. 2019;7:677. doi: 10.3389/fchem.2019.00677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S. S. Choi G. E. Lee H. J. Kim Y. Choy J. H. Jeong B. ACS Appl. Mater. Interfaces. 2017;9:42668–42675. doi: 10.1021/acsami.7b17173. [DOI] [PubMed] [Google Scholar]
- Berdimurodov E. Eliboyev I. Berdimuradov K. Kholikov A. Akbarov K. Dagdag O. Rbaa M. El Ibrahimi B. Verma D. K. Haldhar R. Arrousse N. Carbohydr. Polym. 2022;292:119719. doi: 10.1016/j.carbpol.2022.119719. [DOI] [PubMed] [Google Scholar]
- Yuan X. Wan J. Yang Y. Huang L. Zhou C. Su J. Hua S. Pu H. Zou Y. Zhu H. Jiang X. Xiao J. Carbohydr. Polym. 2023;304:120492. doi: 10.1016/j.carbpol.2022.120492. [DOI] [PubMed] [Google Scholar]
- Xu J. Feng Q. Lin S. Yuan W. Li R. Li J. Wei K. Chen X. Zhang K. Yang Y. Wu T. Wang B. Zhu M. Guo R. Li G. Bian L. Biomaterials. 2019;210:51–61. doi: 10.1016/j.biomaterials.2019.04.031. [DOI] [PubMed] [Google Scholar]
- Fan W. Li J. Yuan L. Chen J. Wang Z. Wang Y. Guo C. Mo X. Yan Z. Drug Deliv. 2018;25:1004–1012. doi: 10.1080/10717544.2018.1461279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X. Chen Y. Mao A. S. Xuan C. Wang Z. Gao H. An G. Zhu Y. Shi X. Mao C. Biomaterials. 2020;232:119644. doi: 10.1016/j.biomaterials.2019.119644. [DOI] [PubMed] [Google Scholar]
- Chen C. Huang S. Chen Z. Liu Q. Cai Y. Mei Y. Xu Y. Guo R. Yan C. Bioeng. Transl. Med. 2023;8:e10364. doi: 10.1002/btm2.10364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fallahi H. Daemi H. Bagheri F. Baghaban Eslaminejad M. Biomed. Mater. 2022;17:065002. doi: 10.1088/1748-605X/ac8bbd. [DOI] [PubMed] [Google Scholar]
- Ding W. Zhou J. Zeng Y. Wang Y. N. Shi B. Carbohydr. Polym. 2017;157:1650–1656. doi: 10.1016/j.carbpol.2016.11.045. [DOI] [PubMed] [Google Scholar]
- Li C. Liu Y. Weng T. Yang M. Wang X. Chai W. Pharmaceutics. 2023;15:1949. doi: 10.3390/pharmaceutics15071949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dehghan-Baniani D. Chen Y. Wang D. Bagheri R. Solouk A. Wu H. Colloids Surf., B. 2020;192:111059. doi: 10.1016/j.colsurfb.2020.111059. [DOI] [PubMed] [Google Scholar]
- Gao W. Zhang Y. Zhang Q. Zhang L. Ann. Biomed. Eng. 2016;44:2049–2061. doi: 10.1007/s10439-016-1583-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zare P. Pezeshki-Modaress M. Davachi S. M. Zare P. Yazdian F. Simorgh S. Ghanbari H. Rashedi H. Bagher Z. Carbohydr. Polym. 2021;266:118123. doi: 10.1016/j.carbpol.2021.118123. [DOI] [PubMed] [Google Scholar]
- Yan W. Xu X. Xu Q. Sun Z. Lv Z. Wu R. Yan W. Jiang Q. Shi D. Am. J. Sports Med. 2020;48:3233–3244. doi: 10.1177/0363546520957346. [DOI] [PubMed] [Google Scholar]
- Fan W. Yuan L. Li J. Wang Z. Chen J. Guo C. Mo X. Yan Z. Mater. Sci. Eng., C. 2020;110:110705. doi: 10.1016/j.msec.2020.110705. [DOI] [PubMed] [Google Scholar]
- Lin T.-H. Wang H.-C. Cheng W.-H. Hsu H.-C. Yeh M.-L. Int. J. Mol. Sci. 2019;20:326. doi: 10.3390/ijms20020326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y. Zhu Z. S. Guan J. Wu S. J. Acta Biomater. 2021;125:57–71. doi: 10.1016/j.actbio.2021.02.018. [DOI] [PubMed] [Google Scholar]
- Kim S. H. Yeon Y. K. Lee J. M. Chao J. R. Lee Y. J. Seo Y. B. Sultan M. T. Lee O. J. Lee J. S. Yoon S. I. Hong I. S. Khang G. Lee S. J. Yoo J. J. Park C. H. Nat. Commun. 2018;9:1620. doi: 10.1038/s41467-018-03759-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahya S. Ai J. Shojae S. Khonakdar H. A. Darbemamieh G. Shirian S. Int. J. Biol. Macromol. 2021;182:82–90. doi: 10.1016/j.ijbiomac.2021.03.106. [DOI] [PubMed] [Google Scholar]
- Jiang W. Xiang X. Song M. Shen J. Shi Z. Huang W. Liu H. Mater. Today Bio. 2022;17:100485. doi: 10.1016/j.mtbio.2022.100485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brinkhof S. Nizak R. Sim S. Khlebnikov V. Quenneville E. Garon M. Klomp D. W. J. Saris D. NMR Biomed. 2021;34:e4463. doi: 10.1002/nbm.4463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong Y. Han Y. Wu J. Zhao X. Cheng J. Gao G. Qian Q. Wang X. Cai W. Zreiqat H. Feng D. Xu J. Cui D. Theranostics. 2020;10:5565–5577. doi: 10.7150/thno.43569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang W. Zhu P. Huang H. Zheng Y. Liu J. Feng L. Guo H. Tang S. Guo R. ACS Appl. Mater. Interfaces. 2019;11:34744–34754. doi: 10.1021/acsami.9b12288. [DOI] [PubMed] [Google Scholar]
- Xiao M. Li Y. Zhao J. Wang Z. Gao M. Gianneschi N. C. Dhinojwala A. Shawkey M. D. Chem. Mater. 2016;28:5516–5521. [Google Scholar]
- Jiang Z. Zhang Z. Li S. Lin S. Yuan H. Int. J. Nanomed. 2022;17:5511–5524. doi: 10.2147/IJN.S381815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahapatra C. Kim J. J. Lee J. H. Jin G. Z. Knowles J. C. Kim H. W. J. Tissue Eng. 2019;10:2041731419826433. doi: 10.1177/2041731419826433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xuan H. Hu H. Geng C. Song J. Shen Y. Lei D. Guan Q. Zhao S. You Z. Acta Biomater. 2020;105:97–110. doi: 10.1016/j.actbio.2020.01.015. [DOI] [PubMed] [Google Scholar]
- Sun X. Wang J. Wang Y. Zhang Q. Artif. Cells, Nanomed. Biotechnol. 2018;46:1957–1966. doi: 10.1080/21691401.2017.1397000. [DOI] [PubMed] [Google Scholar]
- Wang J. Wang Y. Sun X. Liu D. Huang C. Wu J. Yang C. Zhang Q. Artif. Cells, Nanomed. Biotechnol. 2019;47:1710–1721. doi: 10.1080/21691401.2019.1607866. [DOI] [PubMed] [Google Scholar]
- Hong Y. Liu N. Zhou R. Zhao X. Han Y. Xia F. Cheng J. Duan M. Qian Q. Wang X. Cai W. Zreiqat H. Feng D. Xu J. Cui D. ACS Biomater. Sci. Eng. 2020;6:6276–6284. doi: 10.1021/acsbiomaterials.0c00724. [DOI] [PubMed] [Google Scholar]
- Zhang P. Chen J. Sun Y. Cao Z. Zhang Y. Mo Q. Yao Q. Zhang W. J. Mater. Chem. B. 2023;11:1240–1261. doi: 10.1039/d2tb02203f. [DOI] [PubMed] [Google Scholar]
- Liu X. Wei Y. Xuan C. Liu L. Lai C. Chai M. Zhang Z. Wang L. Shi X. Adv. Healthc. Mater. 2020:e2000076. doi: 10.1002/adhm.202000076. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable to this article as no new data were created or analyzed in this study.