Abstract
Clostridium perfringens bacteremia is a rare but rapidly fatal condition, especially in patients exhibiting massive intravascular hemolysis (MIH), gas gangrene, and septic shock. Herein, we present an autopsy case of C. perfringens septicemia exhibiting MIH, gas gangrene, and cytokine storm. The patient was an 84-year-old female with a history of biliary reconstruction surgery for congenital biliary dilatation. She developed MIH, elevated inflammatory mediator levels, thrombocytopenia, and coagulopathy. She went into shock within 1 h of the presentation and died within a few hours. Rapid progression was associated with the transformation of liver abscesses into gas-filled abscesses on computed tomography scan, suggesting the rapid outgrowth of gas-producing bacteria. The patient was finally diagnosed with MIH and gas gangrene due to C. perfringens infection based on the presence of this bacterium in the blood and bile. On autopsy, gas gangrene was observed in almost all organs, originating from the bile duct. Polymerase chain reactions targeting C. perfringens toxins identified the isolated bacterium as C. perfringens type A expressing α-toxin (CPA), perfringolysin O (PFO), and collagenase (ColA). Elevated interleukin 6 and tumor necrosis factor-α expression levels were observed in the serum, and such proinflammatory responses were partially mediated by Toll-like receptor 2. This study elucidated the association between the toxin profiles of clinically isolated C. perfringens and the host cytokine responses in the patient.
Keywords: Clostridium perfringens, Gas gangrene, Intravascular hemolysis, Liver abscess, Cytokine storm
Introduction
Clostridium perfringens is a Gram-positive anaerobic bacterium that colonizes the gastrointestinal tract, soil, and foods [1], [2], [3]. It can survive in harsh environments such as high temperatures, low nutrient levels, and the presence of oxygen by forming spores [1], [2], [3]. The widespread distribution of C. perfringens is associated with various infections and diseases, including foodborne gastroenteritis, gas gangrene, hepatobiliary infections, and massive intravascular hemolysis (MIH) [1], [2], [3]. Although most cases of foodborne gastroenteritis caused by C. perfringens are mild and self-limiting, those with C. perfringens bacteremia have a poor prognosis [1], [2], [3]. MIH, gas gangrene, and cytokine storm are considered to contribute to the lethal outcome of C. perfringens bacteremia [1], [2], [3]. Another critical aspect of C. perfringens bacteremia is its exceptionally rapid disease progression, which is attributed to an extremely rapid doubling time of < 10 min [4]. MIH and septic shock caused by this bacterium can lead to death within few hours of diagnosis [1], [5], [6]. However, no specific treatment other than antibiotics for lethal C. perfringens infections has been established because the immunopathogenesis of this disorder has not been fully elucidated.
The molecular mechanisms underlying host-C. perfringens interactions have been poorly understood. C. perfringens secretes a broad range of toxins that can destroy host organ architecture and active host immune systems [1], [2], [3]. Previous studies on the pathogenesis of C. perfringens bacteremia have mainly utilized in vitro culture systems where host immune cells are exposed to recombinant toxins and/or C. perfringens strains. Although these studies have provided insights into individual properties of each toxin, the association between clinical C. perfringens isolates and disease manifestations or immune responses remains unclear. Herein, we have presented an autopsy case of C. perfringens bacteremia exhibiting gas gangrene, MIH, and cytokine storm. Polymerase chain reactions (PCRs) targeting toxins identified the expression of C. perfringens α (CPA) toxin, perfringolysin O (PFO), and collagenase (ColA) in the isolated strain. In addition, immunological analyses revealed that the presence of CPA+PFO+ColA+ in C. perfringens type A contributed to the cytokine storm partially through Toll-like receptor 2 (TLR2) signaling. This study sheds light on the relationship between toxin profiles and host cytokine responses in lethal C. perfringens infections.
Case presentation
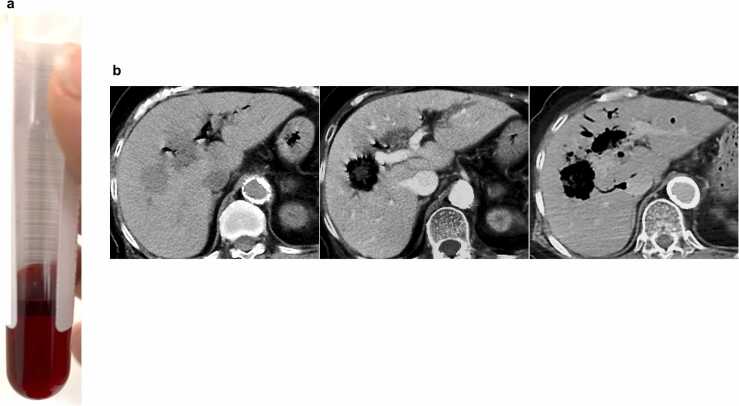
An 84-year-old Japanese female was brought to our emergency room with severe back pain and low-grade fever. She had a history of noninsulin-dependent diabetes mellitus (DM) treated with hypoglycemic agents for 20 years. Her DM was well controlled, with a recent hemoglobin A1c level of 6.6 %. She underwent biliary reconstruction surgery for congenital biliary dilatation 40 years ago. On physical examination, she appeared unwell with tachypnea but had a normal level of consciousness. Initial vital signs were blood pressure of 151/73 mmHg, pulse rate of 98 beats/min, respiratory rate of 30 breaths/min, axillary temperature of 36.9 °C, and oxygen saturation of 97 % on ambient air. Blood tests revealed an abnormally bright red color of the serum, indicating intravascular hemolysis [7] (Fig. 1a). The presence of MIH was confirmed by a marked decrease in hemoglobin levels (1.8 g/dL) and an increase in serum lactate dehydrogenase levels (6691 U/L) (Table 1). The patient also had leukocytosis, elevated C-reactive protein and procalcitonin levels, severe thrombocytopenia, coagulopathy with elevated D-dimer levels, and metabolic acidosis with a base excess of −9.8 mmol/L (pH; 7.382, PaCO2; 26.4 mmHg). These laboratory findings indicated septicemia with MIH, necessitating immediate vigorous fluid resuscitation.
Fig. 1.
Patient with lethal Clostridium perfringens infection with massive intravascular hemolysis and gas gangrene. (a) Serum of the patient at presentation. (b) Abdominal computed tomography (CT) images obtained at presentation (left panel), 1.5 h (middle panel), and 2.5 h after death (right panel). The lesion in the right lobe of the liver, initially seen as a low-density area at presentation (left panel), was replaced by a gas-filled cavity 1.5 h later (middle panel). A postmortem CT scan revealed rapid and massive expansion of gas-filled cavities in the right and left lobes of the liver (right panel).
Table 1.
Laboratory findings on admission.
| Laboratory tesr | Presentation | Normal range |
|---|---|---|
| Hematology | ||
| White blood cells (μL) | 20490 | 3300–8600 |
| Neutrophil (%) | 83.9 | 38–77 |
| Monocyte (%) | 0.5 | 2.7–9.3 |
| Lymphocyte (%) | 14.8 | 20.2–53.2 |
| Red blood cells (μL) | 197 × 104 | 386 × 104–492 × 104 |
| Hemoglobin (g/dL) | 1.8 | 11.6–14.8 |
| Platelets (μL) | 4.6 × 104 | 15.8 × 104–34.8 × 104 |
| Biochemistry | ||
| Total protein (g/dL) | 8.5 | 6.6–8.1 |
| Albumin (g/dL) | 2.9 | 4.1–5.1 |
| Aspartate aminotransferase (U/L) | 716 | 13–30 |
| Alanine aminotransferase (U/L) | 205 | 7–23 |
| Lactate dehydrogenase (U/L) | 6691 | 124–222 |
| Alkaline phosphatase (U/L) | 196 | 38–113 |
| γ-glutamyl transpeptidase (U/L) | 22 | 9–32 |
| Total bilirubin (mg/dL) | 2.4 | 0.4–1.5 |
| Amylase (U/L) | 36 | 44–132 |
| Blood urea nitrogen (mg/dL) | 43 | 8–20 |
| Creatinine (mg/dL) | 1.26 | 0.46–0.79 |
| C-reactive protein (mg/dL) | 8.78 | 0–0.14 |
| Lactic acid (mg/dL) | 54.5 | 4–16 |
| Procalcitonin (ng/mL) | 6.37 | 0–0.5 |
| Hemoglobin A1c (%) | 6.6 | 4.6–6.2 |
| Coagulation | ||
| PT-INR | 1.70 | |
| APTT (sec) | 33.2 | 24–39 |
| D-dimer (μg/mL) | 7.0 | 0–1 |
| Fibrin degradation products (μg/mL) | 20.5 | 0–5 |
Computed tomography (CT) scan of the abdomen revealed low-density areas (LDAs) in the right lobe of the liver along with pneumobilia (Fig. 1b, left panel), suggesting liver abscesses associated with post-choledochojejunostomy reflux cholangitis. While awaiting blood test results, the patient experienced increasing back pain and restlessness. A contrast-enhanced CT scan performed 1.5 h after the initial plain CT scan clearly showed the transformation of the initial LDAs into gas-filled cavities (Fig. 1b, middle panel), confirming the diagnosis of septicemia due to a rapidly progressive gas-producing liver abscess. Empiric antibiotic treatment (meropenem 0.5 g) was initiated after two sets of blood cultures were obtained. Despite intensive treatment, the patient’s condition deteriorated rapidly, leading to cardiopulmonary arrest and death within 2.5 h of presentation to the emergency room. Postmortem CT imaging revealed massive expansion of gas-producing abscesses in the liver (Fig. 1b, right panel). Due to the unexpected death and rapid progression of the disease, the patient’s family consented to an autopsy, which was performed 7 h after death.
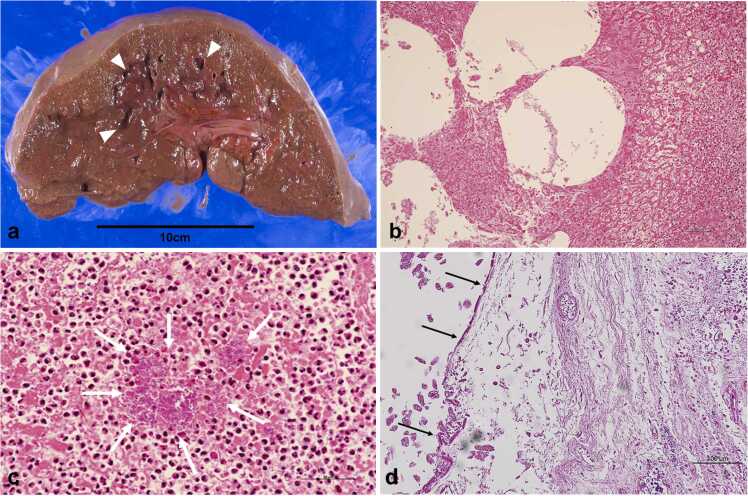
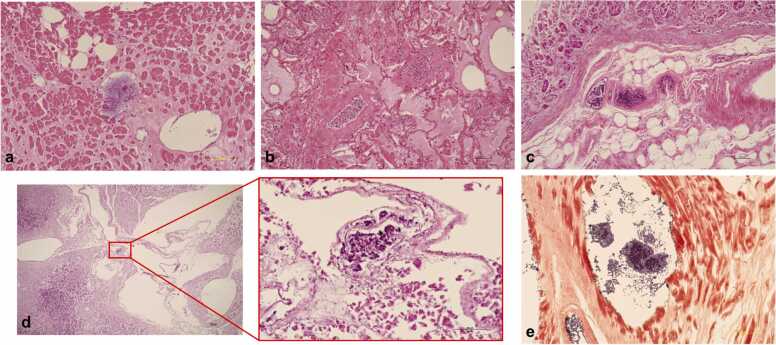
Macroscopic examination upon performing autopsy revealed liver abscesses with a spongiform appearance in both lobes (Fig. 2a). Microscopically, massive hepatocyte necrosis with gas bubble formations, indicative of gas gangrene, was observed (Fig. 2b). Neutrophil infiltration, coagulation necrosis, and numerous bacilli colonies were observed within the liver abscess (Fig. 2c). Due to severe necrosis and autolysis, biliary epithelial cells were not found around the choledochojejunostomy site (Fig. 2d). Gas gangrene was not confined to the liver, as other organs like the heart (Fig. 3a), lung (Fig. 3b), stomach (Fig. 3c), pancreas (Fig. 3d), small intestine, colon, kidneys, adrenal glands, and thyroid gland were also affected. In particular, the stomach exhibited severe emphysematous changes with a spongiform appearance, and colonies of bacilli were found in the interstitium and vascular spaces of all affected organs (Fig. 3a-d). Gram staining of the vacuolated areas of the heart revealed the proliferation of oblong-shaped Gram-positive bacilli (Fig. 3e). Based on the autopsy findings, the present case was finally diagnosed as multiple organ failure due to gas gangrene caused by a Gram-positive bacillus infection. Although C. perfringens and Edwardsiella tarda were detected in the blood and bile, C. perfringens was identified as the likely causative bacterium based on the presence of numerous Gram-positive bacilli in the affected organs. In particular, the severe destruction at the choledochojejunostomy site and the detection of C. perfringens in the bile support the hypothesis of retrograde bile duct infection with this bacterium leading to multiple liver abscesses, septicemia, and multiorgan gas gangrene.
Fig. 2.
Pathological findings of the liver and bile duct on autopsy. (a) Gross appearance of the liver showing abscess formation with an internal spongiform appearance on the cut surface (arrowheads). (b, c) Microphotographs of the liver (b. low magnification image, scale bar: 100 µm, c. high magnification image, scale bar: 50 µm) showing massive necrosis of hepatocytes with gas bubble formations during hematoxylin and eosin staining. A marked proliferation of bacilli was observed in the liver abscess (arrows). (d) Microphotograph of the choledochojejunostomy site (hematoxylin-eosin stain, scale bar: 100 µm). Biliary epithelial cells could not be found around the choledochojejunostomy site due to severe necrosis and autolysis. Arrows indicate destruction of the bile duct epithelium.
Fig. 3.
Microphotographs of organs affected by gas gangrene. Extensive necrosis and vacuolation with a massive proliferation of bacilli are observed in the heart (a), lung (b), stomach (c), and pancreas (d) (hematoxylin and eosin staining, scale bar: 100 µm). Gram staining of the vacuolated areas of the heart revealed the proliferation and accumulation of oblong-shaped Gram-positive bacilli (e).
The clinical course of this case was typical of C. perfringens bacteremia, as the patient developed MIH, septic shock, hepatobiliary infection, and gas gangrene within a couple of hours [1]. Despite the rapid progression to death, the pathogenesis of lethal C. perfringens infection remains unclear. In this study, we aimed to elucidate the host-C. perfringens interaction that underlies the rapid progression and death of this case. To achieve this, we conducted toxinotyping of C. perfringens isolated from this patient and determined the host cytokine responses.
Materials and methods
C. perfringens has been classified into seven types (types A to G) based on the secretion profiles of toxins: CPA, C. perfringens β toxin (CPB), ε toxin (ETX), ι toxin (ITX), C. perfringens enterotoxin (CPE), and necrotic enteritis B-like toxin (NetB) [2], [4]. In addition, extracellular enzymes such as PFO and ColA have been shown to be involved in the destruction of host tissues and degradation of the extracellular matrix [2]. Genomic PCRs targeting CPA, CPB, ETX, ITX, CPE, NetB, PFO, and ColA were performed using bacterial DNA of C. perfringens isolated from the patient’s blood and a commercially available type A strain of C. perfringens (JCM#1290; RIKEN BioResource Research Center, Tsukuba, Japan). The C. perfringens strains were cultured overnight in thioglycolate broth at 37 °C in an anaerobic environment. Bacterial genomic DNA was extracted using a QIAamp DNA Mini Kit (Qiagen, Hilden, Germany) following the manufacturer’s instructions. Isolated DNA (5 ng) was applied to the thermal cycler for PCRs using Quick TaqHS DyeMix (TOYOBO, Tokyo, Japan). The PCR conditions were as follows: hot start for 150 s at 95 °C, amplification for 40 cycles (denaturation for 1 min at 95 °C, annealing for 1 min at 55 °C, and extension for 1 min at °C), and final extension for 10 min at 72 °C. The PCR products were run on a 2.5 % agarose gel stained with ethidium bromide. The PCR primer sequences and expected band sizes are presented in Table 2.
Table 2.
Primer sequences for detection of toxins and enzymes.
| Toxin gene | Primer directions | Gene sequence (5′−3′) | Size (bp) |
|---|---|---|---|
| cpa | forward | AGTCTACGCTTGGGATGGAA | 900 |
| reverse | TTTCCTGGGTTGTCCATTTC | ||
| cpb | forward | TCCTTTCTTGAGGGAGGATAAA | 611 |
| reverse | TGAACCTCCTATTTTGTATCCCA | ||
| cpe | forward | GGGGAACCCTCAGTAGTTTCA | 506 |
| reverse | ACCAGCTGGATTTGAGTTTAATG | ||
| etx | forward | TGGGAACTTCGATACAAGCA | 396 |
| reverse | TTAACTCATCTCCCATAACTGCAC | ||
| iap | forward | AAACGCATTAAAGCTCACACC | 293 |
| reverse | CTGCATAACCTGGAATGGCT | ||
| pfoA | forward | TGTAGCTTATGGAAGAACTA | 591 |
| reverse | CACCATTCCCAAGCAAGACC | ||
| colA | forward | CCTGATGAATTTTTTCCACCAAA | 281 |
| reverse | GGATATGATGCTAAAAACACTGAGTTCTAT | ||
| netB | forward | CGCTTCACATAAAGGTTGGAAGGC | 316 |
| reverse | TCCAGCACCAGCAGTTTTTCCT |
To visualize host cytokine responses, serum samples from the patient and healthy control (A.H.) were analyzed using cytokine and chemokine arrays (R&D Systems, Minneapolis, USA), as previously described [8], [9]. A total of 200 μL of serum was applied to the membrane. To determine the TLRs involved in the production of proinflammatory cytokines by C. perfringens, female C57BL/6 mice aged 6 weeks were obtained from Japan SLC Inc. (Hamamatsu, Japan). Age- and sex-matched TLR2-deficient (TLR2-/-), TLR4-deficient (TLR4-/-), or TLR2 and TLR4-double deficient (TLR2-/-TLR4-/-) mice were also employed [10], [11]. Splenocytes were prepared from these mice, and cells (2 × 106/mL) were stimulated with heat-killed C. perfringens (3 × 109 CFU/mL) for 24 h. After stimulation, mRNA was isolated from splenic cells and transcribed into cDNA for quantitative PCR (qPCR) analysis, as previously described [10], [12], [13]. mRNA expression was determined using SYBR Green-based qPCR on a LightCycler 480 system (Roche Diagnostics, Tokyo, Japan). PCR primers for the IL6 genes were purchased from Qiagen (Valencia, USA), and Actin mRNA expression was used as a reference gene.
Results
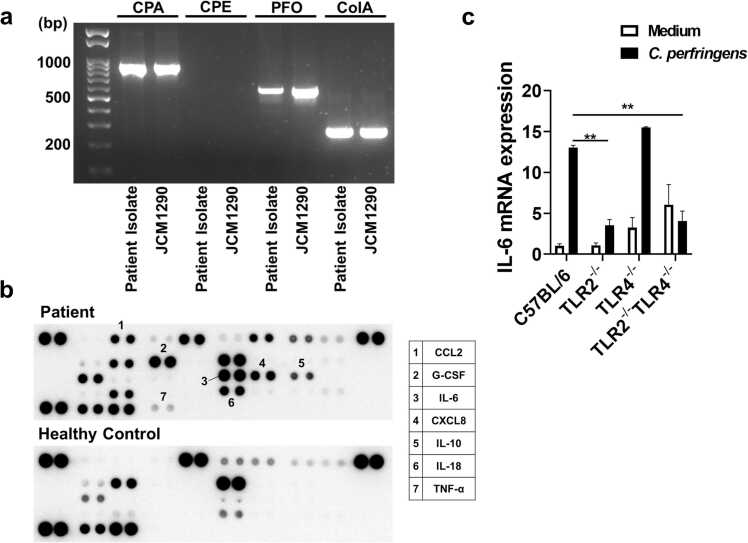
The type of C. perfringens was initially determined based on the expression profiles of the toxins [2], [4]. A commercially available type A strain (JCM#1290) was used as a control for PCRs. C. perfringens isolated from this case expressed CPA, PFO, and ColA, but not CPE (Fig. 4a). In addition, the patient’s C. perfringens did not express CPB, ETX, ITX, or NetB (data not shown). This toxin expression profile (CPA+PFO+ColA+) matched that of JCM#1290, indicating that CPA+PFO+ColA+ C. perfringens type A caused MIH, gas gangrene, and septic shock in the patient.
Fig. 4.
Host cytokine responses against Clostridium perfringens isolated from this case. (a) Toxin profiles of Clostridium perfringens isolated from the patient and a commercially available type A strain (JCM#1290). The leftmost lane represents the 100-bp DNA ladder. Agarose gel electrophoresis of polymerase chain reaction (PCR) products revealed that C. perfringens isolated from the patient expressed CPA, PFO, and ColA, but not CPE. CPA, C. perfringens α toxin; PFO, perfringolysin O; ColA, collagenase. (b) Profiles of serum cytokines and chemokines. Cytokine and chemokine arrays revealed heightened proinflammatory responses in the patient serum. CCL2; C-C chemokine ligand 2, CXCL8; C-X-C motif chemokine ligand 8, G-CSF; granulocyte-colony stimulating factor. (c) Toll-like receptors (TLRs) involved in the production of proinflammatory cytokines by C. perfringens. Splenocytes prepared from C57BL/6 mice or mice deficient in TLR2 and/or TLR4 were stimulated with heat-killed C. perfringens. IL-6 mRNA expression was expressed as the mean ± standard error of the mean. * * P < 0.01.
The host cytokine and chemokine responses to septicemia caused by CPA+PFO+ColA+ C. perfringens type A were then examined. Cytokine and chemokine arrays revealed elevated levels of C-C chemokine ligand 2, C-X-C motif chemokine ligand 8 (CXCL8), granulocyte-colony stimulating factor, interleukin 6 (IL-6), IL-18, and tumor necrosis factor-α (TNF-α) in the patient’s cells (Fig. 4b). This indicated heightened innate immunity in the present case, with cytokine storms likely involving IL-6, IL-18, and TNF-α, mainly produced by macrophages and dendritic cells.
Given that the bacterial sensing of TLRs drives proinflammatory cytokine responses by immune cells [10], [12], [13], [14], we aimed to determine the type of TLRs responsible for enhanced cytokine responses. Robust IL-6 mRNA expression was observed in splenocytes from TLR2- and TLR4-intact C57BL/6 mice upon stimulation with heat-killed CPA+PFO+ColA+ C. perfringens type A isolated from the patient (Fig. 4c). In contrast, IL-6 mRNA expression was markedly reduced in splenocytes from TLR2-/- mice or TLR2-/-TLR4-/- mice, whereas IL-6 expression was comparable in splenocytes from C57BL/6 mice and TLR4-/- mice (Fig. 4c). These findings suggest that retrograde bile duct infection with CPA+PFO+ColA+ C. perfringens type A-induced cytokine storms partially propagate through TLR2, but not TLR4, in this case.
Discussion
C. perfringens is a Gram-positive anaerobic bacterium that colonizes the gastrointestinal tract, soil, and foods [1], [2]. Despite being a commensal bacterium, infection by C. perfringens can result in various conditions, such as food poisoning, enterocolitis, gas gangrene, hepatobiliary infection, sepsis, and MIH [1], [2], [3]. Because of the high incidence of foodborne gastroenteritis caused by C. perfringens, an association between this bacterium and gastrointestinal injury has been well established [1], [2], [3]. In addition to self-limiting foodborne gastroenteritis, C. perfringens can cause lethal conditions like MIH, gas gangrene, and septic shock. Herein, we present an autopsy case of lethal C. perfringens septicemia with the typical manifestations of MIH, septic shock, and gas gangrene. van Bunderen et al. reported that only 8 of 40 patients with C. perfringens septicemia survived, with 21 patients dying within 10 h of admission [6]. The rapid progression and poor prognosis of this case are consistent with previous reports [1], [2], [3], [6]. Nonetheless, the present case is unique in that ordinary liver abscesses transformed into gas-filled nodules due to the extremely rapid overgrowth of C. perfringens, leading to shock and altered consciousness within 1 h of stable vital sign verification. Additionally, it is worth noting that systemic gangrene developed within a couple of hours despite immediate intensive treatment and antibiotic administration, as confirmed by autopsy findings.
The patient in the present case had a history of biliary reconstruction for congenital biliary dilatation. The destruction of the choledochojejunostomy site by gas gangrene, along with the detection of C. perfringens in the bile, strongly suggests that the lethal infection originated from the bile duct. In line with the present case, recent epidemiological and clinicopathological studies have identified the biliary tract as the most common entry site for C. perfringens [15]. In addition, other studies have shown that hepatobiliary diseases and a history of hepatobiliary surgery are associated with C. perfringens septicemia[16], [17]. The patient in this case also had DM, which is another risk factor for lethal infection with this organism [15], [16]. Therefore, it is speculated that the abnormalities of the hepatobiliary system and the presence of DM may have allowed C. perfringens to enter the bile ducts, leading to the formation of gas-producing liver abscesses and systemic bacterial dissemination.
The relationship between bacterial pathogenic factors and host immune responses in a lethal C. perfringens infection has not been well understood. The rapid progression to lethal outcome in this case led us to examine the involvement of C. perfringens toxins and cytokine responses. PCR analyses for the determination of toxin profiles identified C. perfringens as CPA+PFO+ColA+ type A, which is associated with the clinical manifestations. CPA causes gas gangrene by degrading sphingomyelin and phosphatidylcholine in the plasma membrane [2], [18]. The degree of erythrocyte hemolysis is parallel to PFO expression [1], [5]. Of interest, human peripheral blood mononuclear cells produce large amounts of CXCL8, IL-6, and TNF-α upon stimulation with recombinant PFO, indicating a link between cytokine storm and PFO expression [1], [5]. Consistent with these findings, cytokine and chemokine array analyses clearly showed elevated expression of CXCL8, IL-6, and TNF-α in the patient’s serum. Collectively, these studies, along with our data, suggest that retrograde bile duct infection with C. perfringens type A led to MIH, gas gangrene, and cytokine storms through the secretion of PFO and CPA. To the best of our knowledge, the present case is the first to demonstrate the association between toxin profiles and host cytokine responses.
Although proinflammatory cytokine responses by macrophages and dendritic cells depend on the activation of TLRs [14], the specific TLRs-recognizing components of C. perfringens have not been identified. We found that CPA+PFO+ColA+ C. perfringens type A isolated from the present case induces IL-6 expression in a TLR2-dependent and TLR4-independent manner. Consistent with these findings, mRNA expression of IL-6 and TLR2 is upregulated in murine muscle tissues upon C. perfringens infection [19]. However, another study suggests that TLR4-deficient C3H/HeJ mice exhibit reduced IL-6 responses upon intramuscular injection with C. perfringens [20]. These discrepancies in the role of TLR4 in C. perfringens-mediated IL-6 release may be attributable to strain differences in C. perfringens. Further studies are necessary to identify the TLRs involved in the C. perfringens-induced cytokine storm. However, CPA+PFO+ColA+ C. perfringens type A isolated from the patient contributes to the cytokine storm partially through TLR2, which primarily recognizes lipoproteins and peptidoglycans. Immediate and aggressive antibiotic therapy alone may not be sufficient for treating patients infected with CPA+PFO+ColA+ C. perfringens type A if they manifest MIH and gas gangrene, as demonstrated in the present case. Therefore, targeting TLRs and/or proinflammatory cytokines may be useful, although no report on the efficacy of biologics in C. perfringens infection is available.
In conclusion, we present an autopsy case of gas gangrene, MIH, and cytokine storm resulting from CPA+PFO+ColA+ C. perfringens type A infection originating in the biliary tract. Our PCR and cytokine analyses revealed the molecular mechanisms by which the toxin profiles of C. perfringens contribute to the development of MIH, gas gangrene, and cytokine storms.
Ethics statement
This study was approved by the ethical review board of the Kindai University Faculty of Medicine (Number 28–034), and the protocol was performed in accordance with the Helsinki Declaration. Written informed consent was obtained from the patient’s family for the publication of this case report and any accompanying images.
Ethical approval
This study was approved by the ethical review board of the Kindai University Faculty of Medicine (Number 28–034), and the protocol was performed in accordance with the Helsinki Declaration.
Funding
This work was supported in part by Kindai University Research Enhancement Grants (grant numbers IP001, KD2208, KD2301, and KD2405).
CRediT authorship contribution statement
Masatoshi Kudo: Supervision, Writing – review & editing. Tomohiro Watanabe: Conceptualization, Funding acquisition, Methodology, Supervision, Writing – original draft, Writing – review & editing. Kosuke Minaga: Conceptualization, Funding acquisition, Writing – original draft, Data curation, Investigation, Methodology. Akane Hara: Conceptualization, Data curation, Investigation, Writing – original draft. Yasuo Otsuka: Data curation, Investigation, Writing – review & editing. Yasuhiro Masuta: Data curation, Writing – review & editing. Yuko Nakamura: Writing – review & editing. Hiroshi Kajiyama: Investigation, Writing – review & editing. Ah-Mee Park: Investigation, Writing – review & editing.
Declaration of Competing Interest
The authors declare that they have no conflicts of interest.
Acknowledgements
The authors would like to thank Enago (www.enago.jp) for the English language review.
Consent
Written informed consent was obtained from the patient’s family for the publication of this case report and any accompanying images.
Data availability
The datasets generated and/or analyzed during the present study are not publicly available due to ethical considerations but are available from the corresponding author on reasonable request.
References
- 1.Suzaki A., Hayakawa S. Clinical and microbiological features of fulminant haemolysis caused by clostridium perfringens bacteraemia: Unknown pathogenesis. Microorganisms. 2023;11(4):824. doi: 10.3390/microorganisms11040824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Camargo A., Ramirez J.D., Kiu R., Hall L.J., Munoz M. Unveiling the pathogenic mechanisms of Clostridium perfringens toxins and virulence factors. Emerg Microbes Infect. 2024;13(1) doi: 10.1080/22221751.2024.2341968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kiu R., Hall L.J. An update on the human and animal enteric pathogen Clostridium perfringens. Emerg Microbes Infect. 2018;7(1):141. doi: 10.1038/s41426-018-0144-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mehdizadeh Gohari I., M A.N., Li J., Shrestha A., Uzal F., B A.M. Pathogenicity and virulence of Clostridium perfringens. Virulence. 2021;12(1):723–753. doi: 10.1080/21505594.2021.1886777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Suzaki A., Ohtani K., Komine-Aizawa S., Matsumoto A., Kamiya S., Hayakawa S. Pathogenic characterization of clostridium perfringens strains isolated from patients with massive intravascular hemolysis. Front Microbiol. 2021;12 doi: 10.3389/fmicb.2021.713509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.van Bunderen C.C., Bomers M.K., Wesdorp E., Peerbooms P., Veenstra J. Clostridium perfringens septicaemia with massive intravascular haemolysis: a case report and review of the literature. Neth J Med. 2010;68(9):343–346. [PubMed] [Google Scholar]
- 7.Dhaliwal G., Cornett P.A., Tierney L.M. Jr.: Hemolytic anemia. Am Fam Physician. 2004;69(11):2599–2606. [PubMed] [Google Scholar]
- 8.Fujita S., Honjo H., Takada R., Hara A., Masuta Y., Otsuka Y., et al. Ulcerative colitis-associated spondyloarthritis successfully treated with infliximab in the absence of enhanced TNF-alpha responses. Intern Med. 2023;62(17):2493–2497. doi: 10.2169/internalmedicine.1182-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kamata K., Watanabe T., Minaga K., Hara A., Yoshikawa T., Okamoto A., et al. Intestinal dysbiosis mediates experimental autoimmune pancreatitis via activation of plasmacytoid dendritic cells. Int Immunol. 2019;31(12):795–809. doi: 10.1093/intimm/dxz050. [DOI] [PubMed] [Google Scholar]
- 10.Okai N., Masuta Y., Otsuka Y., Hara A., Masaki S., Kamata K., et al. Crosstalk between NOD2 and TLR2 suppresses the development of TLR2-mediated experimental colitis. J Clin Biochem Nutr. 2024;74(2):146–153. doi: 10.3164/jcbn.23-87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tsuji Y., Watanabe T., Kudo M., Arai H., Strober W., Chiba T. Sensing of commensal organisms by the intracellular sensor NOD1 mediates experimental pancreatitis. Immunity. 2012;37(2):326–338. doi: 10.1016/j.immuni.2012.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Watanabe T., Minaga K., Kamata K., Sakurai T., Komeda Y., Nagai T., et al. RICK/RIP2 is a NOD2-independent nodal point of gut inflammation. Int Immunol. 2019;31(10):669–683. doi: 10.1093/intimm/dxz045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Masuta Y., Minaga K., Kurimoto M., Sekai I., Hara A., Omaru N., et al. Activation of nucleotide-binding oligomerization domain 2 by muramyl dipeptide negatively regulates Toll-like receptor 9-mediated colonic inflammation through the induction of deubiquitinating enzyme A expression. Int Immunol. 2023;35(2):79–94. doi: 10.1093/intimm/dxac045. [DOI] [PubMed] [Google Scholar]
- 14.Kawai T., Ikegawa M., Ori D., Akira S. Decoding Toll-like receptors: recent insights and perspectives in innate immunity. Immunity. 2024;57(4):649–673. doi: 10.1016/j.immuni.2024.03.004. [DOI] [PubMed] [Google Scholar]
- 15.Shindo Y., Dobashi Y., Sakai T., Monma C., Miyatani H., Yoshida Y. Epidemiological and pathobiological profiles of Clostridium perfringens infections: review of consecutive series of 33 cases over a 13-year period. Int J Clin Exp Pathol. 2015;8(1):569–577. [PMC free article] [PubMed] [Google Scholar]
- 16.Fujita H., Nishimura S., Kurosawa S., Akiya I., Nakamura-Uchiyama F., Ohnishi K. Clinical and epidemiological features of Clostridium perfringens bacteremia: a review of 18 cases over 8 year-period in a tertiary care center in metropolitan Tokyo area in Japan. Intern Med. 2010;49(22):2433–2437. doi: 10.2169/internalmedicine.49.4041. [DOI] [PubMed] [Google Scholar]
- 17.Yamamoto Y., Itoh N., Sugiyama T., Kurai H. Clinical features of Clostridium bacteremia in cancer patients: a case series review. J Infect Chemother. 2020;26(1):92–94. doi: 10.1016/j.jiac.2019.07.019. [DOI] [PubMed] [Google Scholar]
- 18.Takagishi T., Oda M., Kabura M., Kurosawa M., Tominaga K., Urano S., et al. Clostridium perfringens alpha-toxin induces Gm1a clustering and trka phosphorylation in the host cell membrane. PLoS One. 2015;10(4) doi: 10.1371/journal.pone.0120497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Low L.Y., Harrison P.F., Gould J., Powell D.R., Choo J.M., Forster S.C., et al. Concurrent host-pathogen transcriptional responses in a clostridium perfringens murine myonecrosis infection. mBio. 2018;9(2) doi: 10.1128/mBio.00473-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Takehara M., Kobayashi K., Nagahama M. Toll-like receptor 4 protects against clostridium perfringens infection in mice. Front Cell Infect Microbiol. 2021;11 doi: 10.3389/fcimb.2021.633440. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated and/or analyzed during the present study are not publicly available due to ethical considerations but are available from the corresponding author on reasonable request.