Abstract
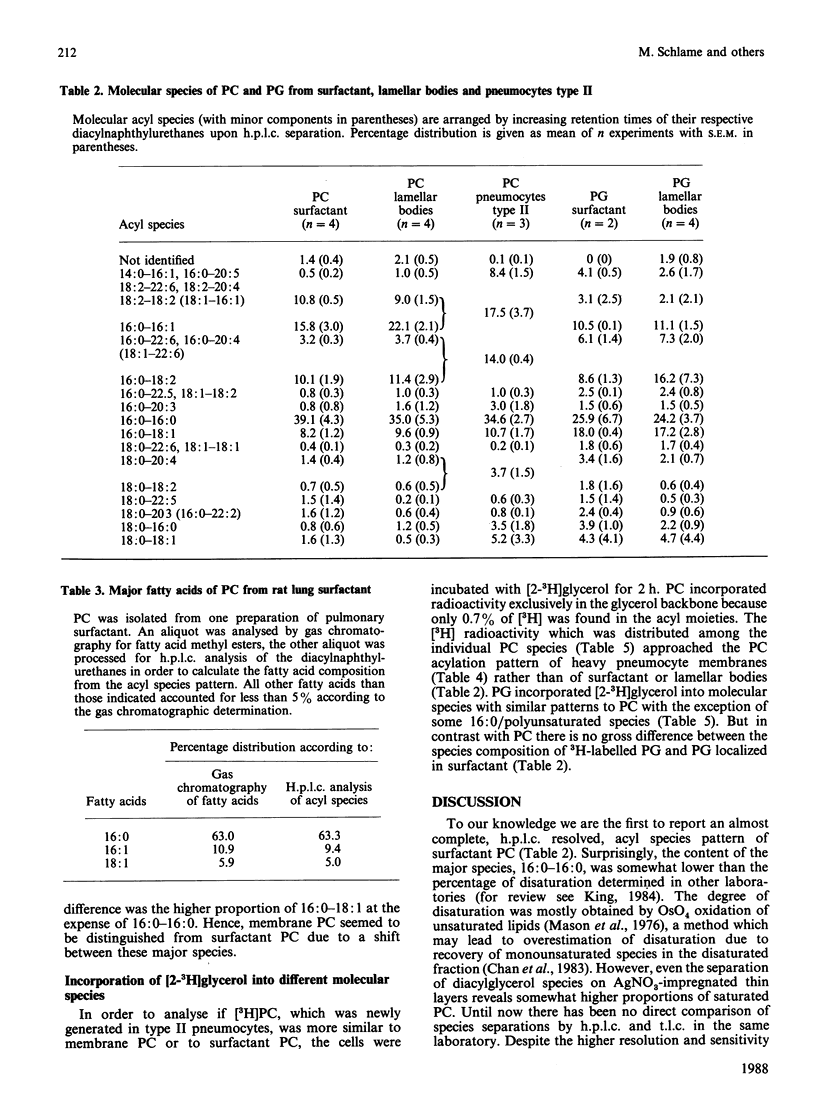
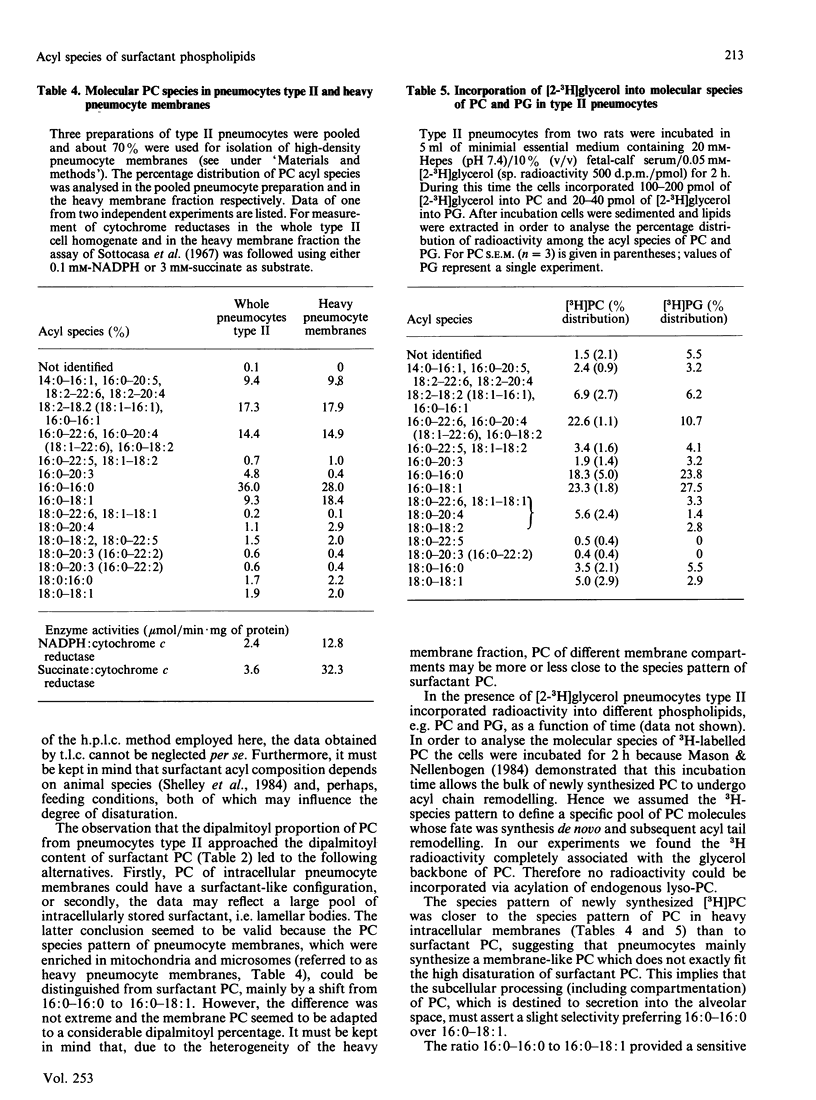
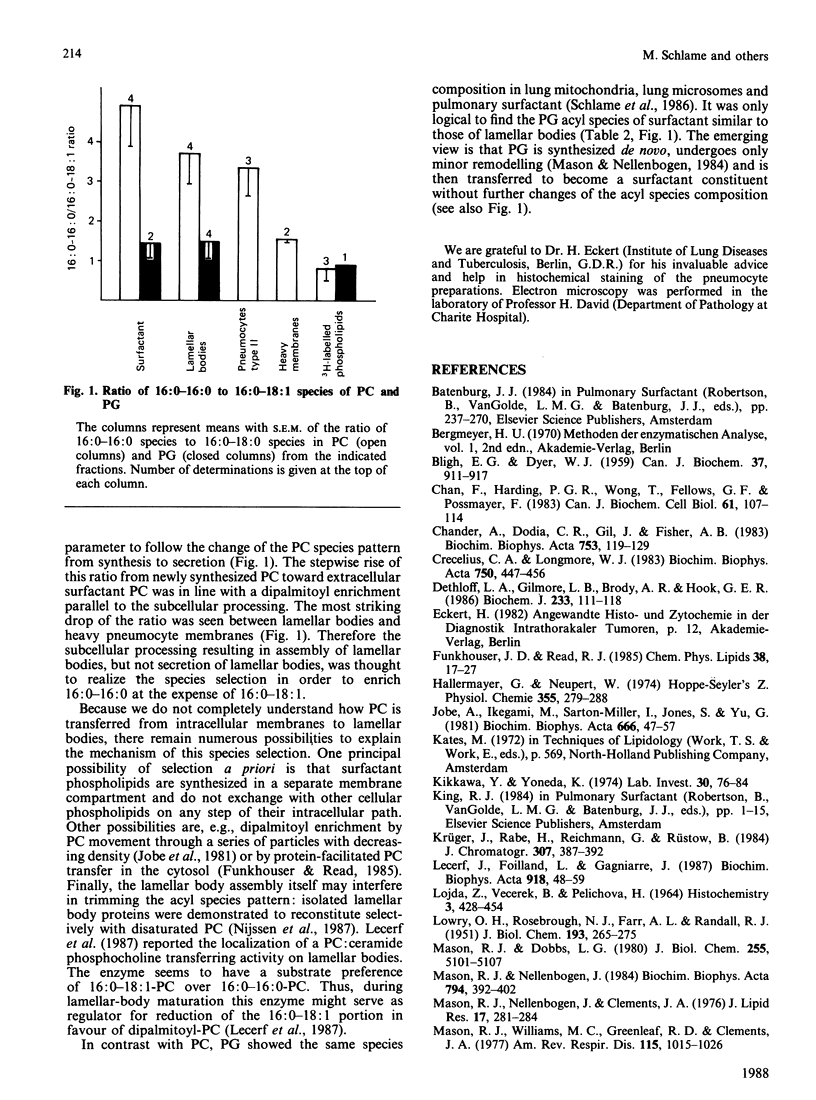
It is not yet completely understood how a cell is able to export specific phospholipids, like dipalmitoylphosphatidylcholine (dipalmitoyl-PC), which is secreted by pneumocytes type II, into pulmonary surfactant. The acyl species composition of [3H]PC which was synthesized in type II cells in the presence of [2-3H]glycerol resembled the species composition of PC localized in intracellular pneumocyte membranes. This species pattern was different from the pattern of PC of lamellar bodies, i.e., intracellularly stored surfactant, by a higher proportion of dipalmitoyl-PC mainly at expense of 1-palmitoyl-2-oleoyl-PC. Lamellar body PC in turn showed the same species distribution as surfactant PC. The data suggest that subcellular compartmentation and/or intracellular transfer of PC destined to storage in lamellar bodies, but not secretion of lamellar bodies, involves an enrichment of dipalmitoyl-PC and a depletion of 1-palmitoyl-2-oleoyl-PC. In contrast, the acyl species pattern of phosphatidylglycerol does not seem to undergo gross changes on the path from synthesis to secretion.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BLIGH E. G., DYER W. J. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959 Aug;37(8):911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- Chan F., Harding P. G., Wong T., Fellows G. F., Possmayer F. Cellular distribution of enzymes involved in phosphatidylcholine synthesis in developing rat lung. Can J Biochem Cell Biol. 1983 Feb-Mar;61(2-3):107–114. doi: 10.1139/o83-016. [DOI] [PubMed] [Google Scholar]
- Chander A., Dodia C. R., Gil J., Fisher A. B. Isolation of lamellar bodies from rat granular pneumocytes in primary culture. Biochim Biophys Acta. 1983 Aug 29;753(1):119–129. doi: 10.1016/0005-2760(83)90105-4. [DOI] [PubMed] [Google Scholar]
- Crecelius C. A., Longmore W. J. Phosphatidic acid phosphatase activity in subcellular fractions derived from adult rat type II pneumocytes in primary culture. Biochim Biophys Acta. 1983 Mar 1;750(3):447–456. doi: 10.1016/0005-2760(83)90184-4. [DOI] [PubMed] [Google Scholar]
- Dethloff L. A., Gilmore L. B., Brody A. R., Hook G. E. Induction of intra- and extra-cellular phospholipids in the lungs of rats exposed to silica. Biochem J. 1986 Jan 1;233(1):111–118. doi: 10.1042/bj2330111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funkhouser J. D., Read R. J. Phospholipid transfer proteins from lung, properties and possible physiological functions. Chem Phys Lipids. 1985 Aug 30;38(1-2):17–27. doi: 10.1016/0009-3084(85)90054-4. [DOI] [PubMed] [Google Scholar]
- Hallermayer G., Neupert W. Lipid composition of mitochondrial outer and inner membranes of Neurospora crassa. Hoppe Seylers Z Physiol Chem. 1974 Mar;355(3):279–288. doi: 10.1515/bchm2.1974.355.1.279. [DOI] [PubMed] [Google Scholar]
- Jobe A., Ikegami M., Sarton-Miller I., Jones S., Yu G. Characterization of phospholipids and localization of some phospholipid synthetic and subcellular marker enzymes in subcellular fractions from rabbit lung. Biochim Biophys Acta. 1981 Oct 23;666(1):47–57. doi: 10.1016/0005-2760(81)90089-8. [DOI] [PubMed] [Google Scholar]
- Kikkawa Y., Yoneda K. The type II epithelial cell of the lung. I. Method of isolation. Lab Invest. 1974 Jan;30(1):76–84. [PubMed] [Google Scholar]
- Krüger J., Rabe H., Reichmann G., Rüstow B. Separation and determination of diacylglycerols as their naphthylurethanes by high-performance liquid chromatography. J Chromatogr. 1984 May 11;307(2):387–392. doi: 10.1016/s0378-4347(00)84110-9. [DOI] [PubMed] [Google Scholar]
- LOJDA Z., VECEREK B., PELICHOVA H. SOME REMARKS CONCERNING THE HISTOCHEMICAL DETECTION OF ACID PHOSPHATASE BY AZO-COUPLING REACTIONS. Z Zellforch Microsk Anat Histochem. 1964 Jan 31;48:428–454. doi: 10.1007/BF00736421. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lecerf J., Fouilland L., Gagniarre J. Evidence for a high activity of sphingomyelin biosynthesis by phosphocholine transfer from phosphatidylcholine to ceramides in lung lamellar bodies. Biochim Biophys Acta. 1987 Mar 13;918(1):48–59. doi: 10.1016/0005-2760(87)90008-7. [DOI] [PubMed] [Google Scholar]
- MORRISON W. R., SMITH L. M. PREPARATION OF FATTY ACID METHYL ESTERS AND DIMETHYLACETALS FROM LIPIDS WITH BORON FLUORIDE--METHANOL. J Lipid Res. 1964 Oct;5:600–608. [PubMed] [Google Scholar]
- Mason R. J., Dobbs L. G. Synthesis of phosphatidylcholine and phosphatidylglycerol by alveolar type II cells in primary culture. J Biol Chem. 1980 Jun 10;255(11):5101–5107. [PubMed] [Google Scholar]
- Mason R. J., Nellenbogen J. Synthesis of saturated phosphatidylcholine and phosphatidylglycerol by freshly isolated rat alveolar type II cells. Biochim Biophys Acta. 1984 Jul 26;794(3):392–402. doi: 10.1016/0005-2760(84)90005-5. [DOI] [PubMed] [Google Scholar]
- Mason R. J., Williams M. C., Greenleaf R. D., Clements J. A. Isolation and properties of type II alveolar cells from rat lung. Am Rev Respir Dis. 1977 Jun;115(6):1015–1026. doi: 10.1164/arrd.1977.115.6.1015. [DOI] [PubMed] [Google Scholar]
- Nijssen J. G., Promes L. W., Hardeman D., van den Bosch H. Phospholipid-protein interactions in rat lung lamellar bodies. Biochim Biophys Acta. 1987 Jan 13;917(1):140–147. doi: 10.1016/0005-2760(87)90294-3. [DOI] [PubMed] [Google Scholar]
- Possmayer F., Yu S. H., Weber J. M., Harding P. G. Pulmonary surfactant. Can J Biochem Cell Biol. 1984 Nov;62(11):1121–1133. doi: 10.1139/o84-146. [DOI] [PubMed] [Google Scholar]
- Post M., Schuurmans E. A., Batenburg J. J., Van Golde L. M. Mechanisms involved in the synthesis of disaturated phosphatidylcholine by alveolar type II cells isolated from adult rat lung. Biochim Biophys Acta. 1983 Jan 7;750(1):68–77. doi: 10.1016/0005-2760(83)90205-9. [DOI] [PubMed] [Google Scholar]
- Rooney S. A. The surfactant system and lung phospholipid biochemistry. Am Rev Respir Dis. 1985 Mar;131(3):439–460. doi: 10.1164/arrd.1985.131.3.439. [DOI] [PubMed] [Google Scholar]
- Rüstow B., Kunze D., Rabe H., Reichmann G. The molecular species of phosphatidic acid, diacylglycerol and phosphatidylcholine synthesized from sn-glycerol 3-phosphate in rat lung microsomes. Biochim Biophys Acta. 1985 Jul 31;835(3):465–476. doi: 10.1016/0005-2760(85)90116-x. [DOI] [PubMed] [Google Scholar]
- Schlame M., Rüstow B., Kunze D., Rabe H., Reichmann G. Phosphatidylglycerol of rat lung. Intracellular sites of formation de novo and acyl species pattern in mitochondria, microsomes and surfactant. Biochem J. 1986 Nov 15;240(1):247–252. doi: 10.1042/bj2400247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shelley S. A., Paciga J. E., Balis J. U. Lung surfactant phospholipids in different animal species. Lipids. 1984 Nov;19(11):857–862. doi: 10.1007/BF02534515. [DOI] [PubMed] [Google Scholar]
- Skillrud D. M., Martin W. J., 2nd The isolation of rat alveolar type II cells: a simplified approach using Percoll density centrifugation. Lung. 1984;162(4):245–252. doi: 10.1007/BF02715652. [DOI] [PubMed] [Google Scholar]
- Sottocasa G. L., Kuylenstierna B., Ernster L., Bergstrand A. An electron-transport system associated with the outer membrane of liver mitochondria. A biochemical and morphological study. J Cell Biol. 1967 Feb;32(2):415–438. doi: 10.1083/jcb.32.2.415. [DOI] [PMC free article] [PubMed] [Google Scholar]


