Abstract
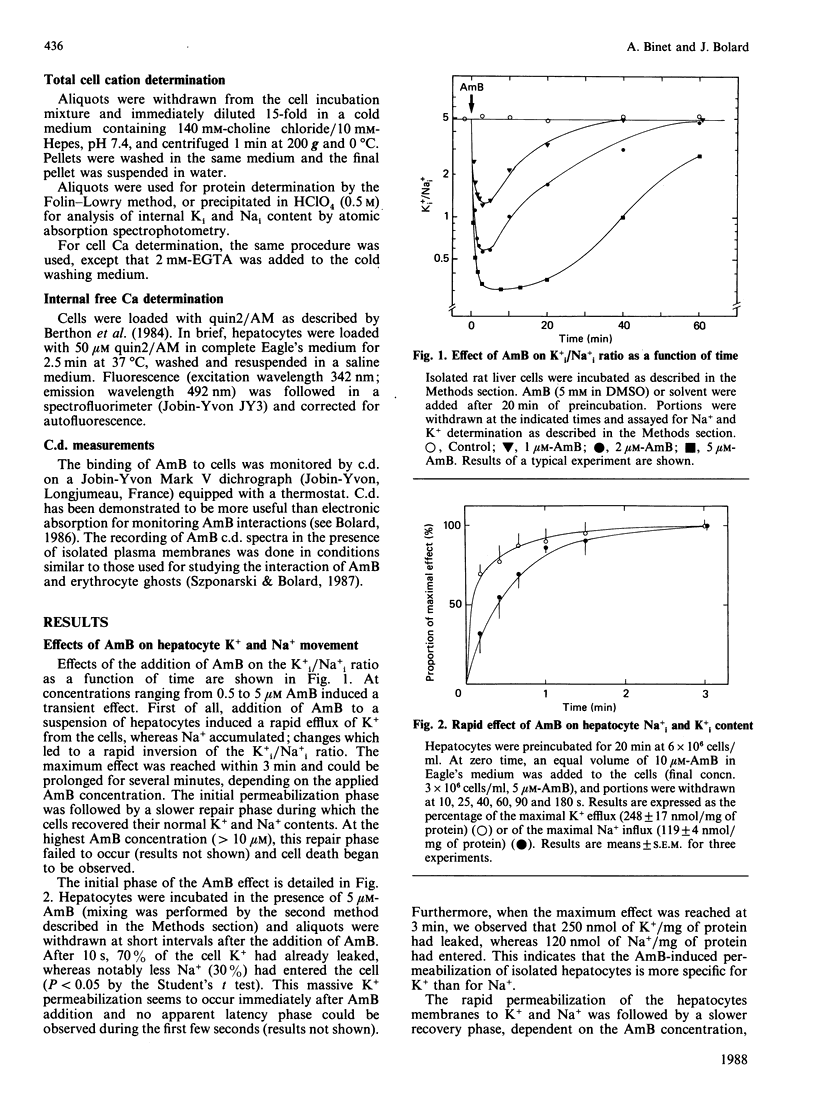
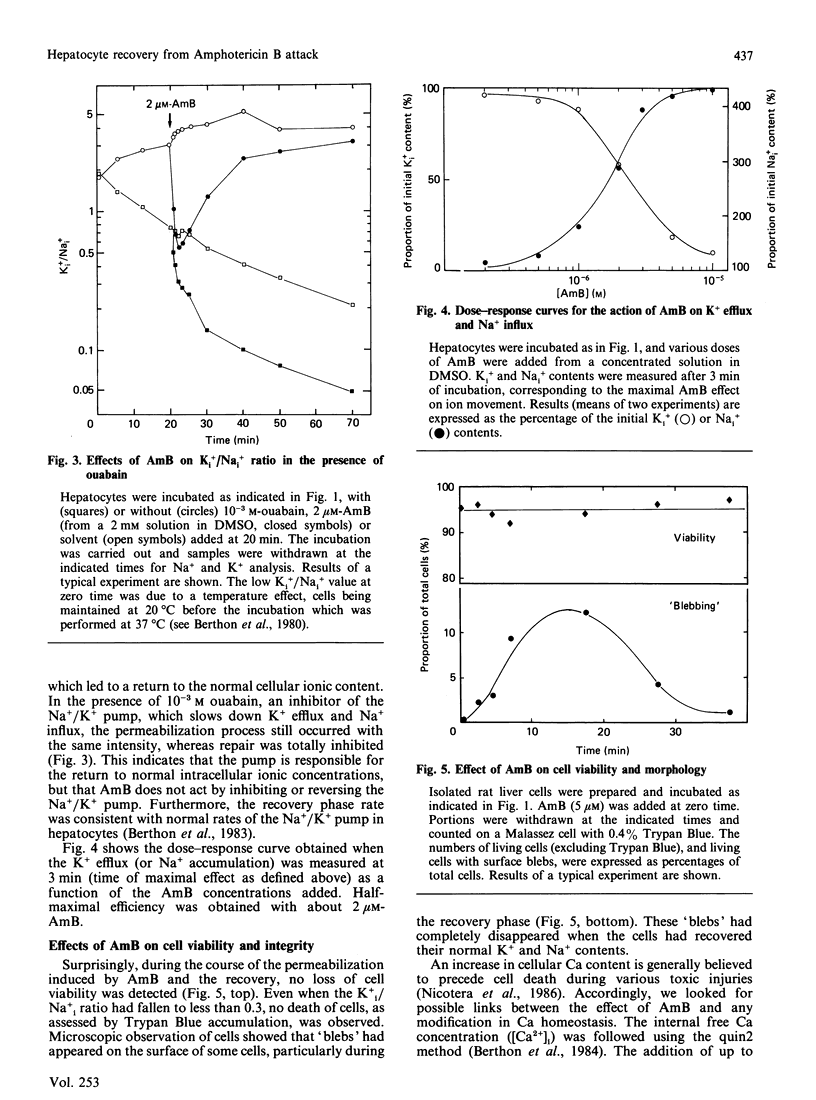
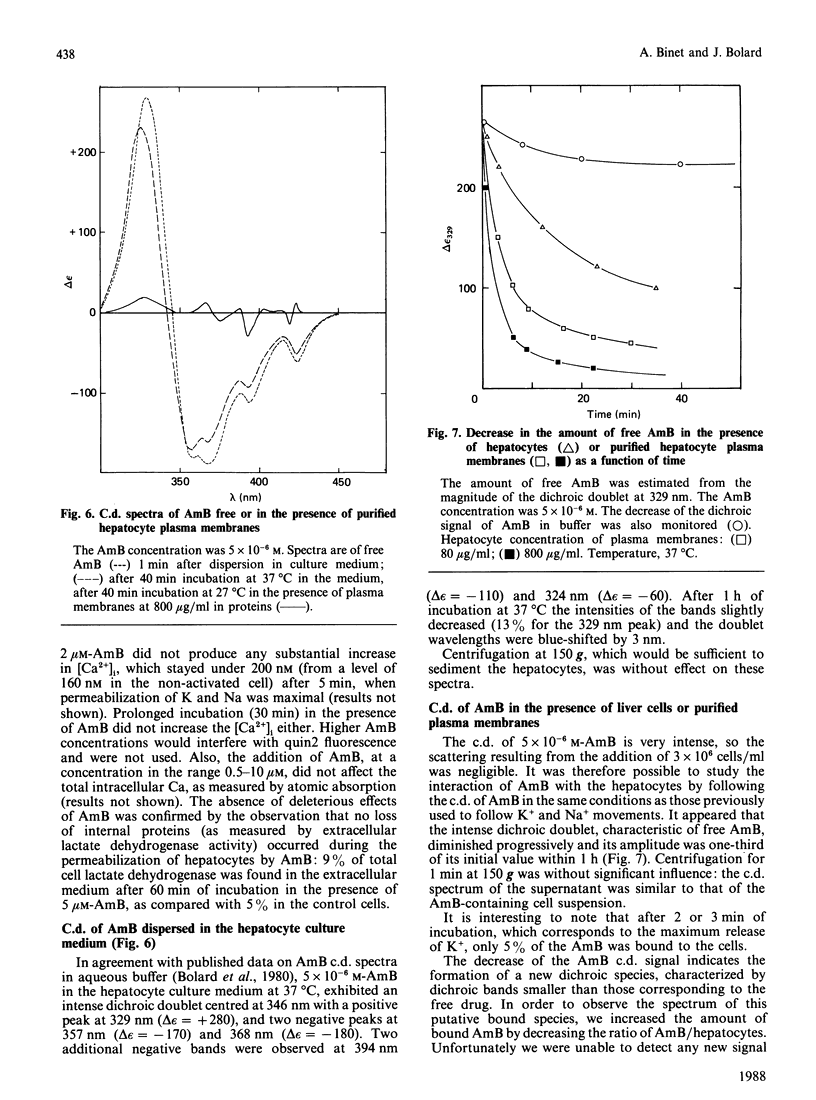
Sublytic amounts of the pore former Amphotericin B (AmB) induced transient movements of Na and K ions across the hepatocyte plasma membranes without altering the intracellular free Ca ion concentration. The presence of 1-5 microM-AmB induced leakage of up to 80% of the intracellular K+ within 3 min, followed by Na+ entry without loss of cell viability. A repair process occurred after 3-10 min, which restored the initial cationic concentrations. Progressive binding of AmB to the cells could be observed by following the disappearance of the intense excitonic dichroic doublet of free AmB. It was shown that the amount of AmB binding, responsible for the Na+ and K+ movements, was low (approx. 16% of total AmB). The recovery process occurred when higher amounts of AmB bound to the cells, and was mediated by Na+/K+-ATPase. The c.d. spectrum of AmB bound to isolated hepatocyte plasma membranes, indicated that during this step AmB formed a complex with cholesterol, similar to that formed by the binary mixture in water.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bashford C. L., Alder G. M., Menestrina G., Micklem K. J., Murphy J. J., Pasternak C. A. Membrane damage by hemolytic viruses, toxins, complement, and other cytotoxic agents. A common mechanism blocked by divalent cations. J Biol Chem. 1986 Jul 15;261(20):9300–9308. [PubMed] [Google Scholar]
- Berthon B., Binet A., Mauger J. P., Claret M. Cytosolic free Ca2+ in isolated rat hepatocytes as measured by quin2. Effects of noradrenaline and vasopressin. FEBS Lett. 1984 Feb 13;167(1):19–24. doi: 10.1016/0014-5793(84)80824-8. [DOI] [PubMed] [Google Scholar]
- Berthon B., Burgess G. M., Capiod T., Claret M., Poggioli J. Mechanism of action of noradrenaline on the sodium-potassium pump in isolated rat liver cells. J Physiol. 1983 Aug;341:25–40. doi: 10.1113/jphysiol.1983.sp014790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berthon B., Claret M., Mazet J. L., Poggioli J. Volume- and temperature-dependent permeabilities in isolated rat liver cells. J Physiol. 1980 Aug;305:267–277. doi: 10.1113/jphysiol.1980.sp013362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binet A., Berthon B., Claret M. Hormone-induced increase in free cytosolic calcium and glycogen phosphorylase activation in rat hepatocytes incubated in normal and low-calcium media. Biochem J. 1985 Jun 15;228(3):565–574. doi: 10.1042/bj2280565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolard J., Cheron M. Association of the polyene antibiotic amphotericin B with phospholipid vesicles: perturbation by temperature changes. Can J Biochem. 1982 Aug;60(8):782–789. doi: 10.1139/o82-097. [DOI] [PubMed] [Google Scholar]
- Bolard J. How do the polyene macrolide antibiotics affect the cellular membrane properties? Biochim Biophys Acta. 1986 Dec 22;864(3-4):257–304. doi: 10.1016/0304-4157(86)90002-x. [DOI] [PubMed] [Google Scholar]
- Bolard J., Seigneuret M., Boudet G. Interaction between phospholipid bilayer membranes and the polyene antibiotic amphotericin B: lipid state and cholesterol content dependence. Biochim Biophys Acta. 1980 Jun 20;599(1):280–293. doi: 10.1016/0005-2736(80)90074-7. [DOI] [PubMed] [Google Scholar]
- Brajtburg J., Elberg S., Medoff J., Kobayashi G. S., Schlessinger D., Medoff G. Stimulatory, permeabilizing, and toxic effects of amphotericin B on L cells. Antimicrob Agents Chemother. 1984 Dec;26(6):892–897. doi: 10.1128/aac.26.6.892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carney D. F., Hammer C. H., Shin M. L. Elimination of terminal complement complexes in the plasma membrane of nucleated cells: influence of extracellular Ca2+ and association with cellular Ca2+. J Immunol. 1986 Jul 1;137(1):263–270. [PubMed] [Google Scholar]
- Christiansen K. J., Bernard E. M., Gold J. W., Armstrong D. Distribution and activity of amphotericin B in humans. J Infect Dis. 1985 Nov;152(5):1037–1043. doi: 10.1093/infdis/152.5.1037. [DOI] [PubMed] [Google Scholar]
- Fisher P. B., Bryson V., Schaffner C. P. Polyene macrolide antibiotic cytotoxicity and membrane permeability alterations. I. Comparative effects of four classes of polyene macrolides on mammalian cells. J Cell Physiol. 1978 Dec;97(3 Pt 1):345–351. doi: 10.1002/jcp.1040970309. [DOI] [PubMed] [Google Scholar]
- Hollander d' F., Chevallier F. Movement of cholesterol in vitro in rat blood and quantitation of the exchange of free cholesterol between plasma and erythrocytes. J Lipid Res. 1972 Nov;13(6):733–744. [PubMed] [Google Scholar]
- Kotler-Brajtburg J., Medoff G., Schlessinger D., Kobayashi G. S. Amphotericin B and filipin effects on L and HeLa cells: dose response. Antimicrob Agents Chemother. 1977 May;11(5):803–808. doi: 10.1128/aac.11.5.803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemasters J. J., DiGuiseppi J., Nieminen A. L., Herman B. Blebbing, free Ca2+ and mitochondrial membrane potential preceding cell death in hepatocytes. Nature. 1987 Jan 1;325(6099):78–81. doi: 10.1038/325078a0. [DOI] [PubMed] [Google Scholar]
- Malewicz B., Jenkin H. M., Borowski E. Dissociation between the induction of potassium efflux and cytostatic activity of polyene macrolides in mammalian cells. Antimicrob Agents Chemother. 1980 Apr;17(4):699–706. doi: 10.1128/aac.17.4.699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malewicz B., Jenkin H. M., Borowski E. Repair of membrane alterations induced in baby hamster kidney cells by polyene macrolide antibiotics. Antimicrob Agents Chemother. 1981 Feb;19(2):238–247. doi: 10.1128/aac.19.2.238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan B. P., Dankert J. R., Esser A. F. Recovery of human neutrophils from complement attack: removal of the membrane attack complex by endocytosis and exocytosis. J Immunol. 1987 Jan 1;138(1):246–253. [PubMed] [Google Scholar]
- Nicotera P., Hartzell P., Davis G., Orrenius S. The formation of plasma membrane blebs in hepatocytes exposed to agents that increase cytosolic Ca2+ is mediated by the activation of a non-lysosomal proteolytic system. FEBS Lett. 1986 Dec 1;209(1):139–144. doi: 10.1016/0014-5793(86)81099-7. [DOI] [PubMed] [Google Scholar]
- Pasternak C. A. Effect of pore formers on intracellular calcium. Cell Calcium. 1986 Dec;7(5-6):387–397. doi: 10.1016/0143-4160(86)90041-2. [DOI] [PubMed] [Google Scholar]
- Prpić V., Green K. C., Blackmore P. F., Exton J. H. Vasopressin-, angiotensin II-, and alpha 1-adrenergic-induced inhibition of Ca2+ transport by rat liver plasma membrane vesicles. J Biol Chem. 1984 Feb 10;259(3):1382–1385. [PubMed] [Google Scholar]
- Rozengurt E., Mendoza S. Monovalent ion fluxes and the control of cell proliferation in cultured fibroblasts. Ann N Y Acad Sci. 1980;339:175–190. doi: 10.1111/j.1749-6632.1980.tb15977.x. [DOI] [PubMed] [Google Scholar]
- Sato Y., Chiba T., Suzuki Y. Characterization of the anion transport channel protein in human erythrocytes. Induced circular dichroism of inhibitors bound to the anion transport channel. Biochim Biophys Acta. 1986 Mar 27;856(1):11–18. doi: 10.1016/0005-2736(86)90003-9. [DOI] [PubMed] [Google Scholar]
- Schanne F. A., Kane A. B., Young E. E., Farber J. L. Calcium dependence of toxic cell death: a final common pathway. Science. 1979 Nov 9;206(4419):700–702. doi: 10.1126/science.386513. [DOI] [PubMed] [Google Scholar]
- Schneider A. S., Herz R., Sonenberg M. Chlortetracycline as a probe of membrane-associated calcium and magnesium: interaction with red cell membranes, phospholipids, and proteins monitored by fluorescence and circular dichroism. Biochemistry. 1983 Mar 29;22(7):1680–1686. doi: 10.1021/bi00276a025. [DOI] [PubMed] [Google Scholar]
- Sims P. J., Wiedmer T. Repolarization of the membrane potential of blood platelets after complement damage: evidence for a Ca++ -dependent exocytotic elimination of C5b-9 pores. Blood. 1986 Aug;68(2):556–561. [PubMed] [Google Scholar]
- Szponarski W., Bolard J. Temperature-dependent modes for the binding of the polyene antibiotic amphotericin B to human erythrocyte membranes. A circular dichroism study. Biochim Biophys Acta. 1987 Feb 26;897(2):229–237. doi: 10.1016/0005-2736(87)90419-6. [DOI] [PubMed] [Google Scholar]
- Vertut-Croquin A., Bolard J., Chabbert M., Gary-Bobo C. Differences in the interaction of the polyene antibiotic amphotericin B with cholesterol- or ergosterol-containing phospholipid vesicles. A circular dichroism and permeability study. Biochemistry. 1983 Jun 7;22(12):2939–2944. doi: 10.1021/bi00281a024. [DOI] [PubMed] [Google Scholar]
- Vertut-Croquin A., Brajtburg J., Medoff G. Two mechanisms of synergism when amphotericin B is used in combination with actinomycin D or 1-(2-chloroethyl)-3-cyclohexyl-1-nitrosourea against the human promyelocytic leukemia cell line HL-60. Cancer Res. 1986 Dec;46(12 Pt 1):6054–6058. [PubMed] [Google Scholar]


