Abstract
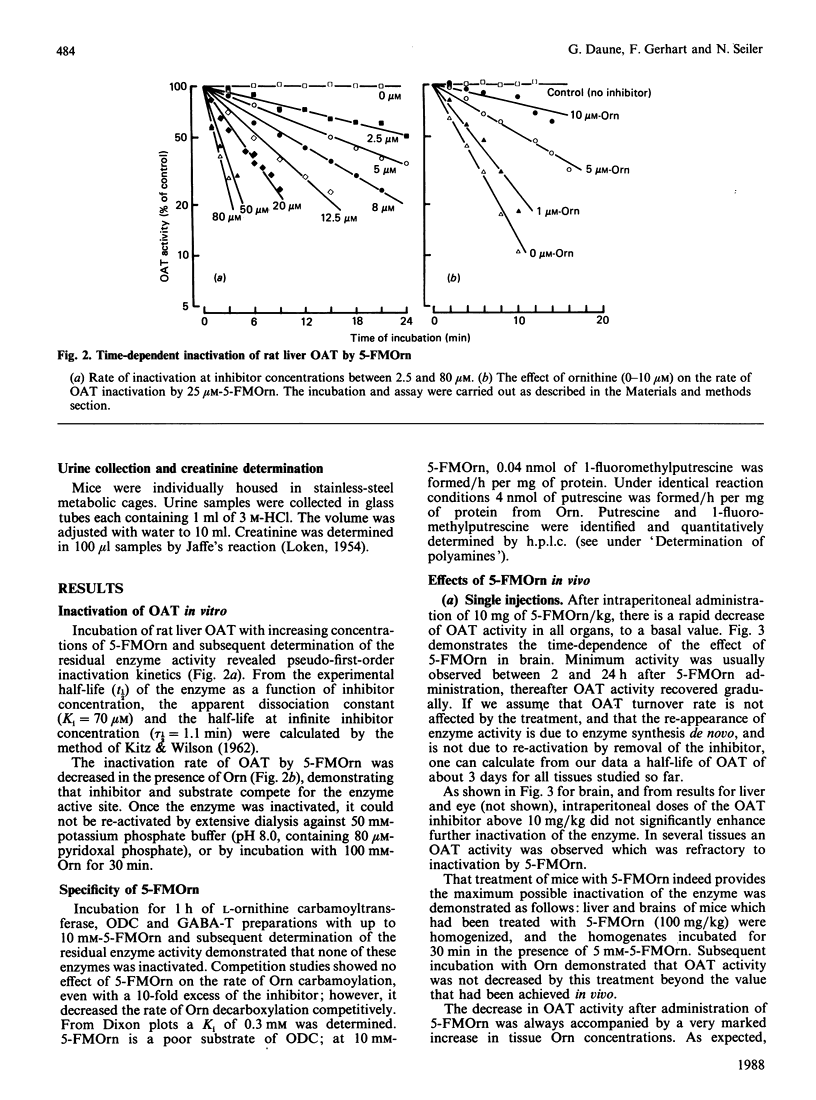
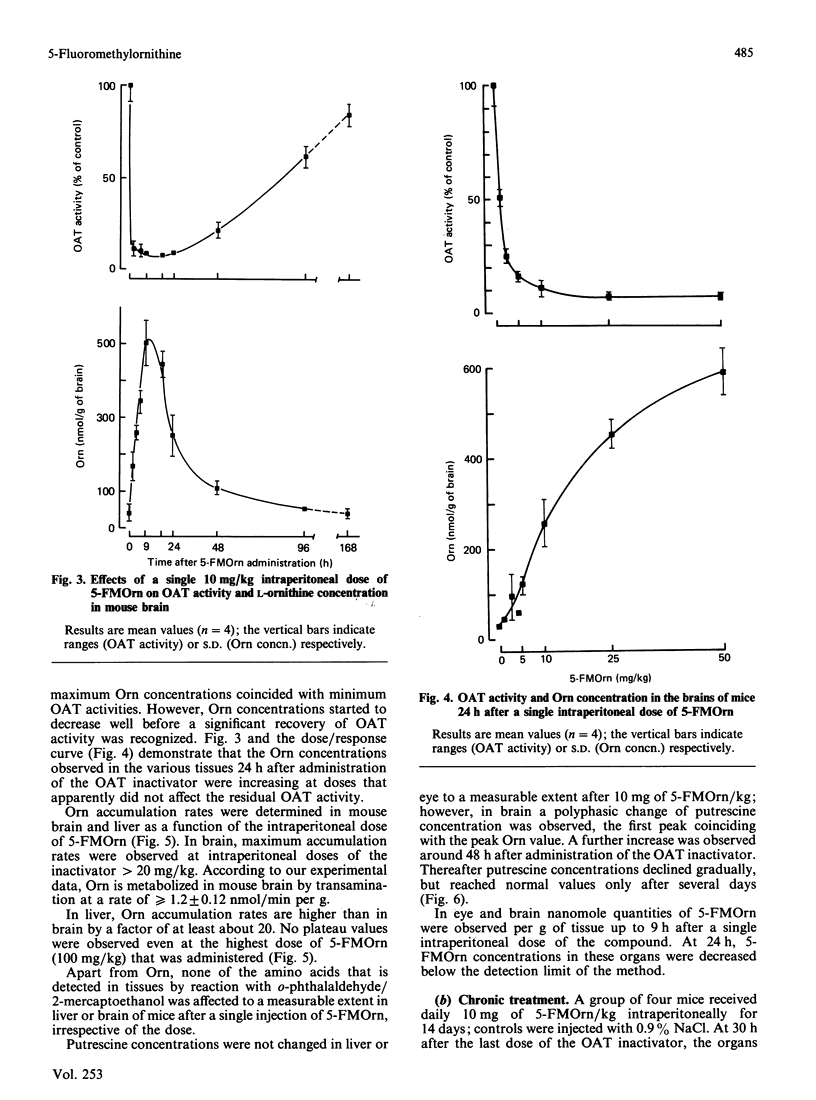
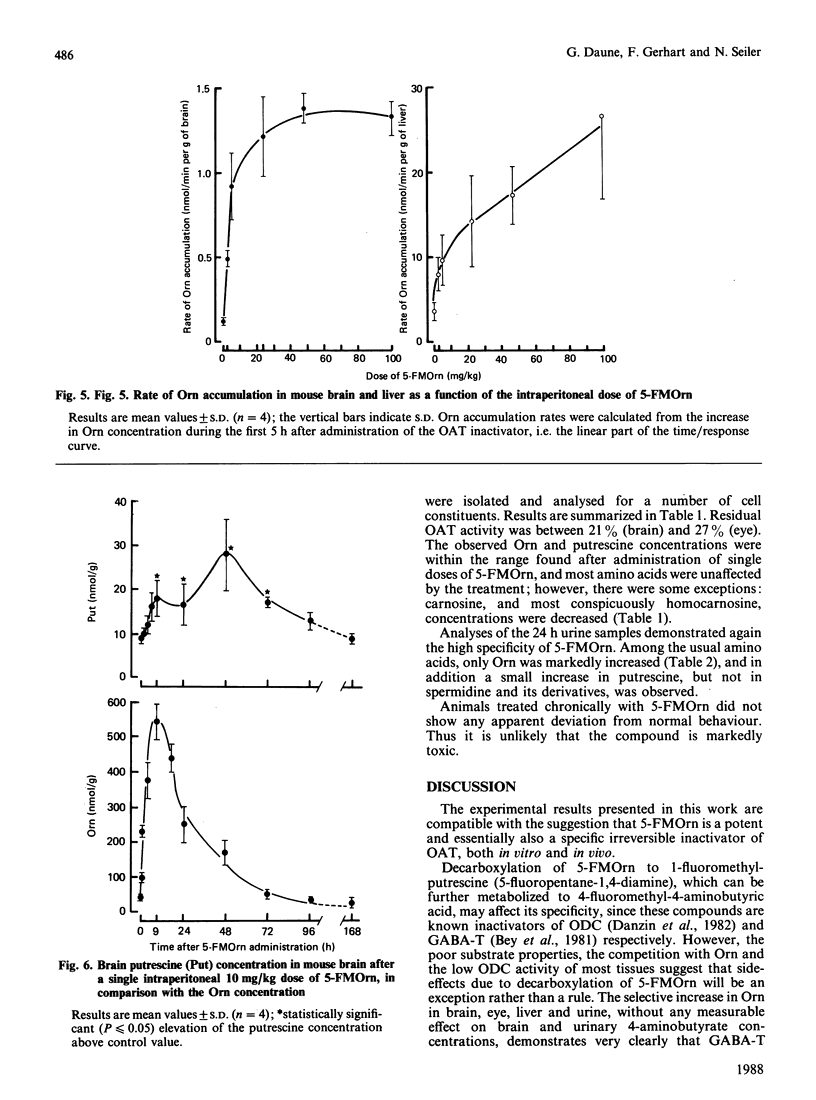
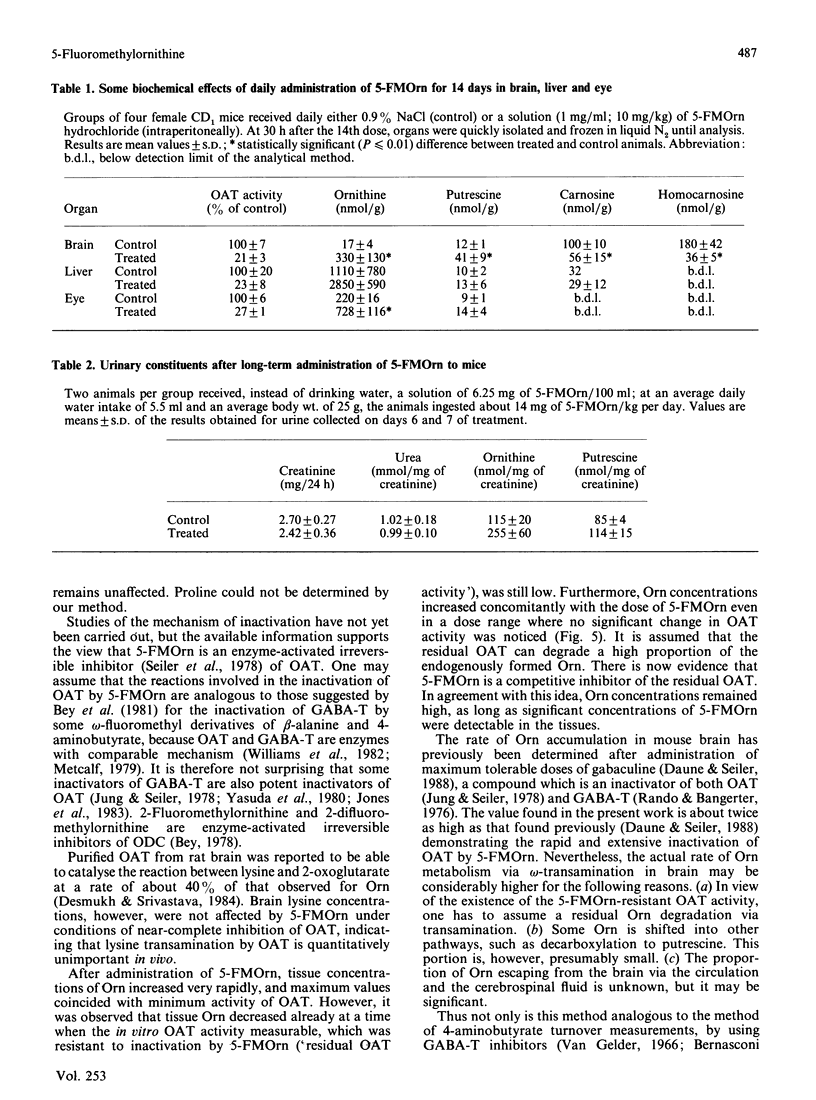
5-Fluoromethylornithine (5-FMOrn) is the first specific irreversible inhibitor of L-ornithine:2-oxoacid aminotransferase (OAT) found. Single doses (greater than 10 mg/kg) of this compound inactivate OAT to a residual OAT-like activity. This activity (10-20% of total activity) is resistant to further inactivation by higher or repeated doses of 5-FMOrn, or incubation with the inactivator in vitro. Ornithine concentrations are greatly enhanced in various tissues, and urinary ornithine is dramatically increased, but no other amino acid is affected after acute treatment with 5-FMOrn. Repeated administration decreases carnosine and homocarnosine concentrations in brain. Toxic effects were not observed. The new inactivator is considered as a tool in the establishment of functions of OAT under physiological and pathological conditions.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bernasconi R., Maitre L., Martin P., Raschdorf F. The use of inhibitors of GABA-transaminase for the determination of GABA turnover in mouse brain regions: an evaluation of aminooxyacetic acid and gabaculine. J Neurochem. 1982 Jan;38(1):57–66. doi: 10.1111/j.1471-4159.1982.tb10853.x. [DOI] [PubMed] [Google Scholar]
- Bey P., Jung M. J., Gerhart F., Schirlin D., Van Dorsselaer V., Casara P. omega-Fluoromethyl analogues of omega-amino acids as irreversible inhibitors of 4-aminobutyrate:2-oxoglutarate aminotransferase. J Neurochem. 1981 Nov;37(5):1341–1344. doi: 10.1111/j.1471-4159.1981.tb04688.x. [DOI] [PubMed] [Google Scholar]
- Boernke W. E., Stevens F. J., Peraino C. Effects of self-association of ornithine aminotransferase on its physicochemical characteristics. Biochemistry. 1981 Jan 6;20(1):115–121. doi: 10.1021/bi00504a020. [DOI] [PubMed] [Google Scholar]
- Danzin C., Bey P., Schirlin D., Claverie N. alpha-Monofluoromethyl and alpha-difluoromethyl putrescine as ornithine decarboxylase inhibitors: in vitro and in vivo biochemical properties. Biochem Pharmacol. 1982 Dec 1;31(23):3871–3878. doi: 10.1016/0006-2952(82)90304-5. [DOI] [PubMed] [Google Scholar]
- Daune G., Seiler N. Interrelationships between ornithine, glutamate, and GABA. II. Consequences of inhibition of GABA-T and ornithine aminotransferase in brain. Neurochem Res. 1988 Jan;13(1):69–75. doi: 10.1007/BF00971857. [DOI] [PubMed] [Google Scholar]
- Deshmukh D. R., Srivastava S. K. Purification and properties of ornithine aminotransferase from rat brain. Experientia. 1984 Apr 15;40(4):357–359. doi: 10.1007/BF01952550. [DOI] [PubMed] [Google Scholar]
- Herzfeld A., Knox W. E. The properties, developmental formation, and estrogen induction of ornithine aminotransferase in rat tissues. J Biol Chem. 1968 Jun 25;243(12):3327–3332. [PubMed] [Google Scholar]
- John R. A., Fowler L. J. Kinetic and spectral properties of rabbit brain 4-aminobutyrate aminotransferase. Biochem J. 1976 Jun 1;155(3):645–651. doi: 10.1042/bj1550645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones E. D., Basford J. M., John R. A. An investigation of the properties of ornithine aminotransferase after inactivation by the 'suicide' inhibitor aminohexynoate and use of the compound as a probe of intracellullar protein turnover. Biochem J. 1983 Jan 1;209(1):243–249. doi: 10.1042/bj2090243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung M. J., Lippert B., Metcalf B. W., Böhlen P., Schechter P. J. gamma-Vinyl GABA (4-amino-hex-5-enoic acid), a new selective irreversible inhibitor of GABA-T: effects on brain GABA metabolism in mice. J Neurochem. 1977 Nov;29(5):797–802. doi: 10.1111/j.1471-4159.1977.tb10721.x. [DOI] [PubMed] [Google Scholar]
- Jung M. J., Seiler N. Enzyme-activated irreversible inhibitors of L-ornithine:2-oxoacid aminotransferase. Demonstration of mechanistic features of the inhibition of ornithine aminotransferase by 4-aminohex-5-ynoic acid and gabaculine and correlation with in vivo activity. J Biol Chem. 1978 Oct 25;253(20):7431–7439. [PubMed] [Google Scholar]
- KATUNUMA N., OKADA M., MATSUZAWA T., OTSUKA Y. STUDIES ON ORNITHINE KETOACID TRANSAMINASE. II. ROLE IN METABOLIC PATHWAY. J Biochem. 1965 Mar;57:445–449. doi: 10.1093/oxfordjournals.jbchem.a128099. [DOI] [PubMed] [Google Scholar]
- KITZ R., WILSON I. B. Esters of methanesulfonic acid as irreversible inhibitors of acetylcholinesterase. J Biol Chem. 1962 Oct;237:3245–3249. [PubMed] [Google Scholar]
- Kekomäki M., Rahiala E. L., Räihä N. C. Canaline: interfering with ornithine metabolism in the isolated perfused rat liver. Ann Med Exp Biol Fenn. 1969;47(1):33–38. [PubMed] [Google Scholar]
- Kito K., Sanada Y., Katunuma N. Mode of inhibition of ornithine aminotransferase by L-canaline. J Biochem. 1978 Jan;83(1):201–206. doi: 10.1093/oxfordjournals.jbchem.a131892. [DOI] [PubMed] [Google Scholar]
- Laitinen S. I., Laitinen P. H., Hietala O. A., Pajunen A. E., Piha R. S. Developmental changes in mouse brain polyamine metabolism. Neurochem Res. 1982 Dec;7(12):1477–1485. doi: 10.1007/BF00965090. [DOI] [PubMed] [Google Scholar]
- LØKEN F. On the determination of creatinine in plasma by the Jaffé reaction, after adsorption to Lloyd's reagent. Scand J Clin Lab Invest. 1954;6(4):325–334. doi: 10.3109/00365515409134871. [DOI] [PubMed] [Google Scholar]
- McGivan J. D., Bradford N. M., Beavis A. D. Factors influencing the activity of ornithine aminotransferase in isolated rat liver mitochondria. Biochem J. 1977 Jan 15;162(1):147–156. doi: 10.1042/bj1620147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metcalf B. W. Inhibitors of GABA metabolism. Biochem Pharmacol. 1979 Jun 1;28(11):1705–1712. doi: 10.1016/0006-2952(79)90529-x. [DOI] [PubMed] [Google Scholar]
- Ono M., Inoue H., Suzuki F., Takeda Y. Studies on ornithine decarboxylase from the liver of thioacetamide-treated rats. Purification and some properties. Biochim Biophys Acta. 1972 Sep 19;284(1):285–297. doi: 10.1016/0005-2744(72)90067-8. [DOI] [PubMed] [Google Scholar]
- Peraino C., Bunville L. G., Tahmisian T. N. Chemical, physical, and morphological properties of ornithine Aminotransferase from rat liver. J Biol Chem. 1969 May 10;244(9):2241–2249. [PubMed] [Google Scholar]
- Rahiala E. L., Kekomäki M., Jänne J., Raina A., Räihä N. C. Inhibition of pyridoxal enzymes by L-canaline. Biochim Biophys Acta. 1971 Feb 10;227(2):337–343. doi: 10.1016/0005-2744(71)90065-9. [DOI] [PubMed] [Google Scholar]
- Rando R. R., Bangerter F. W. The irreversible inhibition of mouse brain gamma-aminobutyric acid (GABA)-alpha-ketoglutaric acid transaminase by gabaculine. J Am Chem Soc. 1976 Oct 13;98(21):6762–6764. doi: 10.1021/ja00437a090. [DOI] [PubMed] [Google Scholar]
- Rosenthal G. A. The biological and biochemical properties of L-canaline, a naturally occurring structural analogue of L-ornithine. Life Sci. 1978 Jul 10;23(2):93–98. doi: 10.1016/0024-3205(78)90255-2. [DOI] [PubMed] [Google Scholar]
- SCOTT E. M., JAKOBY W. B. Soluble gamma-aminobutyric-glutamic transaminase from Pseudomonas fluorescens. J Biol Chem. 1959 Apr;234(4):932–936. [PubMed] [Google Scholar]
- STRECKER H. J. PURIFICATION AND PROPERTIES OF RAT LIVER ORNITHINE DELTA-TRANSAMINASE. J Biol Chem. 1965 Mar;240:1225–1230. [PubMed] [Google Scholar]
- Seiler N., Bink G., Grove J. Regulatory interrelations between GABA and polyamines. I. Brain GABA levels and polyamine metabolism. Neurochem Res. 1979 Aug;4(4):425–435. doi: 10.1007/BF00964637. [DOI] [PubMed] [Google Scholar]
- Seiler N., Knodgen B. Determination of amino acids by separation of their ion pairs with dodecyl sulphate. J Chromatogr. 1985 May 31;341(1):11–21. doi: 10.1016/s0378-4347(00)84005-0. [DOI] [PubMed] [Google Scholar]
- Seiler N., Sarhan S. On the nonoccurrence of ornithine decarboxylase in nerve endings. Neurochem Res. 1980 Jan;5(1):97–100. doi: 10.1007/BF00964463. [DOI] [PubMed] [Google Scholar]
- Shih V. E. Regulation of ornithine metabolism. Enzyme. 1981;26(5):254–258. doi: 10.1159/000459187. [DOI] [PubMed] [Google Scholar]
- Shin W. W., Fong W. F., Pang S. F., Wong P. C. Limited blood-brain barrier transport of polyamines. J Neurochem. 1985 Apr;44(4):1056–1059. doi: 10.1111/j.1471-4159.1985.tb08724.x. [DOI] [PubMed] [Google Scholar]
- Smith R. J., Downing S. J., Phang J. M. Enzymatic synthesis and purification of L-pyrroline-5-carboxylic acid. Anal Biochem. 1977 Sep;82(1):170–176. doi: 10.1016/0003-2697(77)90145-2. [DOI] [PubMed] [Google Scholar]
- Van Gelder N. M. The effect of aminooxyacetic acid on the metabolism of gamma-aminobutyric acid in brain. Biochem Pharmacol. 1966 May;15(5):533–539. doi: 10.1016/0006-2952(66)90019-0. [DOI] [PubMed] [Google Scholar]
- Williams J. A., Bridge G., Fowler L. J., John R. A. The reaction of ornithine aminotransferase with ornithine. Biochem J. 1982 Jan 1;201(1):221–225. doi: 10.1042/bj2010221. [DOI] [PMC free article] [PubMed] [Google Scholar]


