Abstract
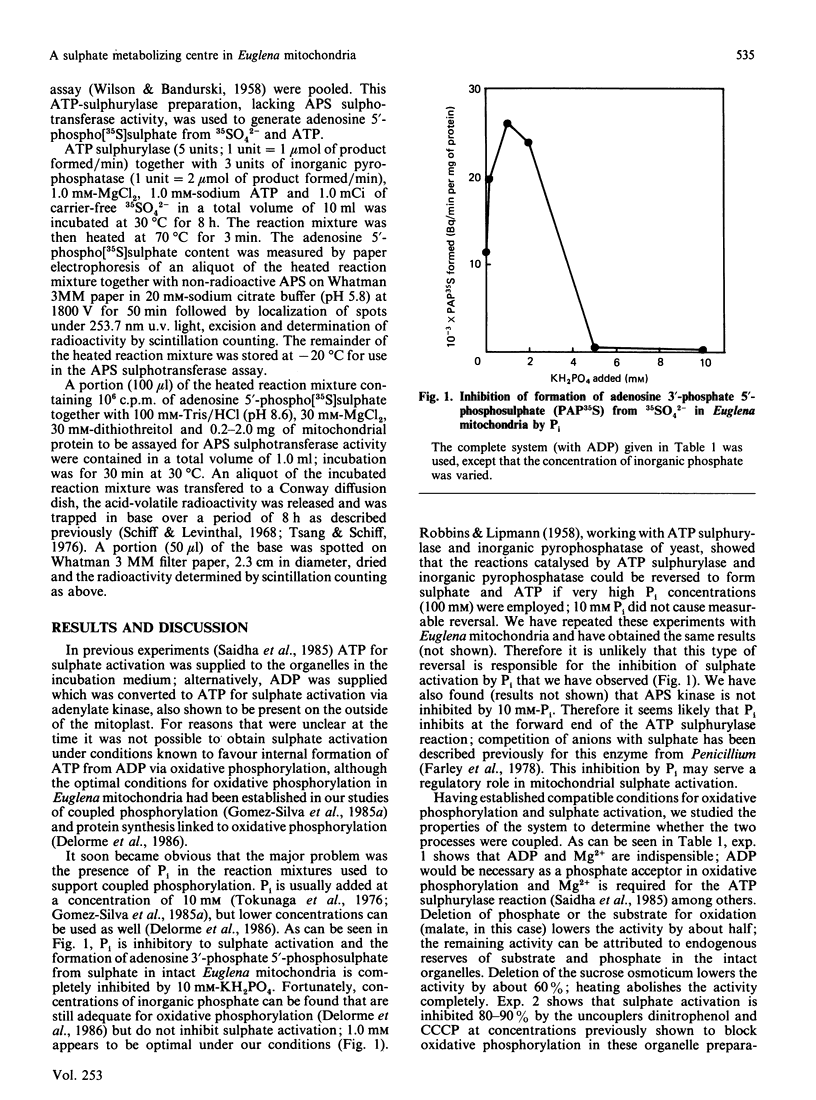
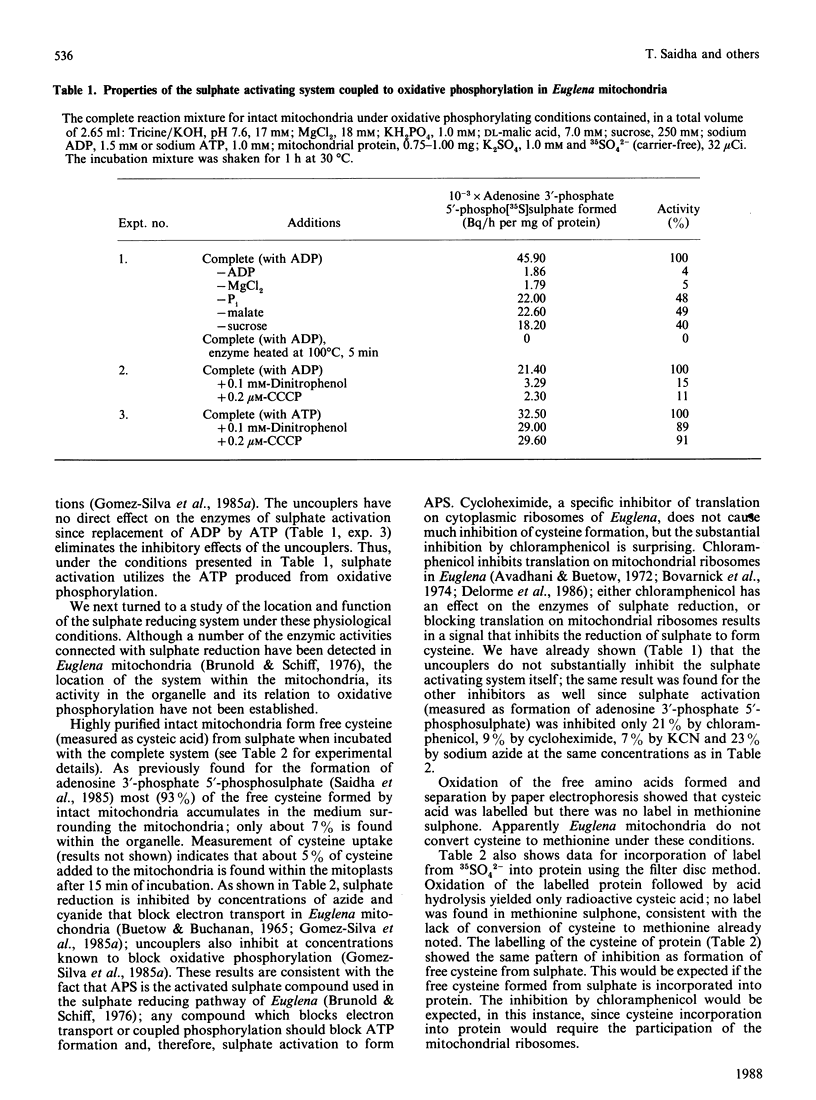
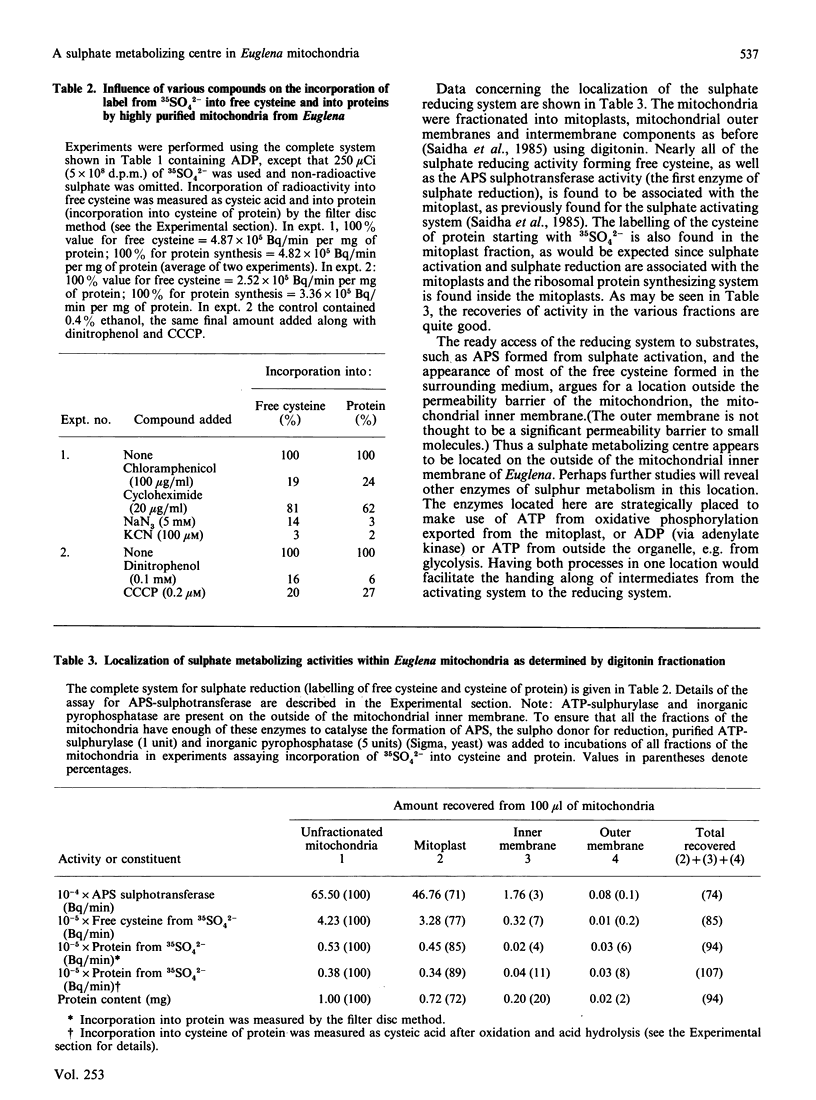
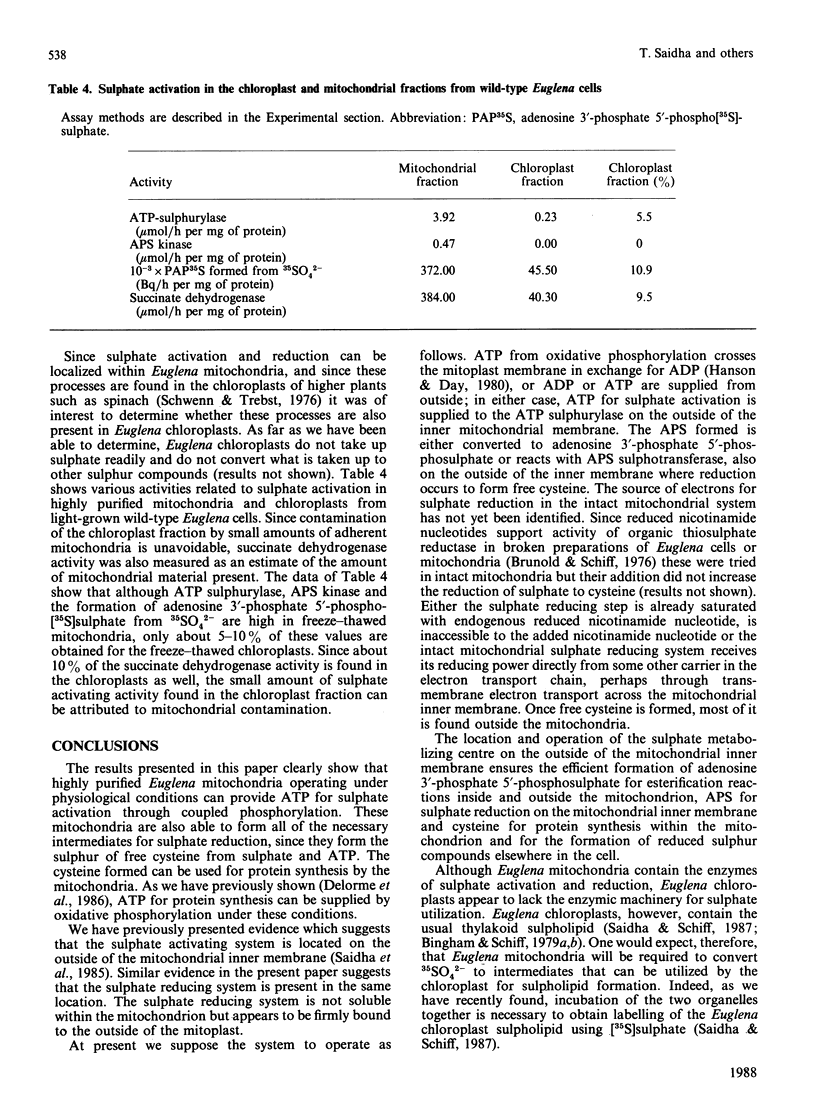
We have previously shown that a sulphate activating system is present on the outside of the inner mitochondrial membrane of Euglena gracilis Klebs. var. bacillaris Cori, but efforts to couple this system to ATP produced from oxidative phosphorylation were unsuccessful. In the present work we show that the concentration of Pi ordinarily used to support oxidative phosphorylation in these mitochondria (10 mM) inhibits sulphate activation completely; by reducing the concentration of Pi 10-fold, both processes proceeded normally. Sulphate activation under these conditions is inhibited nearly completely by the uncouplers of oxidative phosphorylation dinitrophenol (0.1 mM) and carbonyl cyanide m-chlorophenylhydrazone (CCCP) (0.2 microM). Sulphate reduction to form free cysteine, most of which appears outside the organelle, and in the cysteine of mitochondrial protein can be demonstrated in the same preparations, is membrane-bound and is inhibited by chloramphenicol (100 micrograms/ml), NaN3 (5 mM), KCN (100 microM); dinitrophenol (0.1 mM) or CCCP (0.2 microM). Digitonin fractionation of the mitochondria into mitoplasts, outer membranes and an intermembrane fraction show that reduction of 35SO4(2-) to form free cysteine and cysteine of protein is located on the mitoplasts; adenosine 5'-phosphosulphate sulphotransferase, the first enzyme of sulphate reduction, is found in the same location. Sulphate activation is highly enriched in the mitochondrial fraction of Euglena; the small amount found in the chloroplast fraction can be attributed to mitochondrial contamination. Thus, in Euglena, sulphate activation and reduction are contained in a sulphate metabolizing centre on the outside of the mitochondrial inner membrane; this centre appears to supply the mitochondrion and the rest of the cell with the products of sulphate activation as well as with reduced sulphur in the form of cysteine. Mitochondria from wild-type Euglena cells and from W10BSmL, a mutant lacking plastids completely, appear to be similar in the properties studied.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Avadhani N. G., Buetow D. E. Isolation of active polyribosomes from the cytoplasm, mitochondria and chloroplasts of Euglena gracilis. Biochem J. 1972 Jun;128(2):353–365. doi: 10.1042/bj1280353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BRUINSMA J. A comment on the spectrophotometric determination of chlorophyll. Biochim Biophys Acta. 1961 Sep 30;52:576–578. doi: 10.1016/0006-3002(61)90418-8. [DOI] [PubMed] [Google Scholar]
- BUETOW D. E., BUCHANAN P. J. OXIDATIVE PHOSPHORYLATION IN MITOCHONDRIA ISOLATED FROM EUGLENA GRACILIS. Biochim Biophys Acta. 1965 Jan;96:9–17. doi: 10.1016/0005-2787(65)90604-0. [DOI] [PubMed] [Google Scholar]
- Bingham S., Schiff J. A. Events surrounding the early development of Euglena chloroplasts. 15. Origin of plastid thylakoid polypeptides in wild-type and mutant cells. Biochim Biophys Acta. 1979 Sep 11;547(3):512–530. doi: 10.1016/0005-2728(79)90031-8. [DOI] [PubMed] [Google Scholar]
- Bingham S., Schiff J. A. Events surrounding the early development of Euglena chloroplasts. 16. Plastid thylakoid polypeptides during greening. Biochim Biophys Acta. 1979 Sep 11;547(3):531–543. doi: 10.1016/0005-2728(79)90032-x. [DOI] [PubMed] [Google Scholar]
- Bovarnick J. G., Schiff J. A., Freedman Z., Egan J. M. Events surrounding the early development of Euglena chloroplasts: cellular origins of chloroplast enzymes in euglena. J Gen Microbiol. 1974 Jul;83(0):63–71. doi: 10.1099/00221287-83-1-63. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Brunold C., Schiff J. A. Studies of sulfate utilization of algae: 15. Enzymes of assimilatory sulfate reduction in euglena and their cellular localization. Plant Physiol. 1976 Mar;57(3):430–436. doi: 10.1104/pp.57.3.430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnell J. N., Whatley F. R. A new, rapid, and sensitive assay for adenosine 5'-phosphosulphate (APS) kinase. Anal Biochem. 1975 Sep;68(1):281–288. doi: 10.1016/0003-2697(75)90706-x. [DOI] [PubMed] [Google Scholar]
- Cunningham F. X., Schiff J. A. Chlorophyll-Protein Complexes from Euglena gracilis and Mutants Deficient in Chlorophyll b: I. Pigment Composition. Plant Physiol. 1986 Jan;80(1):223–230. doi: 10.1104/pp.80.1.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis B., Merrett M. J. Malate dehydrogenase isoenzymes in division synchronized cultures of euglena. Plant Physiol. 1973 Jun;51(6):1127–1132. doi: 10.1104/pp.51.6.1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farley J. R., Nakayama G., Cryns D., Segel I. H. Adenosine triphosphate sulfurylase from Penicillium chrysogenum equilibrium binding, substrate hydrolysis, and isotope exchange studies. Arch Biochem Biophys. 1978 Jan 30;185(2):376–390. doi: 10.1016/0003-9861(78)90180-7. [DOI] [PubMed] [Google Scholar]
- Hodson R. C., Schiff J. A., Scarsella A. J. Studies of Sulfate Utilization by Algae. 7. In vivo Metabolism of Thiosulfate by Chlorella. Plant Physiol. 1968 Apr;43(4):570–577. doi: 10.1104/pp.43.4.570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osafune T., Schiff J. A. W10BSmL, a mutant of Euglena gracilis var. bacillaris lacking plastids. Exp Cell Res. 1983 Oct 15;148(2):530–535. doi: 10.1016/0014-4827(83)90176-3. [DOI] [PubMed] [Google Scholar]
- ROBBINS P. W., LIPMANN F. Separation of the two enzymatic phases in active sulfate synthesis. J Biol Chem. 1958 Sep;233(3):681–685. [PubMed] [Google Scholar]
- Saidha T., Stern A. I., Lee D. H., Schiff J. A. Localization of a sulphate-activating system within Euglena mitochondria. Biochem J. 1985 Dec 1;232(2):357–365. doi: 10.1042/bj2320357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiff J. A. A green safelight for the study of chloroplast development and other photomorphogenetic. Methods Enzymol. 1972;24:321–322. doi: 10.1016/0076-6879(72)24079-4. [DOI] [PubMed] [Google Scholar]
- Schiff J. A., Levinthal M. Studies of sulfate utilization by algae. 4. Properties of a cell-free sulfate-reducing system from chlorella. Plant Physiol. 1968 Apr;43(4):547–554. doi: 10.1104/pp.43.4.547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WILSON L. G., BANDURSKI R. S. Enzymatic reactions involving sulfate, sulfite, selenate, and molybdate. J Biol Chem. 1958 Oct;233(4):975–981. [PubMed] [Google Scholar]


