Abstract
Plants defend themselves chemically against herbivory through secondary metabolites and phytohormones. Few studies have investigated how constitutive variation in secondary metabolites contributes to systemic herbivory response. We hypothesized that plants with lower constitutive defenses would induce a stronger phytohormone response to spatially separated herbivory than plants with high constitutive defense. We used growth chamber bioassays to investigate how aboveground herbivory by Colorado potato beetle (Leptinotarsa decemlineata, CPB) and belowground herbivory by northern root-knot nematode (Meloidogyne hapla, RKN) altered phytohormones and glycoalkaloids in roots and shoots of two lines of wild potato (Solanum chacoense). These lines had different constitutive levels of chemical defense, particularly leptine glycoalkaloids, which are only present in aboveground tissues. We also determined how these differences influenced the preference and performance of CPB. The susceptible wild potato line responded to aboveground damage by CPB through induction of jasmonic acid (JA) and OPDA. However, when challenged by both RKN and CPB, the susceptible line retained high levels of JA, but not OPDA. Beetles gained more mass after feeding on the susceptible line compared to the resistant line, but were not affected by nematode presence. Belowground, JA, JA-Isoleucine, and OPDA were higher in the resistant line compared to the susceptible line, and some compounds demonstrated response to local herbivory. In contrast, the susceptible line did not induce phytohormone defenses belowground. These findings allow us to predict that constitutive level of defense may influence the threshold of herbivory that may lead to plant-mediated effects on spatially separated herbivores.
Supplementary Information
The online version contains supplementary material available at 10.1007/s10886-024-01538-2.
Keywords: Jasmonic acid, OPDA, Plant-herbivore interactions, Leptinotarsa decemlineata, Meloidogyne hapla, Solanum chacoense
Introduction
Plants can respond dynamically to antagonists, such as insect herbivores, that remove their tissues (Gatehouse 2002), or plant-parasitic nematodes that manipulate the plant to create a nutritional sink (Hewezi and Baum 2013; Jones et al. 2013). Plants have a variety of tools at their disposal to minimize the negative impact of herbivory, but one of the most well-studied and important routes is through chemical defense (e.g. Hare 2011; Dyer et al. 2018). In responding to herbivory, plants induce local responses at the site of feeding to quickly deter herbivory, and also upregulate systemic pathways that protect other parts of the plant through the vascular system, phloem, apoplast, or volatile signals (Kessler and Baldwin 2002; Karban and Baldwin 2007; Ruan et al. 2019). Induced defense responses are often initiated by phytohormone pathways such as jasmonic acid (JA), salicylic acid (SA), ethylene (ET), and abscisic acid (ABA) (Caarls et al. 2015; Ruan et al. 2019; Yang et al. 2019). In many cases, these pathways regulate both defense and primary functions such as growth (Yang et al. 2019), and unsurprisingly, are broadly conserved across plant taxa (Meyer et al. 1984; Walling 2000; Raskin 1992).
In addition to interacting with broadly conserved phytohormone pathways, plant antagonists also interact with secondary metabolites, such as glycoalkaloids in solanaceous plants (Zhao et al. 2021) or glucosinolates in Brassicaceae (Textor and Gershenzon 2009) (Fig. S1). The quantity and identity of these secondary metabolites can have major impacts on plant resistance to herbivory (Kaiser et al. 2020; Hauri et al. 2021) and can even drive insect speciation and plant-insect community diversity (Richards et al. 2015; Glassmire et al. 2016). Many secondary metabolites are constitutive and are present in the plant regardless of herbivory (Hartmann 1996), though levels of these compounds may also increase in response to herbivory (Bezemer and Van Dam 2005; Textor and Gershenzon 2009). Plants with high levels of constitutive defense are predicted to have a higher threshold for inducing a significant defense response to herbivory, since they receive relatively less herbivore damage, and inducing further defenses is costly (Karban et al. 1997; Walters and Heil 2007; Kessler 2015).
Because phytohormone pathways and taxon-specific plant defenses span both above- and belowground tissues, even herbivores that are spatially separated—root and shoot feeders—can influence each other indirectly through plant chemical changes (Soler et al. 2012, 2013; Wondafrash et al. 2013). However, the outcomes of these interactions are variable and often species-specific (Wondafrash et al. 2013; Soler et al. 2013; Hauri and Szendrei 2022). Although several studies have investigated the effects of feeding guild (such as chewing vs. phloem-feeding) on plant-mediated interactions (van Dam et al. 2018) and thus potential crosstalk between phytohormone pathways (Soler et al. 2013; van Dam et al. 2018), there is a knowledge gap in our understanding of how a plant’s constitutive level of secondary metabolites influences the outcome of plant-mediated interactions. Additionally, the outcome of interactions between herbivorous nematodes and insects are dependent on plant family; one possible explanation for this is variation in specialized, taxon-specific secondary metabolites (Hauri and Szendrei 2022) with unique modes of action (e.g., cardenolide inhibition of the enzyme Na+/K+-ATPase (Agrawal et al. 2012), or glucosinolate conversion into isothiocyanates that react with insect protein thiols and amines, leading to loss of function (Jeschke et al. 2016). For example, belowground nematode damage can alter glucosinolate composition (Hol et al. 2013) and quantity (Van Dam et al. 2005) in aboveground tissues, indicating that these compounds play a role in plant-mediated defenses.
Spatially separated herbivores often interact with plant chemical pathways by changing the relative strength of defenses. This can occur because the initial attacker induces systemic pathways, thus leading to a stronger response than for local herbivory alone (Fig. S1B).
Alternatively, an attacker may suppress defense pathways (Fig. S1B). While in some cases plant-parasitic nematodes induce systemic defenses (Van Dam et al. 2005; Arce et al. 2017; Guo and Ge 2017), they are also capable of intimately interacting with plant defenses utilizing stylet secretions to negate or alter plant defense responses to form their feeding site (Hewezi and Baum 2013). However, how significantly plants’ defense strategy is altered by suppression would likely depend on how much the plant invests in constitutive defense vs. induction. This interference may have a reduced impact on spatially separated herbivores in plants that have high levels of constitutive defense. Thus, we hypothesized that plants with high levels of constitutive defense would show less significant local and systemic (root-to-shoot and shoot-to-root) responses to herbivory than plants with low levels of constitutive defense.
To determine how plants with different levels of secondary metabolites respond to spatially separated herbivores above- and belowground, we performed a set of growth chamber and laboratory experiments using two recombinant inbred wild potato (Solanum chacoense) lines that differed quantitatively and qualitatively in glycoalkaloid content. These lines specifically differed in the presence of leptines, which are acetylated glycoalkaloids only present in aerial tissues known to provide resistance to the Colorado potato beetle (Leptinotarsa decemlineata; hereafter, CPB) through cell membrane disruption and cholinesterase inhibition (Kaiser et al. 2021). We exposed plants with high and low levels of constitutive defense to the northern root-knot nematode (Meloidogyne hapla; hereafter, RKN) which forms galls in the plant root; to CPB, a chewing herbivore; to both; or to neither. We then measured levels of phytohormones and secondary metabolites in plant roots and shoots. Specifically, we measured the following phytohormones: jasmonic acid (JA), jasmonic acid isoleucine (JA-Ile), 12-oxo-phytodienoic acid (OPDA), salicylic acid (SA), salicylic acid beta-glucoside (SAG), and abscisic acid (ABA). Jasmonic acid, JA-Ile, and OPDA are all components of the JA pathway, which is typically involved in defense against necrotrophic pathogens (Yang et al. 2019) and wounding due to herbivory (Schilmiller and Howe 2005). OPDA is a JA precursor, and JA-Ile is the biologically active form of JA (Yang et al. 2019). The SA pathway is primarily associated with response to biotrophic pathogens and viruses; SAG is a storage form of SA (Vlot et al. 2009). ABA is involved in drought response and seed development, among other functions (Nakashima and Yamaguchi-Shinozaki 2013). Additionally, we assessed CPB preference and performance when exposed to different combinations of plant line and RKN presence.
These experiments allowed us to answer the following questions: (1) how do root and shoot herbivory, both separately and in combination, influence induction of phytohormone pathways? (2) how does above- and belowground herbivory alter the expression of family-specific secondary metabolites? and, (3) how do plant chemical defense changes in response to belowground herbivory affect an aboveground chewing herbivore? Answering these questions will help us to understand the role of different types of chemical defenses in mediating spatially separated herbivore interactions.
Methods and Materials
Organisms for Experiments
To investigate the effects of leptine on plant-mediated interactions between above and below ground herbivores, we used two breeding lines generated from a cross between the S. chacoense lines USDA 8380-1 and M6 that differed in the presence of leptines I and II (Kaiser et al. 2021). Leptines are acetylated glycoalkaloids only present in aerial tissues known to provide resistance to CPB through cell membrane disruption and cholinesterase inhibition (Kaiser et al. 2021). Line EE501 F5_093_02_05_01 (hereafter, ‘susceptible’), contained 0 mg/g dry weight leptine I or II. Line EE501 F5_278_02_01_03 (hereafter, ‘resistant’), contained an average of 1.6 mg/g dry weight leptine I and 0.22 mg/g leptine II (Table S5 in Kaiser et al. 2021). Plants were maintained in tissue culture on Murashige and Skoog basal medium with vitamins and sucrose (M5501; Murashige and Skoog salts at 8.8 g L− 1, 3% sucrose, pH 5.8, and 0.6% plant agar; Murashige and Skoog 1962) at 22 °C and 16 h:8 h L: D cycle for 2 weeks after propagation. At that point plantlets were transplanted to a 50:50 mix of play sand (Quikrete, Atlanta, GA) or all-purpose sand (KolorScape, Atlanta, GA) and topsoil (Oldcastle Lawn & Garden, Inc., Atlanta, GA) in 9 cm3 pots. Plants were then maintained in growth chambers at 25 °C on a 16:8 L: D cycle and watered ad libitum. All plants were fertilized with a 375 ppm solution of 20-20-20 NPK fertilizer (Jack’s Professional 20-20-20 fertilizer, JR Peters, Allentown, PA) weekly starting one week post-transplant.
Colorado potato beetles were maintained in a colony initiated with field-collected individuals from the Michigan State University Montcalm Potato Research Center (Lakeview, MI) in May 2020. Beetles were maintained on potato (Solanum tuberosum) cv. Atlantic or Russet Norkotah on a 16 h:8 h L: D cycle at 22–28 °C. Egg masses for experiments were transferred to Petri dishes where larvae were allowed to hatch and provided S. tuberosum leaves prior to use in experiments.
Root-knot nematode colonies were maintained on eggplant (Solanum melongena cv. Black Beauty (Burpee, Warminster Township, PA) or tomato (Solanum lycopersicum) cv. New Girl (Johnny’s Selected Seeds, Winslow, ME) in a 50:50 sand: topsoil mix in Michigan State University’s Plant Science Research Greenhouses. Plants were watered ad libitum with a 1:20 ratio of water to NPK fertilizer concentrate (200–300 ppm; Jack’s Professional 20-20-20 fertilizer). Root-knot nematode eggs were elucidated from host plant roots using a slightly adapted, 1% NaOCl shaking protocol (Hussey and Barker 1973). Eggs were stored in plastic tubes with water after extraction and before inoculation, at approximately 17,000 eggs/ml.
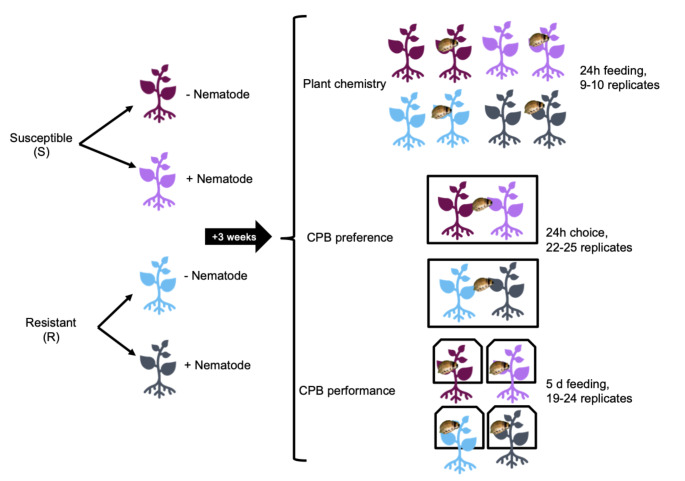
For all experiments, 2-week-old plants in nematode treatments were inoculated with 1100 RKN eggs per 100 cm3 soil. Eggs were pipetted into four holes approximately 1 cm deep and 1–2 cm from the plant stem, made with the non-tip end of a fine point Sharpie marker (Newell Brands, Atlanta, GA) which were then covered with soil; control plants were inoculated with an equal volume of deionized water. Plants were allowed to develop for three weeks before use in experiments to allow for nematode hatching and invasion of the root (Fig. 1).
Fig. 1.
Experimental design. Plants of the susceptible (S) and resistant (R) plant lines were grown with and without root-knot nematodes at a rate of 1100 eggs per cubic centimeter of soil for three weeks in the growth chamber. At that point, plants were either used in experiments to assess plant chemistry (phytohormone and secondary metabolite levels in roots and shoots), CPB preference, or CPB performance. One CPB larva was added per plant for plants in aboveground herbivory treatments
Internal Chemistry
We investigated how different types of herbivory (nematode or beetle) influenced plant secondary metabolite and phytohormone content in roots and shoots. We measured the following phytohormones: JA, JA-Ile, OPDA, SA, SAG, and ABA.
Plants with and without leptines were grown as described above and exposed to one of four herbivory treatments: no herbivory; aboveground only (CPB); belowground only (RKN); or both (CPB and RKN), for a total of eight treatments with 9–10 replicates (one replicate = one plant) per treatment. Three weeks after nematode inoculation, one 2nd instar CPB was bagged on each plant in an aboveground herbivory treatment and allowed to feed for 24 h. All plants were then transferred to the lab, where beetles were removed. Plant roots were gently washed to remove soil and the entire plant was frozen at -80 °C until processing.
Plant tissues were processed for LC-MS analysis according to a modified protocol from Zeng et al. 2011. Approximately 0.07-0.1 g frozen leaf tissue from fully expanded leaflets or 0.03–0.1 g root tissue was weighed and added to 2 ml polypropylene microtubes (USA Scientific, Ocala, FL) with three 3 mm stainless steel balls (SPEX Sample Prep, Metuchen, NJ) per tube. Aboveground samples typically consisted of 2–5 complete leaflets; root biomass was smaller than aboveground biomass, and samples were often comprised of the complete root system of a plant. Frozen tissue was ground in a pre-frozen bead beater at 30/s until fully ground. Samples were extracted with 1 ml extraction buffer (80:20 v/v methanol: water, 0.1% formic acid, with internal standards SA-13C6, ABA-d6, JA-d5, digitoxin). After incubating at 4 °C on a rocking platform for 16 h, samples were centrifuged at 4 °C for 10 min at 14,000 rpm. The supernatant (80 µl) was transferred to high-performance liquid chromatography vials (Restek, Bellefonte, PA) with 250 µl inserts (Agilent Technologies, Santa Clara, CA) for phytohormone analysis, and 10 µl was transferred to an HPLC vial containing 990 µL extraction buffer for glycoalkaloid analysis.
Samples were stored at -20 °C until processing at the Michigan State University’s Mass Spectrometry and Metabolomics Core (East Lansing, MI). Glycoalkaloid samples were analyzed using a Waters Xevo G2-XS Quadrupole-Time-of-flight LC/MS/MS system with a Waters Acquity BEH-C18 UPLC column (2.1 × 100 mm). The machine was operated in positive ion mode. Compounds were eluted using a binary gradient of solvent A (0.1% formic acid in water) and solvent B (acetonitrile) at a flow rate of 0.3 ml min− 1 at 40 °C following a stepwise gradient: 98.0% A, 2.0% B; 0.50 min, 85.0% A, 15.0% B; 5.00 min, 40.0% A, 60.0% B; 7.00 min, 1.0% A, 99.0% B; 8.00 min, 1.0% A, 99.0% B; 8.01 min, 98.0% A, 2.0% B; 10.00 min, 98.0% A, 2.0% B. Jasmonic acid (JA), JA-Ile, OPDA, SA, SAG, and ABA were analyzed with a Waters Xevo TQ-S triple quadrupole LC/MS/MS system with a Waters Acquity BEH-C18 UPLC column (2.1 × 50 mm). Phytohormones were eluted using a binary gradient of solvent A (0.1% formic acid in water) and solvent B (acetonitrile) at a flow rate of 0.4 ml/min at 40 °C following a stepwise gradient: 98% A, 2% B; 0.5 min, 98% A, 2% B; 3 min, 30% A, 70% B; 4 min, 1% A, 99% B; 5 min, 1% A, 99% B, 5.01 min, 98% A, 2%B; 6 min, 98% A, 2% B. MS/MS details for the targeted phytohormone method can be found in Table S1. Data were collected with Waters MassLynx software and processed with Waters Quanlynx MS software. Glycoalkaloids α-solanine and α-chaconine were identified based on comparing retention time, accurate mass and fragmentation patterns with authentic standards (Fig. S2, S3). The other dominant peaks in our untargeted analysis could not be conclusively identified but were annotated as glycoalkaloids based on mass and fragmentation. Prior to statistical analysis, internal chemistry data were normalized to internal standards (phytohormones: JA-d5, ABA-d6, and SA-13C6; untargeted secondary metabolites: digitoxin) and tissue sample mass. Additionally, we excluded compounds from our untargeted analysis that were highest in blanks as well as all compounds with retention times < 0.9 min and > 9 min. This was done to exclude lipids and other compounds we were confident were not relevant secondary metabolites.
Preference and Performance Assays
We used a choice assay to determine how RKN presence influenced CPB larval preference, and no-choice assays to determine how RKN presence influenced CPB larval performance when provided with different plant conditions (susceptible or resistant). The choice assay was performed in metal mesh cages (30 cm3, Bioquip, Rancho Dominguez, CA). A single larva (5–6 days old) was placed on a Petri dish equidistant between two plants of a single line (‘susceptible’, N = 22; or ‘resistant’, N = 25), one inoculated with nematodes and one uninoculated. After 24 h, we recorded the larva’s location. Larvae not located on a plant after 24 h were excluded from the analysis. In no-choice assays, larvae were weighed and randomly assigned to an experimental replicate. For the no-choice assay, a single larva (5–6 days old) was bagged on a plant and allowed to feed for 5 days (susceptible – nematodes: N = 19; susceptible + nematodes: N = 21; resistant – nematodes: N = 24; resistant + nematodes: N = 22). Larvae were then removed, and weights were recorded. We also visually estimated the aboveground biomass removed by herbivory to the nearest 5% and counted the total number of leaflets and the number of damaged leaflets for each plant to check our estimates. The amount of leaf tissue consumed by beetles was calculated by multiplying the total number of leaflets for a plant by the percent removed by herbivory.
Statistical Analyses
All analyses were performed in R version 4.2.2. (R Core Team 2022). Because we measured a small number of phytohormones but a much larger number of secondary metabolites (> 3500 across all samples), we chose to analyze individual phytohormones and secondary metabolite composition (including leptine and non-leptine glycoalkaloids). However, we analyzed α-solanine and α-chaconine quantitatively since they were known to be in our target lines and are often studied in relation to CPB resistance. Secondary metabolite composition by treatment was analyzed with the following functions, all from the package ‘vegan’ (Oksanen et al. 2024): Permutational multivariate analysis of variances (PERMANOVA) were calculated for models using the ‘adonis2’ function; dispersion was calculated with the ‘vegdist’ function (method = ‘bray’) followed by the ‘betadisper’ and ‘permutest’ functions; and pairwise comparisons were performed with the ‘pairwise.adonis2’ function. For PERMANOVA, leaf data were square root transformed and a Bray-Curtis dissimilarity matrix was created using these values. For both PERMANOVA and dispersion analyses, we evaluated models with plant line, CPB presence, and nematode presence as interactive fixed effects. Because this was not possible with the ‘permutest’ function, we evaluated each combination of plant line, nematode presence, and CPB feeding as ‘treatment’. Nonmetric multidimensional scaling (NMDS) plots were created using the ‘metaMDS’ function; 95% confidence intervals were calculated with the ‘anosim’ function in the package ‘vegan’ (Oksanen et al. 2024).
Individual compounds (JA, JA-Ile, OPDA, SA, SAG, ABA, α-solanine, and α-chaconine) were analyzed with generalized linear models with nanomoles g− 1 (phytohormones) or micromoles g− 1 (α-solanine, α-chaconine) of compound as the response variable, and line, treatment, or their interaction as fixed effects. Beetle preference data (location after 24 h) was analyzed using a χ2 test, and performance data (change in mass after 1 week of feeding or amount of tissue consumed) was analyzed using linear mixed models with plant line and nematode as additive or interactive fixed effects, and experiment date as a random effect. Post-hoc testing was performed with the package ‘emmeans’ (Lenth 2024) with a Tukey adjustment to analyze pairwise comparisons across all treatments.
Results
Secondary Metabolite Composition
Leaves
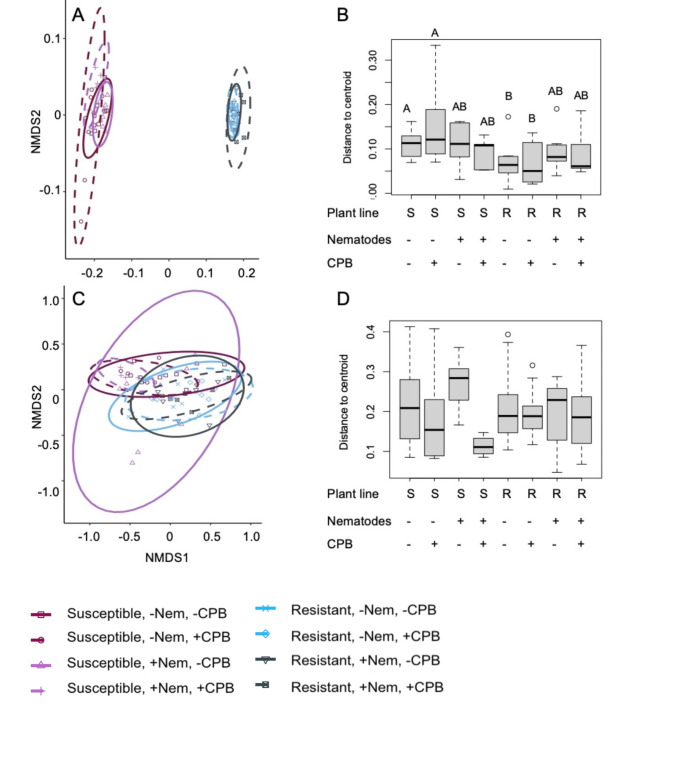
The most abundant peaks in our analysis of the plant internal chemistry were glycoalkaloids. 90% of the variation in leaf secondary metabolite composition was due to plant line (F1,57 = 697.99, p < 0.01, R2 = 0.90, Fig. 2A). Only about 1% of the variance in secondary metabolite composition was explained by CPB feeding (F1,57 = 7.64, p < 0.01, R2 = 0.01, Fig. 2A). Nematode presence did not influence leaf secondary metabolite composition (F1,57 = 1.63, p = 0.19, R2 < 0.01, Fig. 2A). Additionally, the dispersion—distance from points to centroids—of secondary metabolite composition differed among treatments (F7,57 = 2.29, p = 0.04, Fig. 2B). Once again, this was largely driven by plant line; on average, distance from points to centroids was 37.5% lower for resistant line leaf samples than susceptible line leaf samples (F1,63 = 17.88, p < 0.01), indicating that the secondary metabolite composition was more similar between resistant samples than susceptible samples.
Fig. 2.
Secondary metabolite composition is influenced more by plant line than herbivory. Non-metric multidimensional scaling (NMDS) of secondary metabolite composition by treatment in leaves (A, stress = 0.05) and roots (C, stress = 0.11), and boxplots of distance to centroids for leaves (B) and roots (D). Circles represent 95% confidence intervals of secondary metabolite composition for each treatment. Treatment includes plant line (Susceptible, S; Resistant, R), nematode presence (-Nem/+Nem), and CPB presence (-CPB/+CPB) with 9–10 replicates (individual plants) per treatment
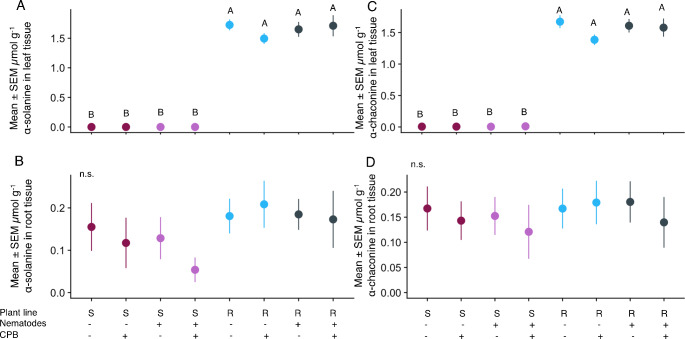
Glycoalkaloids α-solanine and α-chaconine showed similar trends to the overall secondary metabolite composition. Both compounds were higher in the resistant line than the susceptible line (α-solanine: F2,63 = 706, p < 0.01; α-chaconine: F2,63 = 789, p < 0.01; Fig. 3A, C). Neither nematode nor CPB herbivory altered the amount of either compound (F1,61 ≤ 2.29, p ≥ 0.14).
Fig. 3.
Glycoalkaloids α-solanine and α-chaconine vary based on plant line, but not herbivory. Mean ± SEM micromoles per g fresh weight α-solanine in (A) leaves and (B) roots, and α-chaconine in (C) leaves and (D) roots. Letters indicate p < 0.05 in pairwise comparisons across all treatments; n.s. indicates that no post-hoc pairwise comparisons between treatments were significant. N = 9–10 replicates (individual plants) per treatment
Roots
21% of variation in root secondary metabolite composition was due to plant line (F1,54 = 17.71, p < 0.01, R2 = 0.21, Fig. 2C). Neither CPB feeding nor nematode presence affected root secondary metabolite composition (F1,54 ≤ 2.11, p ≥ 0.08, R2 ≤ 0.02, Fig. 2C). Dispersion did not differ between treatments overall (F7,54 = 1.41, p = 0.22, Fig. 2D) nor were there differences by plant line (F1,60 = 2.67, p = 0.1, Fig. 2D). The treatment with the highest average distance to the centroid was the susceptible line with nematodes and without CPB; if CPB were present, the average distance to the centroid was nearly 60% lower (Fig. 2D). On the resistant line, the difference in average distance to centroids between plants with nematodes alone and plants with nematodes and CPB was only 2.56%.
Plant line also influenced the amount of α-solanine in the roots (F2,60 = 36.43, p < 0.01, Fig. 3B, D). However, CPB and nematode herbivory had no effect (F1,59 ≤ 0.39, p ≥ 0.53). We found similar results for α-chaconine (plant line: F2,60 = 54.41, p < 0.01; CPB: F1,58 = 0.36, p = 0.55; nematodes: F1,59 = 0.17, p = 0.68).
Phytohormone Response
Leaves
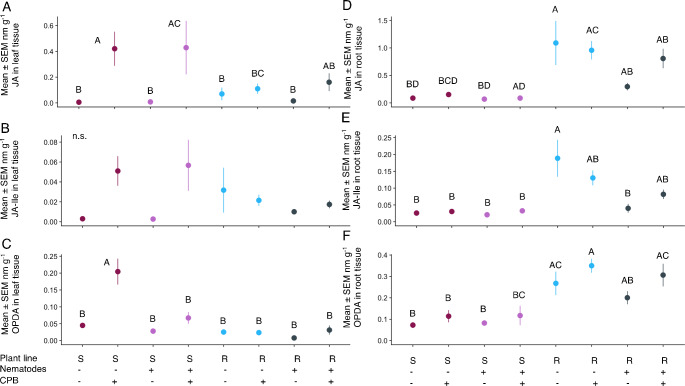
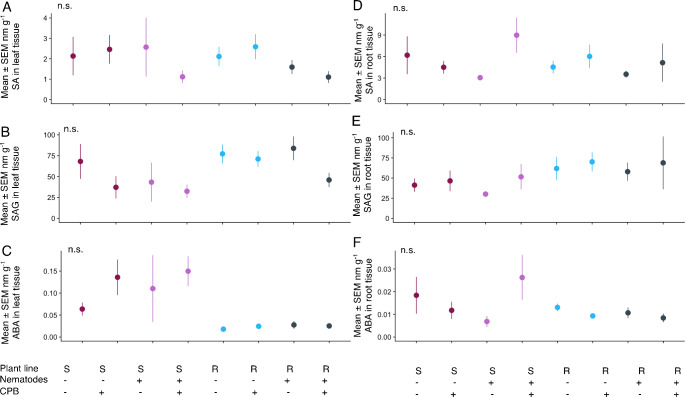
Colorado potato beetle feeding had a significant effect on the amount of all plant phytohormone levels in the JA pathway aboveground—JA, JA-Ile, and OPDA (Table S2, Fig. 4A, B, C)—but the increase was more pronounced in the susceptible line (Table S2, Fig. 4A, B, C, line x CPB interaction). However, post hoc testing across all treatments for each compound did not always yield a significant increase in these compounds (Fig. 4A, B, C). Systemic effects of nematode presence only affected the amount of OPDA in the leaves (Table S2). While plants with CPB alone experienced a 354% increase in OPDA, plants with CPB and nematodes had no significant change in OPDA compared to non-herbivory controls. This suppression did not occur with JA (Table S2, Fig. 4A). SA, SAG, and ABA levels did not differ between CPB or nematode herbivory treatments (Table S2, Fig. 5A-C).
Fig. 4.
Jasmonic acid pathway compounds altered by CPB feeding in the shoots and plant line in the roots. Mean ± SEM nanomoles per g leaf tissue (A) Jasmonic acid (JA), (B) Jasmonic acid isoleucine (JA-Ile), (C) 12-oxo-phytodienoic acid (OPDA); and mean ± SEM nanomoles per g root tissue (D) Jasmonic acid, (E) Jasmonic acid isoleucine (JA-Ile), (F) 12-oxo-phytodienoic acid (OPDA). Letters indicate p < 0.05 in pairwise comparisons across all treatments. N.S. indicates that no post-hoc pairwise comparisons between treatments were significant. N = 9 independent replicates per treatment
Fig. 5.
Plant hormones SA, SAG, and ABA were unaltered by herbivory or plant line. Mean ± SEM nanomoles per g leaf tissue (A) salicylic acid (SA), (B) salicylic acid beta-glucoside (SAG), (C) abscisic acid (ABA); and mean ± SEM nanomoles per g root tissue (D) SA, (E) salicylic acid beta-glucoside (SAG), and (F) ABA. N.S. indicates that no post-hoc pairwise comparisons between treatments were significant. N = 9 independent replicates per treatment
The amount of OPDA, SAG, and ABA in leaf tissue differed between the two plant lines, while SA did not (Table S2, Fig. 5A, B, C). For OPDA, this was largely driven by CPB herbivory alone as discussed above (Fig. 4C). Average SAG was 13% higher in the resistant line (Fig. 5E). In contrast, ABA was 255% higher in the susceptible line (Fig. 5C) regardless of herbivory.
Roots
In root tissue, the amount of JA, JA-Ile, OPDA, and SAG were higher in the resistant line than the susceptible line (Table S2, Figs. 4D, E and F, 5D and 1157% higher, 629% higher, 268% higher, and 50% higher in the resistant line compared to the susceptible line, respectively). Main effects of nematode herbivory were significant for JA and JA-Ile (Table S2). Local nematode presence reduced JA by 36% in the susceptible line and 41% in the resistant line, and JA-Ile by 12% in the susceptible line and 61% in the resistant line (Table S2, Fig. 4D, E, F). In contrast, systemic effects from CPB feeding increased OPDA in root tissue (Table S2) by 49% in the susceptible line and 25% in the resistant line, indicating shoot-to-root-effects (Fig. 4F).
Beetle Response
Preference
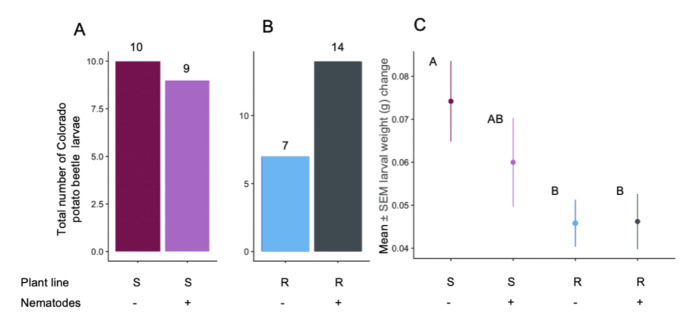
Beetles had no preference between plants with and without nematodes for the susceptible line (χ2 = 0.05, df = 1, p = 0.82; Fig. 6A). Although they chose nematode-infested plants twice as often as control plants on the resistant line, this difference was not statistically significant (χ2 = 2.33, df = 1, p = 0.13; Fig. 6B).
Fig. 6.
Colorado potato beetle (CPB) response to plant and nematode treatments. (A) CPB preference for susceptible plants with and without root-knot nematodes (RKN). Numbers above bars indicate number of CPB larvae found on plants of that treatment after 24 h in choice assays. (B) CPB preference for resistant plants with and without RKN. Numbers above bars indicate number of beetle larvae found on plants of that treatment after 24 h in choice assays. (C) CPB mass change after one week of feeding on susceptible and resistant plants with and without RKN presence. A single larva (5–6 days old) was bagged on a plant and allowed to feed for 5 days (susceptible – nematodes: N = 19; susceptible + nematodes: N = 21; resistant – nematodes: N = 24; resistant + nematodes: N = 22). Letters indicate p < 0.05 in pairwise comparisons
Performance
Larvae feeding on the susceptible line were on average 37.32% smaller than larvae fed on the susceptible line (F1,81.1 = 7.18, p = 0.01, Fig. 6C). Nematode presence did not affect beetle weight change (F1,81, = 0.75, p = 0.39, Fig. 6C). Plants of the resistant line had an average of 84.6% more leaflets per plant than the susceptible line. However, these leaflets were smaller than the leaflets of the susceptible line (K. Hauri, personal observation). Beetles did not consume different amounts of tissue on the two plant lines (F1,86.1 = 0.54, p = 0.46, Fig. S4), nor did nematode presence affect their consumption (F1,86.1 = 0.04, p = 0.83, Fig. S4).
Discussion
We examined the effects of above- and belowground herbivory on two lines of a wild potato relative, Solanum chacoense, one resistant to herbivory due to high levels of glycoalkaloid constitutive defenses and a susceptible line with lower levels of glycoalkaloids. We found that the susceptible line responded to aboveground CPB damage through induction of JA and OPDA, a JA precursor (Ruan et al. 2019), while the resistant line did not differ in levels of JA, OPDA, or JA-Ile, a biologically active form of JA (Ruan et al. 2019) between herbivory treatments. However, when challenged concurrently by RKN and CPB, the susceptible line retained high levels of JA but not OPDA. Consistent with our hypothesis, the susceptible line exhibited root-to-shoot effects aboveground for OPDA, although not for other compounds. In contrast, the resistant line exhibited no root-to-shoot effects. Beetle performance reflected the plant defense response, with higher performance on the susceptible line with a numerical (though not statistically significant) decrease in mass change on plants with nematodes, while there was no difference in beetle mass on plants of the resistant line. Belowground, JA, JA-Ile, and OPDA were higher in the resistant line compared to the susceptible line, and contrary to our hypothesis, we did not see any shoot-to-root effects in the susceptible line. Previous studies have found correlated local expression of phytohormones and secondary metabolites following insect herbivory (Robert et al. 2019); our results suggest that prior to herbivory, high constitutive levels of specialized secondary metabolites can result in systemic elevation of related phytohormones, while plants with low constitutive defenses may induce a response primarily towards the most damaging herbivores.
In our system, one of the main differences between the susceptible and resistant lines was the presence of leptine glycoalkaloids (Kaiser et al. 2021). These acetylated glycoalkaloids reduce CPB herbivory compared to non-acetylated glycoalkaloids, such as α-solanine and α-chaconine, present in commercial potatoes (Sinden et al. 1986; Kaiser et al. 2020, 2021). However, they are only produced in aboveground tissues (Kaiser et al. 2021). This difference was apparent in our samples: the variability in secondary metabolite composition, dominated by glycoalkaloids in our samples, was greater aboveground than belowground, and beetle performance was reduced in the resistant line regardless of nematode presence. An important regulator of the glycoalkaloid signaling pathway in other solanaceous plants is the COI1 gene, which is downstream of the JA signaling pathway (Cárdenas et al. 2016; Montero-Vargas et al. 2018; Zhao et al. 2021). Activation of the JA signaling pathway is one possible explanation for the differences in root responses between the susceptible and resistant lines. While leptine glycoalkaloids are only produced in the shoots, synthesis relies on signaling from an activated JA pathway, which is systemic (Zhao et al. 2021). Therefore, the resistant line—which produces more leptine glycoalkaloids—had higher levels of JA, JA-Ile, and OPDA in root tissues compared to the susceptible line, which had relatively low levels of constitutive glycoalkaloids in comparison. As a result, resistant plants had higher levels of constitutive defense belowground as well, as evidenced by higher levels of JA-pathway compounds in control plants. Future research could test this mechanism with grafting experiments, combining resistant scions with susceptible rootstock, to determine whether aboveground glycoalkaloid production induces resistance in roots.
The differences in levels of JA-pathway compounds may also have influenced root-to-shoot vs. shoot-to-root effects. In our experiments, we only observed root-to-shoot effects in the case of OPDA in the susceptible line, which contrasts with a previous meta-analysis that showed belowground herbivory typically induced root and foliar defenses to a similar extent, while leaf herbivory does not typically induce a response in the roots (Kaplan et al. 2008). While OPDA was elevated after CPB herbivory on nematode-free plants, there was no change after CPB herbivory on plants with nematodes, although JA levels remained high. Because OPDA is a precursor to JA, it is possible that the presence of RKN limited the plant’s ability to upregulate the phytohormone pathway, but that JA was still produced from OPDA that existed in the plant prior to nematode infestation. Long-term, this reduction in OPDA could inhibit the plant’s ability to effectively defend against CPB aboveground. Previous studies have shown a variety of aboveground JA responses to RKN infection, including suppression of Arabidopsis thaliana leaf defenses after infection by Meloidogyne incognita (Hamamouch et al. 2011) and RKN enhanced JA-marker genes in aboveground tissues of Brassica nigra (van Dam et al. 2018). The JA-pathway is critical for defense against RKN colonization, and due to higher constitutive levels of JA in the roots, the resistant line was likely more difficult for nematodes to invade than the susceptible line. OPDA is known to provide resistance to RKN in Arabidopsis (Gleason et al. 2016)d incognita effector protein MilSE5 interferes with the JA signaling pathway early in infection to promote successful parasitism (Shi et al. 2018). There was evidence for this type of interference in the resistant line; the presence of nematodes alone generally caused a numeric, though only statistically significant change for JA-Ile, a reduction in a JA-pathway compound in the root. However, these compounds increased to near control levels when CPB feeding occurred simultaneously, even though CPB feeding alone did not typically elevate levels of root JA-pathway compounds, indicating shoot-to-root effects. In soybean roots, infection by the soybean cyst nematode, Heterodera glycines, caused a systemic induction of JA, but JA-controlled defenses were suppressed locally (Ithal et al. 2007a, b; Wondafrash et al. 2013). A similar mechanism could explain a reduction in JA-pathway compounds with RKN alone, but an increase as systemic JA induction by CPB feeding overwhelms local suppression.
In conclusion, varying the level of family-specific secondary metabolites in plants may partially explain why outcomes of plant-mediated interactions may vary by plant family. Although it is well established that species identity is critical to understanding plant-mediated interactions (e.g. Kaplan et al. 2009, Soler et al. 2013; van Dam et al. 2018), our results will help fine-tune predictions about when an individual species will affect spatially separated herbivores. High constitutive levels of specialized metabolites alter the threshold of damage necessary to induce systemic phytohormone pathways in response to local herbivory. Whether or not a plant reaches that herbivory threshold may in effect alter the timing of the interaction as perceived by organisms feeding on spatially separated parts of the plant. However, maintaining high levels of specialized compounds is reliant on systemic phytohormone pathways; this may offer an opportunity to plants to overwhelm local suppression by invaders or herbivores. Future studies that investigate the effects on belowground herbivores can help determine if selecting for crop varieties with high levels of constitutive defense—even aboveground only—could be deployed in an agricultural context, where belowground damage is less consistent and control options are limited.
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
Supplementary Material 1: Fig. S1. Components of plant chemical defense influencing spatially separated herbivores. Fig. S2. Chaconine and solanine LC-MS chromatograms. Fig. S3. Mass spectra of solanine and chaconine with collision energy on showing fragmentation. Fig. S4. Colorado potato beetle leaflet consumption. Table S1 MS/MS details for targeted phytohormone method. Table S2. Model output (F- and p-values) for plant hormones in the leaves and roots of two Solanum chacoense breeding lines that were damaged by M. hapla nematodes (RKN) and/or Colorado potato beetle (CPB).
Acknowledgements
We thank R. Rantz and S. Kaur for help in the greenhouse and lab, and M. Hufnagel for assistance with tissue culture propagation of plant lines. We thank E. Cinto-Mejia, N. Constancio, N. Carvajal, D. Dillard, L. Marmolejo, J. Zavalnitskaya, M. Eeraerts, and J. Ali for valuable comments and feedback.
Author Contributions
K.C.H. and Z.S. designed the experiments; E.D. and A.H. provided nematode colonies and input on timing and inclusion of nematodes; K.C.H. conducted the experiments; K.C.H. and A.L.S. processed and analyzed leaf and root LC-MS samples; K.C.H. and Z.S. conducted the analyses; K.C.H. and Z.S. wrote the first draft. All authors edited the manuscript.
Funding
This work was supported by the United States Department of Agriculture (USDA) National Institute of Food and Agriculture (NIFA) Predoctoral fellowship (project number 2021–09359), C.S. Mott Predoctoral Fellowship, and the Sustainable Michigan Endowed Project.
Data Availability
Data is available on Figshare (Hauri 2024): 10.6084/m9.figshare.c.7407463.v1.
Declarations
Competing Interests
The authors declare no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Elisabeth Darling and Amanda D. Howland contributed equally to the manuscript.
References
- Agrawal AA, Petschenka G, Bingham RA et al (2012) Toxic cardenolides: chemical ecology and coevolution of specialized plant–herbivore interactions. New Phytol 194:28–45. 10.1111/j.1469-8137.2011.04049.x [DOI] [PubMed] [Google Scholar]
- Arce CCM, Machado RAR, Ribas NS et al (2017) Nematode root herbivory in tomato increases leaf defenses and reduces leaf miner oviposition and performance. J Chem Ecol 43:120–128. 10.1007/s10886-016-0810-z [DOI] [PubMed] [Google Scholar]
- Bezemer T, Van Dam NM (2005) Linking aboveground and belowground interactions via induced plant defenses. Trends Ecol Evol 20:617–624. 10.1016/j.tree.2005.08.006 [DOI] [PubMed] [Google Scholar]
- Caarls L, Pieterse CMJ, Van Wees SCM (2015) How salicylic acid takes transcriptional control over jasmonic acid signaling. Front Plant Sci 6. 10.3389/fpls.2015.00170 [DOI] [PMC free article] [PubMed]
- Cárdenas PD, Sonawane PD, Pollier J et al (2016) GAME9 regulates the biosynthesis of steroidal alkaloids and upstream isoprenoids in the plant mevalonate pathway. Nat Commun 7:10654. 10.1038/ncomms10654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dyer LA, Philbin CS, Ochsenrider KM et al (2018) Modern approaches to study plant–insect interactions in chemical ecology. Nat Rev Chem 2:50–64. 10.1038/s41570-018-0009-7 [Google Scholar]
- Gatehouse JA (2002) Plant resistance towards insect herbivores: a dynamic interaction. New Phytol 156:145–169. 10.1046/j.1469-8137.2002.00519.x [DOI] [PubMed] [Google Scholar]
- Glassmire AE, Jeffrey CS, Forister ML et al (2016) Intraspecific phytochemical variation shapes community and population structure for specialist caterpillars. New Phytol 212:208–219. 10.1111/nph.14038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gleason C, Leelarasamee N, Meldau D, Feussner I (2016) OPDA has key role in regulating plant susceptibility to the root-knot nematode Meloidogyne hapla in Arabidopsis. Front Plant Sci 7. 10.3389/fpls.2016.01565 [DOI] [PMC free article] [PubMed]
- Guo H, Ge F (2017) Root nematode infection enhances leaf defense against whitefly in tomato. Arthropod-Plant Interact 11:23–33. 10.1007/s11829-016-9462-8 [Google Scholar]
- Hamamouch N, Li C, Seo PJ et al (2011) Expression of Arabidopsis pathogenesis-related genes during nematode infection: PR genes in nematode infection. Mol Plant Pathol 12:355–364. 10.1111/j.1364-3703.2010.00675.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hare JD (2011) Ecological role of volatiles produced by plants in response to damage by herbivorous insects. Annu Rev Entomol 56:161–180. 10.1146/annurev-ento-120709-144753 [DOI] [PubMed] [Google Scholar]
- Hartmann T (1996) Diversity and variability of plant secondary metabolism: a mechanistic view. Entomol Exp Appl 80:177–188. 10.1111/j.1570-7458.1996.tb00914.x [Google Scholar]
- Hauri KC, Szendrei Z (2022) A meta-analysis of interactions between insect herbivores and plant-parasitic nematodes. Environ Entomol 51:1–10. 10.1093/ee/nvab131 [DOI] [PubMed] [Google Scholar]
- Hauri KC, Glassmire AE, Wetzel WC (2021) Chemical diversity rather than cultivar diversity predicts natural enemy control of herbivore pests. Ecol Appl 31. 10.1002/eap.2289 [DOI] [PubMed]
- Hewezi T, Baum TJ (2013) Manipulation of plant cells by cyst and root-knot nematode effectors. Mol Plant-Microbe Interactions® 26:9–16. 10.1094/MPMI-05-12-0106-FI [DOI] [PubMed] [Google Scholar]
- Hol WHG, De Boer W, Termorshuizen AJ et al (2013) Heterodera schachtii nematodes interfere with aphid-plant relations on Brassica oleracea. J Chem Ecol 39:1193–1203. 10.1007/s10886-013-0338-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hussey RS, Barker KR (1973) A comparison of methods of collecting inocula of Meloidogyne spp. Including a new technique. Plant Dis Rep 57:1025–1028
- Ithal N, Recknor J, Nettleton D et al (2007a) Parallel genome-wide expression profiling of host and pathogen during soybean cyst nematode infection of soybean. Mol Plant-Microbe Interactions® 20:293–305. 10.1094/MPMI-20-3-0293 [DOI] [PubMed] [Google Scholar]
- Ithal N, Recknor J, Nettleton D et al (2007b) Developmental transcript profiling of cyst nematode feeding cells in soybean roots. Mol Plant-Microbe Interactions® 20:510–525. 10.1094/MPMI-20-5-0510 [DOI] [PubMed] [Google Scholar]
- Jeschke V, Gershenzon J, Vassão DG (2016) A mode of action of glucosinolate-derived isothiocyanates: detoxification depletes glutathione and cysteine levels with ramifications on protein metabolism in Spodoptera Littoralis. Insect Biochem Mol Biol 71:37–48. 10.1016/j.ibmb.2016.02.002 [DOI] [PubMed] [Google Scholar]
- Jones JT, Haegeman A, Danchin EGJ et al (2013) Top 10 plant-parasitic nematodes in molecular plant pathology: top 10 plant-parasitic nematodes. Mol Plant Pathol 14:946–961. 10.1111/mpp.12057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaiser N, Manrique-Carpintero NC, DiFonzo C et al (2020) Mapping Solanum chacoense mediated Colorado potato beetle (Leptinotarsa decemlineata) resistance in a self-compatible F2 diploid population. Theor Appl Genet 133:2583–2603. 10.1007/s00122-020-03619-8 [DOI] [PubMed] [Google Scholar]
- Kaiser NR, Billings G, Coombs J et al (2021) Self-fertility and resistance to the Colorado potato beetle (Leptinotarsa decemlineata) in a diploid Solanum chacoense recombinant inbred line population. Crop Sci 61:3392–3414. 10.1002/csc2.20534 [Google Scholar]
- Kaplan I, Halitschke R, Kessler A et al (2008) Physiological integration of roots and shoots in plant defense strategies links above- and belowground herbivory. Ecol Lett 11:841–851. 10.1111/j.1461-0248.2008.01200.x [DOI] [PubMed] [Google Scholar]
- Karban R, Baldwin IT (2007) Induced responses to herbivory. University of Chicago Press
- Karban R, Agrawal AA, Mangel M (1997) The benefits of induced defenses against herbivores. Ecology 78:1351–1355 [Google Scholar]
- Kessler A (2015) The information landscape of plant constitutive and induced secondary metabolite production. Curr Opin Insect Sci 8:47–53. 10.1016/j.cois.2015.02.002 [DOI] [PubMed] [Google Scholar]
- Kessler A, Baldwin IT (2002) Plant responses to insect herbivory: the emerging molecular analysis. Annu Rev Plant Biol 53:299–328. 10.1146/annurev.arplant.53.100301.135207 [DOI] [PubMed] [Google Scholar]
- Lenth R (2024) emmeans: Estimated Marginal Means, aka Least-Squares Means. R package
- Meyer A, Miersch O, Büttner C et al (1984) Occurrence of the plant growth regulator jasmonic acid in plants. J Plant Growth Regul 3:1–8. 10.1007/BF02041987 [Google Scholar]
- Montero-Vargas JM, Casarrubias-Castillo K, Martínez-Gallardo N et al (2018) Modulation of steroidal glycoalkaloid biosynthesis in tomato (Solanum lycopersicum) by jasmonic acid. Plant Sci 277:155–165. 10.1016/j.plantsci.2018.08.020 [DOI] [PubMed] [Google Scholar]
- Murashige T, Skoog F (1962) A revised medium for rapid growth and bio assays with tobacco tissue cultures. Physiol Plant 15:473–497. 10.1111/j.1399-3054.1962.tb08052.x [Google Scholar]
- Nakashima K, Yamaguchi-Shinozaki K (2013) ABA signaling in stress-response and seed development. Plant Cell Rep 32:959–970. 10.1007/s00299-013-1418-1 [DOI] [PubMed] [Google Scholar]
- Oksanen J, Simpson G, Blanchet F, Kindt R, Legendre P, Minchin P, O’Hara R, Solymos P, Stevens M, Szoecs E, Wagner H, Barbour M, Bedward M, Bolker B, Borcard D, Carvalho G, Chirico M, De Caceres M, Durand S, Evangelista H, FitzJohn R, Friendly M, Furneaux B, Hannigan G, Hill M, Lahti L, McGlinn D, Ouellette M, Ribeiro Cunha E, Smith T, Stier A, Ter Braak C, Weedon J (2024). Vegan: community ecology package. R package version 2.6-6.1
- Raskin I (1992) Role of salicylic acid in plants. Annu Rev Plant Physiol Plant Mol Biol 43:439–463 [Google Scholar]
- Richards LA, Dyer LA, Forister ML et al (2015) Phytochemical diversity drives plant–insect community diversity. Proc Natl Acad Sci 112:10973–10978. 10.1073/pnas.1504977112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robert CAM, Pellissier L, Moreira X et al (2019) Correlated induction of phytohormones and glucosinolates shapes insect herbivore resistance of cardamine species along elevational gradients. J Chem Ecol 45:638–648. 10.1007/s10886-019-01084-2 [DOI] [PubMed] [Google Scholar]
- Ruan J, Zhou Y, Zhou M et al (2019) Jasmonic acid signaling pathway in plants. Int J Mol Sci 20:2479. 10.3390/ijms20102479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schilmiller AL, Howe GA (2005) Systemic signaling in the wound response. Curr Opin Plant Biol 8:369–377. 10.1016/j.pbi.2005.05.008 [DOI] [PubMed] [Google Scholar]
- Shi Q, Mao Z, Zhang X et al (2018) A Meloidogyne incognita effector MiISE5 suppresses programmed cell death to promote parasitism in host plant. Sci Rep 8:7256. 10.1038/s41598-018-24999-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinden SL, Sanford LL, Cantelo WW, Deahl KL (1986) Leptine glycoalkaloids and resistance to the Colorado potato beetle (Coleoptera: Chrysomelidae) in Solanum chacoense. Environ Entomol 15:1057–1062. 10.1093/ee/15.5.1057 [Google Scholar]
- Soler R, Van der Putten WH, Harvey JA et al (2012) Root herbivore effects on aboveground multitrophic interactions: patterns, processes and mechanisms. J Chem Ecol 38:755–767. 10.1007/s10886-012-0104-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soler R, Erb M, Kaplan I (2013) Long distance root–shoot signalling in plant–insect community interactions. Trends Plant Sci 18:149–156. 10.1016/j.tplants.2012.08.010 [DOI] [PubMed] [Google Scholar]
- Textor S, Gershenzon J (2009) Herbivore induction of the glucosinolate–myrosinase defense system: major trends, biochemical bases and ecological significance. Phytochem Rev 8:149–170. 10.1007/s11101-008-9117-1 [Google Scholar]
- Van Dam NM, Raaijmakers CE, Van Der Putten WH (2005) Root herbivory reduces growth and survival of the shoot feeding specialist Pieris rapae on Brassica nigra. Entomol Exp Appl 115:161–170. 10.1111/j.1570-7458.2005.00241.x [Google Scholar]
- van Dam NM, Wondafrash M, Mathur V, Tytgat TOG (2018) Differences in hormonal signaling triggered by two root-feeding nematode species result in contrasting effects on aphid population growth. Front Ecol Evol 6:88. 10.3389/fevo.2018.00088 [Google Scholar]
- version 1 10.1. https://CRAN.R-project.org/package=emmeans
- Vlot AC, Dempsey DA, Klessig DF (2009) Salicylic acid, a multifaceted hormone to combat disease. Annu Rev Phytopathol 47:177–206. 10.1146/annurev.phyto.050908.135202 [DOI] [PubMed] [Google Scholar]
- Walling LL (2000) The myriad plant responses to herbivores. J Plant Growth Regul 19:195–216. 10.1007/s003440000026 [DOI] [PubMed] [Google Scholar]
- Walters D, Heil M (2007) Costs and trade-offs associated with induced resistance. Physiol Mol Plant Pathol 71:3–17. 10.1016/j.pmpp.2007.09.008 [Google Scholar]
- Wondafrash M, Van Dam NM, Tytgat TOG (2013) Plant systemic induced responses mediate interactions between root parasitic nematodes and aboveground herbivorous insects. Front Plant Sci 4. 10.3389/fpls.2013.00087 [DOI] [PMC free article] [PubMed]
- Yang J, Duan G, Li C et al (2019) The crosstalks between jasmonic acid and other plant hormone signaling highlight the involvement of jasmonic acid as a core component in plant response to biotic and abiotic stresses. Front Plant Sci 10:1349. 10.3389/fpls.2019.01349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng W, Brutus A, Kremer JM et al (2011) A genetic screen reveals Arabidopsis stomatal and/or apoplastic defenses against pseudomonas syringae pv. Tomato DC3000. PLoS Pathog 7:e1002291. 10.1371/journal.ppat.1002291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao D-K, Zhao Y, Chen S-Y, Kennelly EJ (2021) Solanum steroidal glycoalkaloids: structural diversity, biological activities, and biosynthesis. Nat Prod Rep 38:1423–1444. 10.1039/D1NP00001B [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material 1: Fig. S1. Components of plant chemical defense influencing spatially separated herbivores. Fig. S2. Chaconine and solanine LC-MS chromatograms. Fig. S3. Mass spectra of solanine and chaconine with collision energy on showing fragmentation. Fig. S4. Colorado potato beetle leaflet consumption. Table S1 MS/MS details for targeted phytohormone method. Table S2. Model output (F- and p-values) for plant hormones in the leaves and roots of two Solanum chacoense breeding lines that were damaged by M. hapla nematodes (RKN) and/or Colorado potato beetle (CPB).
Data Availability Statement
Data is available on Figshare (Hauri 2024): 10.6084/m9.figshare.c.7407463.v1.