Abstract
Lutein, zeaxanthin, and meso-zeaxanthin are three xanthophyll carotenoid pigments that selectively concentrate in the center of the retina. Humans cannot synthesize lutein and zeaxanthin, so these compounds must be obtained from the diet or supplements, with meso-zeaxanthin being converted from lutein in the macula. Xanthophylls are major components of macular pigments that protect the retina through the provision of oxidant defense and filtering of blue light. The accumulation of these three xanthophylls in the central macula can be quantified with non-invasive methods, such as macular pigment optical density (MPOD). MPOD serves as a useful tool for assessing risk for, and progression of, age-related macular degeneration, the third leading cause of blindness worldwide. Dietary surveys suggest that the dietary intakes of lutein and zeaxanthin are decreasing. In addition to low dietary intake, pregnancy and lactation may compromise the lutein and zeaxanthin status of both the mother and infant. Lutein is found in modest amounts in some orange- and yellow-colored vegetables, yellow corn products, and in egg yolks, but rich sources of zeaxanthin are not commonly consumed. Goji berries contain the highest known levels of zeaxanthin of any food, and regular intake of these bright red berries may help protect against the development of age-related macular degeneration through an increase in MPOD. The purpose of this review is to summarize the protective function of macular xanthophylls in the eye, speculate on the compounds’ role in maternal and infant health, suggest the establishment of recommended dietary values for lutein and zeaxanthin, and introduce goji berries as a rich food source of zeaxanthin.
Keywords: age-related macular degeneration, goji berries, lutein, macular pigment optical density, macula, recommended dietary intake, zeaxanthin
INTRODUCTION
Epidemiological studies suggest that diets rich in carotenoids can be beneficial for vision, heart, bone health, cognitive performance, and cancer prevention.1 The current review focuses on the potential role of the xanthophyll carotenoids lutein (L) and zeaxanthin (Z) in eye health, specifically their potential role in reducing the risk of age-related macular degeneration (AMD). A review of the absorption, distribution, and metabolism of L and Z is included, and the current dietary recommendations for these carotenoids, followed by a speculation about their putative role in maternal and infant health. The potential value of goji berries as a food with the highest known amount of Z is also presented.
Carotenoids contribute to the bright red, orange, and yellow color in plants.2 These fat-soluble phytochemicals are classified into 2 categories: carotenes (which include only hydrocarbons) and xanthophylls (eg, L and Z, which also contain oxygen).3,4 While some dietary carotenoids serve as vitamin A precursors (eg, β-carotene, α-carotene, γ-carotene, and β-cryptoxanthin),5,6 most of the approximately 100 carotenoids found in plants do not.7 Among the carotenoids devoid of vitamin A activity are L and Z, along with meso-zeaxanthin (meso-Z), a stereoisomeric metabolite of L.8
AGE-RELATED MACULAR DEGENERATION
AMD is the third leading cause of blindness worldwide, after uncorrected refractive errors and cataracts.9 An estimated 288 million people worldwide are projected to suffer from AMD by 2040.10 In the United States, the prevalence of early-stage AMD was 9.1 million in 2010, and this number is projected to increase to 17.8 million by 2050.11 AMD is characterized by a gradual loss of eyesight from the central visual field.12 Although the exact etiology of AMD is not clear, common pathologic progress includes oxidative stress, lipofuscin toxicity, lipid accumulation, immune dysregulation, and choroidal hyperperfusion.13 Age-related processes such as a decrease in retinal neuronal elements, alterations in the size and shape of retinal pigmented epithelial (RPE) cells, and thickening of Bruch’s membrane further participate in the pathology of AMD.14 Damage to mitochondria in RPE cells has also been suggested to play a role.15 Dry AMD, also termed non-exudative AMD, involves the formation of drusen, which are mainly lipid and protein deposits that accumulate between the RPE and Bruch’s membrane in the macula.16 In contrast, wet AMD, also termed exudative or neovascular AMD, is a consequence of abnormal blood vessel formation arising from the choroid, known as choroidal neovascularization (CNV).17 Clinically, AMD is classified as early- or intermediate-stage based on the size and number of drusen, as well as on the presence of pigmentary changes.18 AMD is considered late- or advanced-stage in the presence of CNV, where fluid accumulation may result in damage to the neurosensory retina and fibrous scarring, or geographic atrophy (GA), respectively, where loss of the RPE results in damage to overlying photoreceptors and underlying choriocapillaris, causing irreversible vision loss.19
The main risk factors for AMD are aging and smoking.20 Other risk factors may include race, obesity, previous cataract surgery, presence of cardiovascular disease, and hypertension.21,22 According to the U.S. National Institutes of Health National Eye Institute, the prevalence of AMD is highest among Caucasians, as compared with other races, and higher in females (65%) than in males (35%).23 Genetic factors are also associated with AMD,24 with several high-risk single-nucleotide polymorphisms identified from genome-wide association studies.25–27 The strongest risk variants include the Y402H variant of complement factor H gene, as well as those in the age-related maculopathy susceptibility 2 (ARMS2) locus.28–30 Whether the color of the iris or sunlight exposure are related to the risk of AMD is still being explored.21,31–35
Diet may also play a role in the risk of developing AMD. Longitudinal studies have reported that a high intake of fruits and vegetables is associated with a lower risk of AMD.36,37 Sub-analyses of the age-related eye disease study (AREDS) and AREDS2 trials reported that the dietary intake of selected vitamins, minerals, omega-3 fatty acids, L, and Z, is inversely associated with the risk of progression to late AMD, GA, and large drusen.38 Similarly, a meta-analysis of 6 longitudinal cohort studies found that the dietary intake of L and Z significantly reduced the risk of GA by 26% and CNV by 32%, with no apparent impact on early stages.39 A systematic review concluded that adherence to dietary patterns that are high in vegetables, fruits, whole grains, and seafood is associated with a deceased risk for AMD, while diets high in red and processed meats and alcohol may increase the risk.40
DIETARY L AND Z
Consuming a diet rich in green leafy vegetables and fish is recommended by the National Eye Institute, for the high carotenoid and omega-3 fatty acid contents.41 Since humans cannot synthesize carotenoids, these compounds, including L and Z, must also be obtained from the intake of certain fruits, vegetables, corn and corn products, egg yolks, and dairy foods. Commonly consumed foods rich in L (μg/g dry matter) include parsley (64–106), spinach (59–79), and kale (48–115).42 Leeks (37), peas (19), lettuce (10–48), green pepper (19), red pepper (2.5–85), and pistachios (8–49) are also good sources. Commonly consumed foods rich in Z (μg/g dry matter) are corn tortillas (105) and corn chips (93),42 as well as cooked egg yolk (5.9) and orange pepper (16.7).43 Though not commonly consumed, goji berries contain Z in an amount greater than almost any other food. One analysis found the amount of Z (μg/g dry matter) was 1155,44 while another study noted the Z content ranged from 2800 μg/g dry matter to 5400 μg/g dry matter.45
The US intake of L and Z has been decreasing. According to the U.S. National Health and Nutrition Examination Survey (NHANES), the average intakes of L plus Z were 2.15 mg/d in males and 2.21 mg/d in females in 1987, and 2.15 mg/d in males and 1.86 mg/d in females in 1992.46 In NHANES 2013–2014, the average intakes of L and Z in males and females was 1.58 mg/d and 1.76 mg/d, respectively.47 Moreover, based on data from NHANES 2003–2004, the reported intake of L was significantly higher than Z in all age groups and ethnicities. However, due to analytical challenges in assessing dietary L and Z separately, most studies present the values together.48 Since the amount of L in most foods is significantly greater than Z, limited emphasis has been given to the separate roles of Z and L in the macula and potentially other physiological functions.
Goji berries: a rich source of zeaxanthin
Goji berry (Lycium barbarum and its closely related species, Lycium chinense), also known as wolfberry or Gou Qi Zi, is a rich dietary source of Z. The bright orange-red–colored oval fruit has been used for millennia in traditional Chinese medicine (TCM) for its role in brain, visual, and immune health, its anti-inflammatory benefits, and to help regulate liver and kidney meridians (Figure 1).44,49,50 Commercially available goji berries and their products come primarily from the Ningxia and Xinjiang autonomous regions in western China,51 although the hardy, drought-tolerant deciduous shrub can be grown most places in the world in USDA hardiness zones 5–9 (low temperatures from –28.9°F to 30°F; –23.3°C to –1.1°C). Cultivation of goji berries exists in other parts of China, as well as in Japan, Korea, and Taiwan. The Castelli Romani region of Italy (near Rome) cultivates the berries, and they are commercially available for home gardeners in the United Kingdom and the United States, among other locations.
Figure 1.
Dried goji berries.
As noted above, goji berries are one of the highest dietary sources of Z.52,53 The carotenoids are present in both saponifiable and nonsaponifiable fractions of the berry. Additionally, other bioactive compounds found in goji berries include Lycium barbarum polysaccharides (LBP), flavonoids, vitamins, minerals, betaine, cerebrosides, phenolic acids, and certain amino acids, which may also support the overall health of the eye, particularly when functioning synergistically.44,51,54 Although the TCM use of goji berry also includes the leaves and bark of the plant, this review will discuss the potential benefits only from the fruit (fructus lycii) on eye health.
In addition to a robust amount of Z, goji berries contain modest amounts of L, β-cryptoxanthin, β-carotene, and neoxanthin.49,52,55 The Z and L content among different varieties of dried goji berries cultivated in Ningxia province ranged from 25 mg/100 g to 152 mg/100 g, and 0.3 mg/100 g to 1.9 mg/100 g, respectively.55 According to the United States Department of Agriculture food database, one serving of goji berries is 28 g, which would provide up to 42.6 mg of L + Z, depending on the cultivar.56 Moreover, the predominant form of Z in goji berries is a dipalmitate ester linkage.45 The ratio of Z dipalmitate to total carotenoids was up to 55% and 88%, in fresh and dried goji berry fruit, respectively.45,57 This esterified form of Z showed a significantly higher intestinal absorption in a caco-2 human intestinal cell model compared with a free Z monoester due to the high efficacy of ester hydrolysis, mainly by carboxyl ester lipase.58 The high bioavailability of Z from goji berries has also been demonstrated in humans. Plasma Z was significantly increased in individuals consuming 15 g goji berries daily for 28 days, compared with those following a habitual diet.59 Participants consuming 5 mg of Z dipalmitate extracted from goji berries also showed a higher plasma Z concentration than when they consumed the same amount as unesterified Z over a 9-hour to 24-hour period.60
Absorption, distribution, metabolism, and excretion
Among the carotenoids, L and Z are unique in their ability to cross the human blood–retina barrier for accumulation into the macula.61 After xanthophylls are released from the food matrix by mechanical mixing, digestive enzymes, and gastric acids, these carotenoids follow an absorption path similar to that of other lipids.61–63 As noted above, esterified forms of xanthophylls appear to be more bioavailable, yet are efficiently hydrolyzed prior to chylomicron insertion by pancreatic cholesterol ester hydrolase or other lipases at the brush border level or within the enterocyte.64,65 After the transfer from lipid emulsions to intestinal mixed micelles,63,65 intestinal absorption is thought to occur via both facilitated transport and passive diffusion.66 Facilitated transport involves enterocyte uptake by CD36, scavenger receptor class B type I, and Niemann-Pick C1-like transporter 1 at the apical membrane.64 Carotenoids with pro-vitamin A activity undergo cleavage by β-carotene-15′,15′-monoxygenase (BCO1) and β-carotene-9′,10′-dioxygenase (BCO2) in the intestinal mucosal cells.67 However, xanthophylls do not undergo cleavage and are packaged directly within chylomicrons.62,63 The chylomicrons are transported to the liver via the lymph, where they are repackaged into lipoproteins for tissue transport.62
The distribution of L and Z across lipoprotein fractions in plasma has been documented. While 80%–85% of carotenoids, including α- and β-carotene and lycopene, are associated with low-density lipoprotein (LDL) particles, 13%–18% are associated with high-density lipoprotein (HDL) particles, and less than 4% are associated with very low density lipoprotein (VLDL) and intermediate-density lipoprotein (IDL).68 Therefore, some have suggested that L and Z transport into the retina may be dependent on the serum lipid profile.67,69 A number of studies suggest that HDL dysregulation increases the risk for AMD.70–72 Some evidence also suggests that macular pigment optical density (MPOD) is inversely associated with the percentage of body fat, after adjusting for age and dietary intake.69 HDL particles have been shown to be the primary transport mechanism for Z, less so for L, into the retinal pigment epithelium using an ApoA-I knockout mouse model.73 In humans consuming eggs enriched with L and Z, the L from HDL particles was positively correlated with MPOD.74 In contrast, other studies have shown that LDL particles are the primary transport vehicles for L and β-carotene, whereas HDL particles are more effective at transporting Z.65 Lipoprotein particles exchange materials via a variety of transfer proteins (eg, cholesteryl ester transfer protein, phospholipid transfer protein), and some evidence exists that these exchange activities in plasma and interstitial fluid regulate the distribution of carotenoids as well as L and Z across the lipoprotein fractions.75 However, the exact mechanisms for carotenoid binding to lipoproteins has not been identified.62 Genetic polymorphisms in key lipoprotein metabolic proteins (eg, lipoprotein lipase, ATP-binding cassette transporter G5) have also been linked with alterations in L and Z concentrations in the retina and with risk of AMD.76,77 Although the literature on excretion mechanisms is incomplete, some evidence exists that, as expected for lipophilic substances, L and Z are excreted via bile.78
Interestingly, in premenopausal women, as much as 34% of L + Z is associated with HDL particles, with enrichment specifically in the HDL2 fraction, which consists of larger HDL particles. Hormonal fluctuations associated with the menstrual cycle are linked with changes in the concentrations of L and Z, with higher xanthophyll content in both LDL and HDL2 in the late follicular phase, when estrogen concentrations are higher than in the luteal phase.
MACULAR PIGMENTS
Lutein, Z, and meso-Z impart a distinctive yellow color to the fovea of primates, the specialized central area of the macular region of the retina rich in cone photoreceptors and optimized for high-acuity central color vision. The compounds have a maximal absorbance at a wavelength near 460 nm and are most concentrated in the inner and outer plexiform layers, which consist primarily of axonal connections between the retinal layers. Lutein and Z are mainly localized in the membranes of photoreceptor axons in Henle’s nerve fiber layers and in their outer segments, and are transported into the retina by different binding proteins, including the steroidogenic acute regulatory domain 3 (StARD3) protein (which binds L) and the GSTP1 (which appears to bind Z in the macula of the retina).62,79,80 Tubulins are abundant in Henle’s fibers and also appear to bind carotenoids with high affinity.81 In primates, the BCO2 gene has been reported to be enzymatically inactive, which partially explains some, if not all, of the accumulation of macular xanthophylls in human retinal tissues.82,83 The accumulation of macular xanthophylls in the tissues and macula of BCO2-knockout mice fed with L and Z further confirms this finding.84
The combined density of L and Z is greatest in the center of the macula and decreases with increasing retinal eccentricity.85,86 In the central fovea, the concentration of Z and meso-Z is higher than that of L at a ratio of 2.4:1. Lutein is most abundant in the peripheral macula, with a Z + meso-Z to L ratio of 1:2 when measured by high-performance liquid chromatography.87 More specifically, these authors reported the mean L + Z content from 6 adult donor eyes to range from 13.3 ng/mm when assessing the range of 0–0.25 mm from the fovea, 5.7 ng/mm in the range of 0–0.75 mm from the fovea, 2.9 ng/mm at a distance of 0.75–1.6 mm from the fovea, and 0.81 ng/mm and decreasing concentrations when determined at 1.6–2.5 mm from the fovea. Newer data obtained by confocal resonance Raman microscopy suggests that the Z+meso-Z to L ratio is as high as 9:1 at the central fovea.88 The concentration of L and Z in different parts of the macula appear driven by the spatial constriction of their binding proteins.62 Interestingly, although the retina is considered an extension of the central nervous system, the relative concentration of L and Z in the retina compared with the brain is nearly 40-fold higher in the retina, suggesting specific mechanisms of xanthophyll transport.89,90
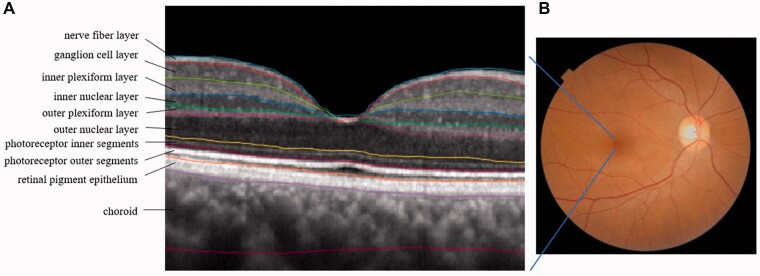
Protection from blue light is critical for eye health. Compared with longer wavelengths of visible light, short blue wavelengths are higher in energy and generate reactive oxygen species (ROS).91,92 Zeaxanthin can provide stronger oxidant defense than L during photooxidation,93 while lutein has a greater capacity to absorb short-wavelength light irradiation in lipid membranes.94 Compared with other carotenoids (eg, lycopene or β-carotene), L and Z are more effective in scavenging ROS and can also reduce phospholipid peroxidation.95,96 The RPE complex in the outer retina is particularly susceptible to ROS damage due to its high polyunsaturated lipid content (Figure 2).97 Quenching of singlet oxygen appeared best when L, Z, and meso-Z were mixed in equal ratios rather than separately, when assessed in an eye tissue model, suggesting some synergy between the these macular pigments in their antioxidant properties.98
Figure 2.
A. Spectral domain–optical coherence tomography of a macular region, showing anatomical layers. B. Fundus image of a healthy adult retina. The dark area is the macula; the light area is the optic nerve.
The most common method for quantifying xanthophylls in the retina is assessment of MPOD. This parameter is measured through techniques such as heterochromatic flicker photometry (HFP), a non-invasive psychophysical technique,99 fundus reflectometry, resonance Raman spectroscopy, or autofluorescence imaging.100 The MPOD index is associated with the plasma levels of L and Z,101–103 and has been used to assess the risk for AMD.104 Some studies report no significant correlation between MPOD and the risk of AMD,105 which suggests that other ocular measures may also be useful for obtaining a more complete profile of AMD risk. In human donor eyes, the amount of L and Z was inversely associated with AMD.106 Supplementation of L, Z, and meso-Z have been shown to significantly increase MPOD in both healthy individuals and patients diagnosed with AMD.41,107 However, a recent narrative review of 10 studies using foods rich in L and Z has produced inconsistent results, which may be due to the relatively modest amounts of these carotenoids, particularly Z, in foods compared with supplements.108 Indeed, a number of limitations among the studies was noted, including the different amounts of L and Z ingested or administered, variable duration of interventions, and lack of information about the background diet of the study participants. Importantly, the plasma concentrations of L and Z have been more strongly associated with MPOD than the correlation between MPOD and dietary intake.109
Results from dietary interventions
Dietary interventions using L- and Z-rich foods have generated inconsistent results regarding the risk of AMD.110,111 In a cohort study that assessed dietary carotenoid consumption among individuals without AMD at baseline over more than 20 years, increased predicted plasma carotenoid score of L, Z, β-carotene, α-carotene, and β-cryptoxanthin were associated with a lower risk of advanced, but of not early or intermediate AMD.112 Another meta-analysis concluded that supplementation with L, Z, and meso-Z significantly increased MPOD levels in both AMD patients and healthy individuals in a dose–response manner.113
The AREDS intervention was a multi-center study that assessed the efficacy of dietary antioxidant supplements on subjects who were 50 years to 80 years old, with and without AMD or cataracts, for more than 7 years.114 The initial study used a formula containing 15 mg of β-carotene, 500 mg of vitamin C, 400 IU of vitamin E, with or without 80 mg of zinc and 2 mg of copper. Lutein and Z were not included, because the scientific evidence suggesting inclusion these two carotenoids was not yet clear. Compared with the placebo group, participants consuming the antioxidants plus zinc and copper showed a 28% reduction in progression to advanced AMD after 5 years.115 Subsequently, the AREDS2 was conducted with a newer formulation that included vitamins C and E, either 10 mg of L plus 2 mg of Z, and either 350 mg of docosahexaenoic acid (DHA) plus 650 mg of eicosapentaenoic acid (EPA), or both. Patients were also given either 25 mg or 80 mg of zinc, each with 2 mg of copper. Beta-carotene was eliminated from the supplement due to a potential increased risk of lung cancer among smokers, who were already at high risk for AMD.116 Primary analyses of the AREDS2 data found no additional benefit in reducing progression to advanced AMD, in comparison with the original AREDS formula. However, in a secondary analysis of combined data from AREDS and AREDS2, the progression risk in those receiving L and Z was significantly lower than in other groups.117 A recent analysis of the AREDS2 dataset assessing the 10-year risk of developing late AMD evaluated the results from 3882 participants with an average age at baseline of 72 years.118 Comparisons were made between participants who consumed the initial formula devoid of L and Z, but instead included beta-carotene, with those who consumed the second AREDS formula, where L and Z were included in place of beta-carotene. The hazard ratio for progression to late AMD was 0.91 when comparing those who consumed the second formula containing L and Z with participants who consumed the initial formula devoid of L and Z. Neither of the AREDS clinical trials monitored the MPOD status. To date, the AREDS2 formula remains the standard of care for management of patients with intermediate AMD.
A recent post hoc analysis of a subset of individuals in the AREDS2 study examined the adherence to a Mediterranean diet and the rate of GA enlargement using annual color fundus photographs.119 Enlargement of GA over a 3.1-year follow-up was significantly slower among those in the highest quartile for dietary intake of whole fruit, alcohol, and the ratio of monounsaturated : polyunsaturated fatty acids, while the rate was faster among participants with the highest red meat intake. The dietary survey data did not distinguish which fruits were associated with the favorable outcomes, but estimated that 14 medium size servings per week qualified for the highest quartile. The association between red meat intake and rate of enlargement of GA is consistent with a systemic review reporting this pattern to be associated with an increased risk of AMD.40 However, the observation that moderate alcohol intake was associated with a lower rate of GA enlargement contrasts with a previous systemic review that noted an increased risk of AMD with alcohol intake. The authors conclude that a Mediterranean diet pattern appears to be strongly associated with reduced disease progression across a wide range of AMD diseases. Such a diet is rich in colorful fruits and vegetables abundant in carotenoids, including L and Z.120 More attention to dietary factors that can address the rising incidence of AMD is warranted.
The high Z content of goji berries has led to them being proposed as a dietary source to reduce the risk of AMD, although studies are limited.63 In one study, circulating Z levels were significantly higher in healthy older individuals who consumed 10 mg of Z extract from goji berries daily for 90 days.121 No change in macular pigmentation or small drusen was observed, but MPOD was not measured. In an uncontrolled trial, individuals with early-stage AMD who consumed a beverage containing 12 mg of L and 2 mg of Z derived from marigold flower and goji berry, respectively, daily for 5 months, showed higher circulating levels of L and Z, lower intraocular pressures, and better best-corrected visual acuity (BCVA) scores.122 Unfortunately, the study lacked a control group, and did not clarify whether the form of Z extracted from goji berry was the dipalmitate ester. Another study investigating the effects of a herbal formula among healthy adults with dry eyes noted that chewable tablets providing L (6 mg, 10 mg, or 14 mg) and Z (1.2 mg, 2.2 mg, or 2.8 mg) from extracts of blackcurrant, chrysanthemum, and goji berry showed dose-dependent reductions in eye fatigue symptoms, and tear secretion, as well as improved MPOD, compared with the placebo.123 The basis of this formula was derived from TCM, so the multicomponent formulation could not directly inform the role of any single ingredient. A study in patients with early AMD reported that the MPOD was significantly higher in those consuming 25 g/day of goji berries (containing approximately 15 mg of Z and 2.5 mg of L) for 90 days, compared with their baseline levels and with a habitual diet control group. The BCVA was also significantly improved in the goji berry group compared with their baseline values.124 The MPOD and skin carotenoid scores were recently reported to be significantly increased in healthy middle-aged individuals consuming 28 g/day of goji berries (containing approximately 28.8 mg of Z and an estimated 0.15 mg of L) 5 times a day for 90 days, compared with a group taking a supplement with 6 mg of L and 4 mg of Z.125 These results illustrate that MPOD levels can increase in healthy individuals, even without early signs of AMD. While these results are encouraging, longer intervention periods with a larger number of participants are necessary for a more complete understanding.
In addition to AMD, goji berries have been studied as a therapy for retinitis pigmentosa, an inherited retinal disease. Patients who consume 0.35 g/d of LBP for 12 months showed a significant improvement in visual acuity and macular thickness, compared with control subjects who did not consume L or Z.126 Examples of human studies that evaluated the effects of supplements containing goji berries on retinal health are shown in Table 1.121–126
Table 1.
Human studies on the effects of consumption of goji berries (directly or in supplements) on eye health
| Reference | Sample | Intervention | Frequency and duration | Outcome measures | Results |
|---|---|---|---|---|---|
| Bucheli et al. (2011)121 | 150 healthy seniors (aged 65–70 y) | 13.7 g/d of a milk-based formulation with 10 mg Z and 68.5 mg vitamin C derived from goji berry OR placebo | Daily for 90 d | Funduscopic exams | The placebo group showed increased hypopigmentation and soft drusen accumulation in the macula, while the treatment group remained stable |
| Chan et al. (2019)126 | 42 patients with retinitis pigmentosa (aged 26–69 y) | Goji berry OR placebo granules | Daily for 12 mo | Visual acuity, Ganzfeld full-field electroretinogram, Humphrey Visual Field Analysis, and Spectral-domain Optical Coherent Tomography | The treatment group maintained contrast visual acuity and macular thickness, while the placebo group showed a decline in all measures |
| Kan et al. (2020)123 | 303 healthy individuals with dry eye symptoms (aged 18–65 y) | Three treatment arms giving chewable tablets with the highest L of 14 mg, Z of 2.8 mg, goji berry extract of 175 mg, chrysanthemum extract of 175 mg, and blackcurrant extract of 233 mg, OR placebo | Daily for 90 d | Eye fatigue symptom score, MPOD, Schirmer test, optical coherence tomography, and keratography | The treatment groups showed significantly reduced eye soreness, blurred vision, dry eye, foreign body sensation, and tearing; improved tear secretion and increased first tear break-up time, average tear break-up time, tear meniscus height, and MPOD, compared with the placebo group |
| Li et al. (2018)124 | 114 patients with early AMD (aged 51–92 y) | 25 g/d of dried goji berry OR habitual diet | Daily for 90 d | MPOD, serum L + Z, BCVA | Increased serum Z and MPOD in the goji berry group compared with their baseline levels or with those of the control group |
| Li et al. (2021)125 | 27 healthy individuals (aged 45–65 y) | 28 g/d of dried goji berry OR supplement with 6 mg L and 4 mg Z | Five times a week for 90 d | MPOD, skin carotenoids | Increased MPOD and skin carotenoid score in the goji berry group compared with no significant changes in the supplement group |
| Peng et al. (2016)122 | 56 patients with early AMD (aged 35–50 y) | 60 mL/d of beverage containing 12 mg of L from marigold flower and 2 mg of Z from goji berry | Daily for 5 mo | Serum L and Z, plasma oxidative indices, antioxidant enzymes in erythrocytes, anti-inflammatory markers, BCVA, intraocular pressure, photostress recovery, ocular comfort index, MPOD | The treatment group showed significantly increased serum L and Z, antioxidant capacity, antioxidant enzymes, ocular comfort index, and MPOD; and decreased oxidative stress index, inflammatory markers, BCVA, and interocular pressure compared with baseline values |
Abbreviations: AMD, age-related macular degeneration; BCVA, best-corrected visual acuity; L, lutein; MPOD, macular pigment optical density; Z, zeaxanthin.
Based on preclinical evidence, potential benefits from goji berries on glaucoma and diabetic retinopathy may also exist. Goji berry extract ameliorated the high glucose–induced blood–retinal barrier disruption in human retinal pigment epithelial cells.127 Studies further reported that LBP showed significant neuroprotective effects over retinal ganglion cells in male C57BL/6N mice and Sprague-Dawley rats with ocular hypertension.128–130 In db/db mice, goji berry extract restored the thickness of the retina, the ganglion cell number, and the integrity of RPE after daily intake over 8 weeks.131
Although research on the upper limit of goji berry intake is scarce, goji berry allergy risk has been associated with the existence of cross-reactivity to nonspecific lipid transfer proteins from peaches, tomatoes, tobacco, tree nuts, and select pollens.132,133 In addition, bleeding symptoms after consuming goji berry juice, tea, or wine have been described in case reports among patients taking warfarin, an anticoagulant medicine.134,135
INFANT MACULAR DEVELOPMENT AND MATERNAL AMD RISK
The average dietary consumption of L and Z among females in the United States is estimated to be 1.76 mg/d, which is substantially below a range of 5 mg/d to 20 mg/d estimated from a recent meta-analysis as being the amount needed to significantly improve MPOD levels among adults with healthy eyes.107 Therefore, increased maternal intakes of L and Z either through diet or supplementation for the duration of pregnancy and lactation may be of value in the support of fetal and infant eye health.
During development, L and Z are not interchangeable. In a study of 30 mother–infant pairs, serum Z in the newborns and in their mothers was strongly correlated with the MPOD of the babies, but no relationship was noted for either maternal or infant levels of L.136 The highest plasma levels of maternal Z and L at delivery were associated with a 38% and 32%, respectively, lower risk of visual acuity problems in children at 3 years of age.137
The accumulation of L and Z in the macula starts in utero in primates and plays a critical role in visual development and maturation later in life.138 L and Z were detected from as early as 20 weeks of gestation in macular tissue from human fetuses inspected at autopsy.87 Unlike in fully matured human eyes, L is the dominant macular pigment in infants under the age of 2 years, regardless of eccentricities. The retina is less mature at birth compared with other eye structures, with complete differentiation requiring 4 years to 5 years.139 The maturation of the macula is associated with a change in the L:Z ratio over the first 4 years of life, which correlates with the development of cone photoreceptors.140
L and Z may also protect against oxidative damage in premature infants, especially those with retinopathy of prematurity (ROP).141 Premature infants with ROP usually have poor visual acuity, even after laser treatment or intravitreal injection of anti-vascular endothelial growth factor (anti-VEGF) agents.142 In a model of oxygen-induced retinopathy, mouse pups given L showed less vessel leakage and a lower avascular area compared with those given a L-free control.143 The authors suggested that the anti-oxidant properties of L may have contributed to these results, although ROS levels were not measured. Studies that investigate L and Z supplementation in ROP babies have produced inconsistent results. In a multi-center, double-blind, randomized controlled trial of very-low-birth-weight infants, no difference was found in the incidence of ROP between those supplemented with daily oral L and Z (0.14 mg L plus 0.6 µg Z) or placebo.144 However, the progression rate of threshold ROP showed a lower trend in the supplemented group. No adverse events were noted with L and Z supplementation, suggesting that they were well tolerated. Another study examining the effects of daily oral L (0.5 mg/kg) and Z (0.02 mg/kg) supplementation in preterm infants from the seventh day after birth until 40 weeks of age or until hospital discharge found no change in the rate or severity of ROP compared with placebo.145 Further, a meta-analysis of 3 randomized controlled trials also found no protective association between L and Z supplementation and the risk of ROP.146
Human breast milk and infant formula
The L and Z in human milk is particularly important for infant eye and brain development, and may provide long-term benefits to vision and cognition.147 During gestation, maternal lipoprotein synthesis increases, which accelerates the transport of carotenoids to the fetus.148 This transfer may deplete maternal stores if the dietary intake of carotenoids in general, and L and Z specifically, is inadequate for maintaining body stores. Low maternal skin and serum carotenoid levels have been reported in mothers of newborn infants.136
The concentration of β-carotene, lycopene, L, and Z, the main carotenoids in breast milk, are associated with maternal dietary intake over the first 6 months of lactation.149 Daily maternal supplementation of either 6 mg of L with 96 µg of Z, or 12 mg of L with 192 µg of Z, over 6 weeks resulted in a dose-dependent increase in L and Z levels in maternal breast milk and plasma, as well as infant plasma levels 3 months to 4 months postpartum.150 Another study reported that more carotenes were present than xanthophylls in maternal plasma, whereas more xanthophylls than carotenes were presented in breast milk.151 While more than 1 study is needed to clarify these dynamics, these preliminary findings suggest that maternal–infant transfer of carotenoids may occur, possibly at the expense of the mother. Future studies are needed to clarify whether breastfeeding or L and Z intake may impact their AMD risk.
Studies in premature infants illustrate the importance of L and Z in visual development.152 In preterm human neonates, extremely low levels of serum L and Z are associated with an undetectable MPOD.153 When a carotenoid-fortified formula containing 211 µg/L of combined L and Z was given to preterm infants, plasma carotenoid levels became comparable with those of breastfed preterm infants, and were significantly higher than those fed formulas without L or Z fortification.154 In a small study that monitored the concentration of L and Z in various infant formulas and breastmilk from different mothers, Z was not detected in any formula but was present in all breast milk samples, while L was consistently higher in breast milk.155 Serum L was also noted to be 6-fold higher in breastfed infants compared with those fed with a formula devoid of L.156 Further studies are warranted to assess the prospective effects of L- and Z-fortified formula on MPOD and visual development in infants as they enter adulthood.
The L-ZIP supplementation trial is currently exploring whether prenatal supplementation of 10 mg of L and 2 mg of Z will maintain maternal body stores, prevent potential macular pigment depletion during pregnancy, or enhance systemic and ocular carotenoid stores for both mothers and infants.157 Clinical trials on the long-term effects of perinatal L and Z intake on MPOD changes among mothers and infants are also warranted.158
Unfortunately, longitudinal studies on AMD in females often do not include breastfeeding history. A useful study design would be to investigate MPOD levels and relative risks of AMD between multiparous and nulliparous women, and in mothers practicing breastfeeding compared with formula feeding. Dietary intake of L and Z would be important to assess. Another challenge in retrospective studies is that breastfeeding history may not be accurate. Therefore, studies on the maternal transfer of L and Z during pregnancy and lactation with MPOD changes in infants and throughout the lifespan could be important but difficult to conduct. Future research should also focus on the measurement of L and Z status and MPOD in mother–infant pairs of twins as well as mother-babies with short birth intervals.
Estrogen exposure
Reproductive hormone status may be a confounding factor when assessing AMD risk. The pathogenesis of AMD involves oxidative stress and immune dysregulation.14 Estrogen has been shown to reduce oxidative stress and inflammation in RPE cells as well as systemically.159,160 Lifetime estrogen exposure, affected by factors such as the number of pregnancies, menopause, reproductive period, oral contraceptive use, and hormone replacement therapy, may influence the risk of developing AMD,161 but the current evidence is inconsistent. Also unknown is whether maternal L and Z intake during pregnancy and lactation can influence AMD risk for both the mother and, longer-term, for the infant. One study reported that postmenopausal hormone use decreased the risk of neovascular AMD but increased the risk of early AMD, while parous women showed a reduced risk of early AMD but not of neovascular AMD.162 Two nationwide studies from South Korea among postmenopausal women noted that exogenous estrogen exposure was not a protective factor for AMD. A cohort study found that hormone replacement therapy and a longer reproductive period was associated with an increased risk of neovascular AMD.163 A cross-sectional study showed that oral contraceptive use was associated with an increased risk of late AMD.164 In addition, a review summarizing the effect of estrogen exposure and the risk of all age-related eye diseases concluded that hormone replacement therapy, or the use of oral contraceptives, could be either positively or negatively associated with the risk of AMD.161 In contrast, some studies have reported that a longer duration of breastfeeding may be protective against late but not early AMD,164,165 even when the estrogen level was low during lactation. Unfortunately, none of the studies cited above explored L or Z dietary intake. Future studies on the interaction of different reproductive and estrogen exposure histories and AMD risk are needed, as well as the effect of supplemental L and Z during pregnancy and lactation.
A DIETARY REFERENCE INTAKE FOR L AND Z?
L and Z fulfill many criteria as essential nutrients, including high concentrations in select tissues, biological plausibility for eye health, depletion outcomes such as vision impairment in primates, and inverse associations with certain diseases.166 In addition to their role in eye health, L and Z are involved in cognitive function at all stages of life.167 A randomized controlled trial reported that L and Z supplementation improved neural efficiency and learning performance by increasing the interaction of numerous brain regions in older adults.168 Other reports have demonstrated an association between increasing L intake or circulating levels and preventing age-related cognitive decline, reducing the risks of certain cancers, coronary heart disease, stroke, and metabolic syndrome, and achieving higher levels of physical activity.169–171 A systematic review of in vivo, ex vivo, and in vitro studies concluded that L may benefit vascular health by improving endothelial function, reducing inflammation, regulating favorable lipid profiles, and maintaining glucose homeostasis.172 Systematic reviews summarizing the amount of L needed for cognitive functions and enhancement of gray matter volume estimated that at least 10 mg/d for 12 months could be beneficial.173,174
Age and sex are considered when creating dietary reference intake (DRI) values for established nutrients. A similar context may be appropriate for L and Z. Although the current clinical evidence is not completely consistent regarding the association between age and MPOD,175 a trend has indicated that the values are lower in older compared with younger individuals.176 Whether the proposed intake of L and Z should be based on the amount that can increase MPOD, reduce AMD risk, benefit visual maturation in newborns, protect cognitive health, or reduce the risk of other diseases requires further consideration. Sex differences in AMD prevalence must also be considered, especially in relation to pregnancy and lactation, as discussed above.
Currently, L and Z are not included in the United States DRI due to inadequate details from food databases, limited large-scale dietary intake studies, and insufficient knowledge regarding their metabolism, biological functions and interindividual variability.177 A DRI for L has, however, been promoted for a number of years, since the compound satisfies a well-established set of criteria for consideration.178,179 Recently, a new 4-step framework for recommended intakes of bioactive dietary compounds was advanced that features L and Z as key examples of substances that promote health but are not essential for preventing classic deficiency diseases.180 As the authors note, knowledge maps may be useful tools to help expand the criteria for a DRI, as well as using enhancement of physiological function (in this case, L and Z for improving MPOD and reducing the risk of AMD). Establishing a DRI for L and Z, either separately or together, would further emphasize the importance of consuming fruits and vegetables rich in a variety of colors, adding more specificity beyond a general recommendation of the number of servings of fruits and vegetables in general. A specific recommendation for L and Z would also provide a benchmark for intake and enable better assessment for determining whether various populations are meeting their needs for visual health.
Dietary supplements
Numerous dietary supplements containing L and Z are available in the United States and worldwide, perhaps due in part to the notation on the US National Institutes of Health, Office of Dietary Supplements website identifying the AREDS2 supplement containing L and Z as one of 4 examples of effective dietary supplements.181 However, the amount of L and Z, along with other ingredients, varies widely in dietary supplements promoted for eye health, with some replicating the AREDS2 formulation, while others contain both higher amounts of select vitamins and minerals, and additional botanicals.182 Even with the evidence that the AREDS2 formula may reduce the risk of progression from intermediate to advanced AMD, and the multitude of product claims for products that “promote”, “protect”, or “support” vision and eye health, no dietary supplements on the market have been shown to help with primary prevention of AMD or other eye diseases, or to reduce the risk of progression from early to intermediate AMD.
The amount of L and Z in foods and dietary supplements appears to be safe.183 No adverse events were found in clinical trials administering L at 30 mg/d for 120 days or 40 mg/d for 63 days.184,185 The only reported adverse effect after a daily supplementation of 15 mg L in a 20-week trial was a single case of self-reported carotenodermia, a reversible condition of orange skin color.186 Although a higher amount has been used in human studies, after assessing the potential risks, the observed upper safety level for L has been proposed as 20 mg/d.187 The European Food Safety Authority concluded that safe upper limits for L and Z for use in dietary supplements were 1 mg/kg body weight/d and 0.75 mg/kg body weight/d, respectively.188,189
In primate models, rhesus monkeys fed a xanthophyll-free diet for 3 years to 6.5 years developed extremely deficient or absent macular yellow pigment and drusen-like bodies.190 When 2.2 mg/kg (3.9 µmol/kg) per day of L or Z were supplemented for 24 weeks to 101 weeks, the rhesus monkeys showed significant increases in their corresponding serum, retinal, and adipose tissue concentrations.191 In their retina samples, L and meso-Z, but not Z, appeared in the L-supplemented group, while only Z was found in the group supplemented with Z. In humans, a study investigating the serum and macular responses of L, Z, and meso-Z from dietary supplements found that 13.13 mg/d for 12 weeks provided maximum MPOD increases, whereas 7.44 mg/d was the amount that increased serum levels with the highest efficacy.192 These and other studies support the need to consider dietary recommendations for L and Z, particularly as conditionally essential nutrients, due to their protective effects on eye health.193
CONCLUSION
Macular xanthophylls cannot be synthesized de novo in primates. The oxidative defense and blue light filtering characteristics of L, Z, and meso-Z are important for visual function and potentially reducing the risk of AMD. Because L and Z storage in the maternal body during pregnancy and lactation may become depleted due to active transfer to the offspring, more attention to safe and robust intake of foods and dietary supplements containing these xanthophylls is warranted. Since many infant formula products are not fortified with L or Z, addition of these two xanthophylls should also be considered.
The impact of foods and dietary supplements rich in L and Z on MPOD and visual function among AMD patients and in healthy individuals deserves further attention. Since Z may play a different role from that of L in terms of macular pigment development or protection, better analytical techniques are needed to reassess food composition databases to distinguish these compounds, with greater attention paid to Z as a stand-alone food component, rather than grouping it with other xanthophylls. A reexamination of the US DRI status for L and Z is also warranted.
Goji berries have the highest known content of Z of any commonly consumed food, which also comes in a unique dipalmitate ester form. Given the increasing rates of AMD worldwide, goji berries, along with L and Z supplements, may help reduce this escalation.
Acknowledgments
Author contributions. X.L. conceived the review and drafted the manuscript; R.M.H., C.L.K., R.R.H., A.M.Z., L.S.M., and G.Y. provided revisions and critically reviewed the paper. X.L., R.M.H., and G.Y. prepared the figures. All authors have read and agreed on the final version.
Funding. X.L. received graduate student awards from the UC Davis College of Agricultural and Environmental Sciences and the UC Davis Department of Nutrition in support of this manuscript preparation.
Declaration of interest. The authors have no relevant interests to declare.
Contributor Information
Xiang Li, are with the Department of Nutrition, UC Davis, Davis, California, USA.
Roberta R Holt, are with the Department of Nutrition, UC Davis, Davis, California, USA.
Carl L Keen, are with the Department of Nutrition, UC Davis, Davis, California, USA; is with the Department of Internal Medicine, UC Davis, Sacramento, California, USA.
Lawrence S Morse, are with the Department of Ophthalmology and Vision Science, UC Davis Medical Center, Sacramento, California, USA.
Angela M Zivkovic, re with the Department of Nutrition, UC Davis, Davis, California, USA.
Glenn Yiu, are with the Department of Ophthalmology and Vision Science, UC Davis Medical Center, Sacramento, California, USA.
Robert M Hackman, are with the Department of Nutrition, UC Davis, Davis, California, USA.
REFERENCES
- 1. Eggersdorfer M, Wyss A. Carotenoids in human nutrition and health. Arch Biochem Biophys. 2018;652:18–26. [DOI] [PubMed] [Google Scholar]
- 2. Burns J, Fraser PD, Bramley PM. Identification and quantification of carotenoids, tocopherols and chlorophylls in commonly consumed fruits and vegetables. Phytochemistry. 2003;62:939–947. [DOI] [PubMed] [Google Scholar]
- 3. Rodriguez-Concepcion M, Avalos J, Bonet ML, et al. A global perspective on carotenoids: metabolism, biotechnology, and benefits for nutrition and health. Prog Lipid Res. 2018;70:62–93. [DOI] [PubMed] [Google Scholar]
- 4. El-Agamey A, Cantrell A, Land EJ, et al. Are dietary carotenoids beneficial? Reactions of carotenoids with oxy-radicals and singlet oxygen. Photochem Photobiol Sci. 2004;3:802–811. [DOI] [PubMed] [Google Scholar]
- 5. Scott KJ, Rodriquez-Amaya D. Pro-vitamin A carotenoid conversion factors: retinol equivalents – fact or fiction? Food Chem. 2000;69:125–127. [Google Scholar]
- 6. Bai C, Twyman RM, Farré G, et al. A golden era-pro-vitamin A enhancement in diverse crops. In Vitro Cell Dev Biol Plant. 2011;47:205–221. [Google Scholar]
- 7. Moran NE, Mohn ES, Hason N, et al. Intrinsic and extrinsic factors impacting absorption, metabolism, and health effects of dietary carotenoids. Adv Nutr. 2018;9:465–492. doi: 10.1093/ADVANCES/NMY025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Nolan JM, Meagher K, Kashani S, et al. What is meso-zeaxanthin, and where does it come from? Eye (Lond). 2013;27:899–905. doi: 10.1038/eye.2013.98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Adelson JD, Bourne RRA, Briant PS, et al. Blindness and Vision Impairment. The Lancet Global Health. 2021. Available at: https://www.who.int/news-room/fact-sheets/detail/blindness-and-visual-impairment. Accessed August 10, 2021.
- 10. Wong WL, Su X, Li X, et al. Global prevalence of age-related macular degeneration and disease burden projection for 2020 and 2040: a systematic review and meta-analysis. Lancet Glob Heal. 2014;2:e106–e116. [DOI] [PubMed] [Google Scholar]
- 11. Rein DB, Wittenborn JS, Zhang X, et al. Forecasting age-related macular degeneration through 2050. JAMA Ophthalmol. 2009;127:533–540. [DOI] [PubMed] [Google Scholar]
- 12. Mitchell P, Liew G, Gopinath B, et al. Age-related macular degeneration. Lancet. 2018;392:1147–1159. [DOI] [PubMed] [Google Scholar]
- 13. Michalska-Małecka K, Kabiesz A, Nowak M, et al. Age related macular degeneration – challenge for future: pathogenesis and new perspectives for the treatment. Eur Geriatr Med. 2015;6:69–75. [Google Scholar]
- 14. Ding X, Patel M, Chan CC. Molecular pathology of age-related macular degeneration. Prog Retin Eye Res. 2009;28:1–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Fisher CR, Ferrington DA. Perspective on AMD pathobiology: a bioenergetic crisis in the RPE. Invest Ophthalmol Vis Sci. 2018;59:AMD41–AMD47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Handa JT, Bowes Rickman C, Dick AD, et al. A systems biology approach towards understanding and treating non-neovascular age-related macular degeneration. Nat Commun. 2019;10:3347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Arunkumar R, Calvo CM, Conrady CD, et al. What do we know about the macular pigment in AMD: the past, the present, and the future. Eye (Lond). 2018;32:992–1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ferris FL, Wilkinson CP, Bird A, et al. Clinical classification of age-related macular degeneration. Ophthalmology. 2013;120:844–851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Lim LS, Mitchell P, Seddon JM, et al. Age-related macular degeneration. Lancet. 2012;379:1728–1738. [DOI] [PubMed] [Google Scholar]
- 20. Armstrong RA, Mousavi M. Overview of risk factors for age-related macular degeneration (AMD). J Stem Cells. 2015;10:171–191. [PubMed] [Google Scholar]
- 21. Lambert NG, ElShelmani H, Singh MK, et al. Risk factors and biomarkers of age-related macular degeneration. Prog Retin Eye Res. 2016;54:64–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Chakravarthy U, Wong TY, Fletcher A, et al. Clinical risk factors for age-related macular degeneration: a systematic review and meta-analysis. BMC Ophthalmol. 2010;10:31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.National Eye Institute. Age-Related Macular Degeneration (AMD) Data and Statistics. Published 2019. Available at: https://www.nei.nih.gov/learn-about-eye-health/eye-health-data-and-statistics/age-related-macular-degeneration-amd-data-and-statistics/age-related-macular-degeneration-amd-tables. Accessed August 31, 2022.
- 24. SanGiovanni JP, Neuringer M. The putative role of lutein and zeaxanthin as protective agents against age-related macular degeneration: promise of molecular genetics for guiding mechanistic and translational research in the field. Am J Clin Nutr. 2012;96:1223S–1233S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Fritsche LG, Igl W, Bailey JNC, et al. A large genome-wide association study of age-related macular degeneration highlights contributions of rare and common variants. Nat Genet. 2016;48:134–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Fritsche LG, Chen W, Schu M, et al. Seven new loci associated with age-related macular degeneration. Nat Genet. 2013;45:433–439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Holliday EG, Smith AV, Cornes BK, et al. ; Wellcome Trust Case Control Consortium 2. Insights into the genetic architecture of early stage age-related macular degeneration: a genome-wide association study meta-analysis. PLoS One. 2013;8:e53830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Mattapallil MJ, Caspi RR. Compliments of factor H: what’s in it for AMD? Immunity. 2017;46:167–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Toomey CB, Johnson LV, Bowes Rickman C. Complement factor H in AMD: bridging genetic associations and pathobiology. Prog Retin Eye Res. 2018;62:38–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Yan Q, Ding Y, Liu Y, et al. ; AREDS2 Research Group. Genome-wide analysis of disease progression in age-related macular degeneration. Hum Mol Genet. 2018;27:929–940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Khan JC, Shahid H, Thurlby DA, et al. ; Genetic Factors in AMD Study. Age related macular degeneration and sun exposure, iris colour, and skin sensitivity to sunlight. Br J Ophthalmol. 2006;90:29–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Nicolas CM, Robman LD, Tikellis G, et al. Iris colour, ethnic origin and progression of age-related macular degeneration. Clin Exp Ophthalmol. 2003;31:465–469. [DOI] [PubMed] [Google Scholar]
- 33. Schick T, Ersoy L, Lechanteur YTE, et al. History of sunlight exposure is a risk factor for age-related macular degeneration. Retina. 2016;36:787–790. [DOI] [PubMed] [Google Scholar]
- 34. Mitchell P, Smith W, Wang JJ. Iris color, skin sun sensitivity, and age-related maculopathy: the Blue Mountains Eye Study. Ophthalmology. 1998;105:1359–1363. [DOI] [PubMed] [Google Scholar]
- 35. Frank RN, Puklin JE, Stock C, et al. Race, iris color, and age-related macular degeneration. Trans Am Ophthalmol Soc. 2000;98:109–117. [PMC free article] [PubMed] [Google Scholar]
- 36. Kim EK, Kim H, Vijayakumar A, et al. Associations between fruit and vegetable, and antioxidant nutrient intake and age-related macular degeneration by smoking status in elderly Korean men. Nutr J. 2017;16:77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. de Koning-Backus APM, Buitendijk GHS, Kiefte-de Jong JC, et al. Intake of vegetables, fruit, and fish is beneficial for age-related macular degeneration. Am J Ophthalmol. 2019;198:70–79. [DOI] [PubMed] [Google Scholar]
- 38. Agrón E, Mares J, Clemons TE, et al. ; AREDS and AREDS2 Research Groups. Dietary nutrient intake and progression to late age-related macular degeneration in the age-related eye disease studies 1 and 2. Ophthalmology. 2021;128:425–442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Ma L, Dou HL, Wu YQ, et al. Lutein and zeaxanthin intake and the risk of age-related macular degeneration: a systematic review and meta-analysis. Br J Nutr. 2012;107:350–359. [DOI] [PubMed] [Google Scholar]
- 40. Chapman NA, Jacobs RJ, Braakhuis AJ. Role of diet and food intake in age-related macular degeneration: a systematic review. Clin Exp Ophthalmol. 2019;47:106–127. [DOI] [PubMed] [Google Scholar]
- 41. Broadhead GK, Grigg JR, Chang AA, et al. Dietary modification and supplementation for the treatment of age-related macular degeneration. Nutr Rev. 2015;73:448–462. [DOI] [PubMed] [Google Scholar]
- 42. Abdel-Aal ESM, Akhtar H, Zaheer K, et al. Dietary sources of lutein and zeaxanthin carotenoids and their role in eye health. Nutrients. 2013;5:1169–1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Perry A, Rasmussen H, Johnson EJ. Xanthophyll (lutein, zeaxanthin) content in fruits, vegetables and corn and egg products. J Food Compos Anal. 2009;22:9–15. [Google Scholar]
- 44. Amagase H, Farnsworth NR. A review of botanical characteristics, phytochemistry, clinical relevance in efficacy and safety of Lycium barbarum fruit (Goji). Food Res Int. 2011;44:1702–1717. [Google Scholar]
- 45. Karioti A, Bergonzi MC, Vincieri FF, et al. Validated method for the analysis of Goji berry, a rich source of Zeaxanthin dipalmitate. J Agric Food Chem. 2014;62:12529–12535. [DOI] [PubMed] [Google Scholar]
- 46. Nebeling LC, Forman MR, Graubard BI, et al. Changes in carotenoid intake in the United States: the 1987 and 1992 National Health Interview Surveys. J Am Diet Assoc. 1997;97:991–996. [DOI] [PubMed] [Google Scholar]
- 47. U.S. Department of Agriculture Food Surveys Research Group. What we eat in America: NHANES 2013-2014. 2014. Available at: www.ars.usda.gov/ARSUserFiles/80400530/pdf/1314/Table_1_NIN_GEN_13.pdf. Accessed December 18, 2021.
- 48. Krinsky NI, Johnson EJ. Carotenoid actions and their relation to health and disease. Mol Aspects Med. 2005;26:459–516. [DOI] [PubMed] [Google Scholar]
- 49. Potterat O. Goji (Lycium barbarum and L. chinense): phytochemistry, pharmacology and safety in the perspective of traditional uses and recent popularity. Planta Med. 2010;76:7–19. [DOI] [PubMed] [Google Scholar]
- 50. Chang RC-C, So K-F. Use of anti-aging herbal medicine, Lycium barbarum, against aging-associated diseases. What do we know so far? Cell Mol Neurobiol. 2008;28:643–652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Neelam K, Dey S, Sim R, et al. Fructus lycii: a natural dietary supplement for amelioration of retinal diseases. Nutrients. 2021;13:246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Inbaraj BS, Lu H, Hung CF, et al. Determination of carotenoids and their esters in fruits of Lycium barbarum Linnaeus by HPLC–DAD–APCI–MS. J Pharm Biomed Anal. 2008;47:812–818. [DOI] [PubMed] [Google Scholar]
- 53. Sajilata MG, Singhal RS, Kamat MY. The carotenoid pigment zeaxanthin – a review. Comp Rev Food Sci Food Safety. 2008;7:29–49. [Google Scholar]
- 54. Zhou ZQ, Xiao J, Fan HX, et al. Polyphenols from wolfberry and their bioactivities. Food Chem. 2017;214:644–654. [DOI] [PubMed] [Google Scholar]
- 55. Zhao LQ, Qiu ZQ, Narasimhamoorthy B, et al. Development of a rapid, high-throughput method for quantification of zeaxanthin in Chinese wolfberry using HPLC-DAD. Ind Crops Prod. 2013;47:51–57. [Google Scholar]
- 56. U.S. Department of Agriculture. FoodData Central. 2018. https://fdc.nal.usda.gov/fdc-app.html#/food-details/173032/nutrients. Accessed November 5, 2020.
- 57. Peng Y, Ma C, Li Y, et al. Quantification of zeaxanthin dipalmitate and total carotenoids in Lycium fruits (Fructus Lycii). Plant Foods Hum Nutr. 2005;60:161–164. [DOI] [PubMed] [Google Scholar]
- 58. Chitchumroonchokchai C, Failla ML. Hydrolysis of zeaxanthin esters by carboxyl ester lipase during digestion facilitates micellarization and uptake of the xanthophyll by caco-2 human intestinal cells. J Nutr. 2006;136:588–594. [DOI] [PubMed] [Google Scholar]
- 59. Cheng CY, Chung WY, Szeto YT, et al. Fasting plasma zeaxanthin response to Fructus barbarum L. (wolfberry; Kei Tze) in a food-based human supplementation trial. Br J Nutr. 2005;93:123–130. [DOI] [PubMed] [Google Scholar]
- 60. Breithaupt DE, Weller P, Wolters M, et al. Comparison of plasma responses in human subjects after the ingestion of 3R,3R′-zeaxanthin dipalmitate from wolfberry (Lycium barbarum) and non-esterified 3R,3R′-zeaxanthin using chiral high-performance liquid chromatography. Br J Nutr. 2004;91:707–713. [DOI] [PubMed] [Google Scholar]
- 61. Tudor C, Pintea A. A brief overview of dietary zeaxanthin occurrence and bioaccessibility. Molecules. 2020;25:4067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Arunkumar R, Gorusupudi A, Bernstein PS. The macular carotenoids: a biochemical overview. Biochim Biophys Acta Mol Cell Biol Lipids. 2020;1865:158617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Widomska J, Sangiovanni JP, Subczynski WK. Why is zeaxanthin the most concentrated xanthophyll in the central fovea? Nutrients. 2020;12:1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Reboul E. Mechanisms of carotenoid intestinal absorption: where do we stand? Nutrients. 2019;11:838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Harrison EH. Mechanisms of transport and delivery of vitamin A and carotenoids to the retinal pigment epithelium. Mol Nutr Food Res. 2019;63:1801046. [DOI] [PubMed] [Google Scholar]
- 66. Kotake-Nara E, Nagao A. Absorption and metabolism of xanthophylls. Mar Drugs. 2011;9:1024–1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Erdman JW, Bierer TL, Gugger ET. Absorption and transport of carotenoids. Ann N Y Acad Sci. 1993;691:76–85. [DOI] [PubMed] [Google Scholar]
- 68. Forman MR, Johnson EJ, Lanza E, et al. Effect of menstrual cycle phase on the concentration of individual carotenoids in lipoproteins of premenopausal women: a controlled dietary study. Am J Clin Nutr. 1998;67:81–87. [DOI] [PubMed] [Google Scholar]
- 69. Goulinet S, Chapman MJ. Plasma LDL and HDL subspecies are heterogenous in particle content of tocopherols and oxygenated and hydrocarbon carotenoids. Arterioscler Thromb Vasc Biol. 1997;17:786–796. [DOI] [PubMed] [Google Scholar]
- 70. Colijn JM, Verzijden T, Meester-Smoor MA, et al. ; EYE-RISK Consortium. Increased high-density lipoprotein levels associated with age-related macular degeneration: evidence from the EYE-RISK and European Eye Epidemiology Consortia. Ophthalmology. 2019;126:393–406. doi: 10.1016/j.ophtha.2018.09.045 [DOI] [PubMed] [Google Scholar]
- 71. Saunier V, Merle BMJ, Delyfer MN, et al. Incidence of and risk factors associated with age-related macular degeneration: four-year follow-up from the ALIENOR study. JAMA Ophthalmol. 2018;136:473–481. doi: 10.1001/jamaophthalmol.2018.0504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Lin H, Mares JA, LaMonte MJ, et al. Association between dietary xanthophyll (lutein and zeaxanthin) intake and early age-related macular degeneration: the Atherosclerosis Risk in Communities study. Ophthalmic Epidemiol. 2017;24:311–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Li B, Vachali P, Chang FY, et al. HDL is the primary transporter for carotenoids from liver to retinal pigment epithelium in transgenic ApoA-I−/−/Bco2−/− mice. Arch Biochem Biophys. 2022;716:109111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Schnebelen-Berthier C, Acar N, Simon E, et al. The ALGOVUE clinical trial: effects of the daily consumption of eggs enriched with lutein and docosahexaenoic acid on plasma composition and macular pigment optical density. Nutrients. 2021;13:3347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Niesor EJ, Chaput E, Mary JL, et al. Effect of compounds affecting ABCA1 expression and CETP activity on the HDL pathway involved in intestinal absorption of lutein and zeaxanthin. Lipids. 2014;49:1233–1243. [DOI] [PubMed] [Google Scholar]
- 76. Herron KL, McGrane MM, Waters D, et al. The ABCG5 polymorphism contributes to individual responses to dietary cholesterol and carotenoids in eggs. J Nutr. 2006;136:1161–1165. [DOI] [PubMed] [Google Scholar]
- 77. Merle BMJ, Maubaret C, Korobelnik JF, et al. Association of HDL-related loci with age-related macular degeneration and plasma lutein and zeaxanthin: the Alienor study. PLoS One. 2013;8:e79848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Leo MA, Ahmed S, Aleynik SI, et al. Carotenoids and tocopherols in various hepatobiliary conditions. J Hepatol. 1995;23:550–556. [DOI] [PubMed] [Google Scholar]
- 79. Li B, Vachali P, Frederick JM, et al. Identification of StARD3 as a lutein-binding protein in the macula of the primate retina. Biochemistry. 2011;50:2541–2549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Bhosale P, Larson AJ, Frederick JM, et al. Identification and characterization of a Pi isoform of glutathione S-transferase (GSTP1) as a zeaxanthin-binding protein in the macula of the human eye. J Biol Chem. 2004;279:49447–49454. [DOI] [PubMed] [Google Scholar]
- 81. Bernstein PS, Tsong ED, Balashov NA, et al. Retinal tubulin binds macular carotenoids. Investig Ophthalmol Vis Sci 1997;38:167–175. [PubMed] [Google Scholar]
- 82. Li B, Vachali PP, Gorusupudi A, et al. Inactivity of human β,β-carotene-9′, 10-′dioxygenase (BCO2) underlies retinal accumulation of the human macular carotenoid pigment. Proc Natl Acad Sci USA. 2014;111:10173–10178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Giordano E, Quadro L. Lutein, zeaxanthin and mammalian development: metabolism, functions and implications for health. Arch Biochem Biophys. 2018;647:33–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Li B, Vachali PP, Shen Z, et al. Retinal accumulation of zeaxanthin, lutein, and β-carotene in mice deficient in carotenoid cleavage enzymes. Exp Eye Res. 2017;159:123–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Snodderly DM, Auran JD, Delori FC. The macular pigment. II. Spatial distribution in primate retinas. Invest Ophthalmol Vis Sci. 1984;25:674–685. [PubMed] [Google Scholar]
- 86. Snodderly DM, Brown PK, Delori FC, et al. The macular pigment. I. Absorbance spectra, localization, and discrimination from other yellow pigments in primate retinas. Investig Ophthalmol Vis Sci. 1984;25:660–673. [PubMed] [Google Scholar]
- 87. Bone RA, Landrum JT, Fernandez L, et al. Analysis of the macular pigment by HPLC: retinal distribution and age study. Investig Ophthalmol Vis Sci. 1988;29:843–849. [PubMed] [Google Scholar]
- 88. Li B, George EW, Rognon GT, et al. Imaging lutein and zeaxanthin in the human retina with confocal resonance Raman microscopy. Proc Natl Acad Sci USA. 2020;117:12352–12358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Connor WE, Duell PB, Kean R, et al. The prime role of HDL to transport lutein into the retina: evidence from HDL-deficient WHAM chicks having a mutant ABCA1 transporter. Invest Ophthalmol Vis Sci. 2007;48:4226–4231. [DOI] [PubMed] [Google Scholar]
- 90. Maoka T. Carotenoids as natural functional pigments. J Nat Med. 2020;74:1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Roberts JE, Dennison J. The photobiology of lutein and zeaxanthin in the eye. J Ophthalmol. 2015;2015:687173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Terman A, Brunk UT. Lipofuscin: mechanisms of formation and increase with age. APMIS. 1998;106:265–276. [DOI] [PubMed] [Google Scholar]
- 93. Kim SR, Nakanishi K, Itagaki Y, et al. Photooxidation of A2-PE, a photoreceptor outer segment fluorophore, and protection by lutein and zeaxanthin. Exp Eye Res. 2006;82:828–839. [DOI] [PubMed] [Google Scholar]
- 94. Sujak A, Gabrielska J, Grudziński W, et al. Lutein and zeaxanthin as protectors of lipid membranes against oxidative damage: the structural aspects. Arch Biochem Biophys. 1999;371:301–307. [DOI] [PubMed] [Google Scholar]
- 95. Lim BP, Nagao A, Terao J, et al. Antioxidant activity of xanthophylls on peroxyl radical–mediated phospholipid peroxidation. Biochim Biophys Acta. 1992;1126:178–184. [DOI] [PubMed] [Google Scholar]
- 96. Nilsson SEG, Sundelin SP, Wihlmark U, et al. Aging of cultured retinal pigment epithelial cells: oxidative reactions, lipofuscin formation and blue light damage. Doc Ophthalmol. 2003;106:13–16. [DOI] [PubMed] [Google Scholar]
- 97. Beatty S, Koh HH, Phil M, et al. The role of oxidative stress in the pathogenesis of age-related macular degeneration. Surv Ophthalmol. 2000;45:115–134. [DOI] [PubMed] [Google Scholar]
- 98. Li B, Ahmed F, Bernstein PS. Studies on the singlet oxygen scavenging mechanism of human macular pigment. Arch Biochem Biophys. 2010;504:56–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Abell RG, Hewitt AW, Andric M, et al. The use of heterochromatic flicker photometry to determine macular pigment optical density in a healthy Australian population. Graefes Arch Clin Exp Ophthalmol. 2014;252:417–421. [DOI] [PubMed] [Google Scholar]
- 100. Bernstein PS, Li B, Vachali PP, et al. Lutein, zeaxanthin, and meso-zeaxanthin: the basic and clinical science underlying carotenoid-based nutritional interventions against ocular disease. Prog Retin Eye Res. 2016;50:34–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Curran-Celentano J, Hammond BR, Ciulla TA, et al. Relation between dietary intake, serum concentrations, and retinal concentrations of lutein and zeaxanthin in adults in a Midwest population. Am J Clin Nutr. 2001;74:796–802. [DOI] [PubMed] [Google Scholar]
- 102. Burke JD, Curran-Celentano J, Wenzel AJ. Diet and serum carotenoid concentrations affect macular pigment optical density in adults 45 years and older. J Nutr. 2005;135:1208–1214. [DOI] [PubMed] [Google Scholar]
- 103. Alassane S, Binquet C, Cottet V, et al. Relationships of macular pigment optical density with plasma lutein, zeaxanthin, and diet in an elderly population: the Montrachet study. Invest Ophthalmol Vis Sci. 2016;57:1160–1167. [DOI] [PubMed] [Google Scholar]
- 104. Bernstein PS, Zhao DY, Wintch SW, et al. Resonance Raman measurement of macular carotenoids in normal subjects and in age-related macular degeneration patients. Ophthalmology. 2002;109:1780–1787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Mares J. Lutein and zeaxanthin isomers in eye health and disease. Annu Rev Nutr. 2016;36:571–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Bone RA, Landrum JT, Mayne ST, et al. Macular pigment in donor eyes with and without AMD: a case–control study. Investig Ophthalmol Vis Sci. 2001;42:235–240. [PubMed] [Google Scholar]
- 107. Wilson LM, Tharmarajah S, Jia Y, et al. The effect of lutein/zeaxanthin intake on human macular pigment optical density: a systematic review and meta-analysis. Adv Nutr. 2021;12:2244–2254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Fitzpatrick N, Chachay V, Bowtell J, et al. An appraisal of trials investigating the effects on macular pigment optical density of lutein and zeaxanthin dietary interventions: a narrative review. Nutr Rev. 2022;80:513–524. [DOI] [PubMed] [Google Scholar]
- 109. Mares JA, LaRowe TL, Snodderly DM, et al. ; CAREDS Macular Pigment Study Group and Investigators. Predictors of optical density of lutein and zeaxanthin in retinas of older women in the Carotenoids in Age-Related Eye Disease Study, an ancillary study of the Women’s Health Initiative. Am J Clin Nutr. 2006;84:1107–1122. [DOI] [PubMed] [Google Scholar]
- 110. Eisenhauer B, Natoli S, Liew G, et al. Lutein and zeaxanthin – food sources, bioavailability and dietary variety in age‐related macular degeneration protection. Nutrients. 2017;9:120.28208784 [Google Scholar]
- 111. Nwachukwu ID, Udenigwe CC, Aluko RE. Lutein and zeaxanthin: production technology, bioavailability, mechanisms of action, visual function, and health claim status. Trends Food Sci Technol. 2016;49:74–84. [Google Scholar]
- 112. Wu J, Cho E, Willett WC, et al. Intakes of lutein, zeaxanthin, and other carotenoids and age- related macular degeneration during 2 decades of prospective follow-up. JAMA Ophthalmol. 2015;133:1415–1424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Ma L, Liu R, Du JH, et al. Lutein, zeaxanthin and meso-zeaxanthin supplementation associated with macular pigment optical density. Nutrients. 2016;8:426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Age-Related Eye Disease Study Research Group. The Age-Related Eye Disease Study (AREDS): design implications AREDS Report No.1. Control Clin Trials. 1999;20:573–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Age-Related Eye Disease Study Research Group. A randomized, placebo-controlled, clinical trial of high-dose supplementation with vitamins C and E, beta carotene, and zinc for age-related macular degeneration and vision loss: AREDS report no. 8. Arch Ophthalmol. 2001;119:1417–1436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Chew EY, Clemons TE, SanGiovanni JP, et al. Lutein + zeaxanthin and omega-3 fatty acids for age-related macular degeneration: the Age-Related Eye Disease Study 2 (AREDS2) randomized clinical trial. JAMA. 2013;309:2005–2015. [DOI] [PubMed] [Google Scholar]
- 117. Chew EY, Clemons TE, SanGiovanni JP, et al. ; Age-Related Eye Disease Study 2 (AREDS2) Research Group. Secondary analyses of the effects of lutein/zeaxanthin on age-related macular degeneration progression AREDS2 report no. 3. JAMA Ophthalmol. 2014;132:142–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Chew EY, Clemons TE, Agrón E, et al. ; AREDS2 Research Group. Long-term outcomes of adding lutein/zeaxanthin and ω-3 fatty acids to the AREDS supplements on age-related macular degeneration progression: AREDS2 report 28. JAMA Ophthalmol. 2022;140:692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Agrón E, Mares J, Chew EY, et al. Adherence to a Mediterranean diet and geographic atrophy enlargement rate: age-related eye disease study 2 report 29. Ophthalmol Retina. 2022;S2468-6530(22)00152-X. doi: 10.1016/j.oret.2022.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Marhuenda-Muñoz M, Hurtado-Barroso S, Tresserra-Rimbau A, et al. A review of factors that affect carotenoid concentrations in human plasma: differences between Mediterranean and Northern diets. Eur J Clin Nutr. 2019;72:18–25. [DOI] [PubMed] [Google Scholar]
- 121. Bucheli P, Vidal K, Shen L, et al. Goji berry effects on macular characteristics and plasma antioxidant levels. Optom Vis Sci. 2011;88:257–262. [DOI] [PubMed] [Google Scholar]
- 122. Peng ML, Chiu HF, Chou H, et al. Influence/impact of lutein complex (marigold flower and wolfberry) on visual function with early age-related macular degeneration subjects: a randomized clinical trial. J Funct Foods. 2016;24:122–130. [Google Scholar]
- 123. Kan J, Wang M, Liu Y, et al. A novel botanical formula improves eye fatigue and dry eye: a randomized, double-blind, placebo-controlled study. Am J Clin Nutr. 2020;112:334–342. [DOI] [PubMed] [Google Scholar]
- 124. Li S, Liu N, Lin L, et al. Macular pigment and serum zeaxanthin levels with Goji berry supplement in early age-related macular degeneration. Int J Ophthalmol. 2018;11:970–975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Li X, Holt RR, Keen CL, et al. Goji berry intake increases macular pigment optical density in healthy adults: a randomized pilot trial. Nutrients. 2021;13:4409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Chan HH, Lam H-I, Choi K-Y, et al. Delay of cone degeneration in retinitis pigmentosa using a 12-month treatment with Lycium barbarum supplement. J Ethnopharmacol. 2019;236:336–344. [DOI] [PubMed] [Google Scholar]
- 127. Pavan B, Capuzzo A, Forlani G. High glucose-induced barrier impairment of human retinal pigment epithelium is ameliorated by treatment with Goji berry extracts through modulation of cAMP levels. Exp Eye Res. 2014;120:50–54. [DOI] [PubMed] [Google Scholar]
- 128. Mi X-S, Feng Q, Lo ACY, et al. Protection of retinal ganglion cells and retinal vasculature by Lycium barbarum polysaccharides in a mouse model of acute ocular hypertension. PLoS One. 2012;7:e45469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Mi X-S, Chiu K, Van G, et al. Effect of Lycium barbarum polysaccharides on the expression of endothelin-1 and its receptors in an ocular hypertension model of rat glaucoma. Neural Regen Res. 2012;7:651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Lakshmanan Y, Wong FSY, Zuo B, et al. Posttreatment intervention with Lycium barbarum polysaccharides is neuroprotective in a rat model of chronic ocular hypertension. Invest Ophthalmol Vis Sci. 2019;60:4606–4618. [DOI] [PubMed] [Google Scholar]
- 131. Tang L, Zhang Y, Jiang Y, et al. Dietary wolfberry ameliorates retinal structure abnormalities in db/db mice at the early stage of diabetes. Exp Biol Med (Maywood). 2011;236:1051–1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Lopez-Matas MA, Carnes J, Larramendi CH, et al. Goji berries, a novel potent allergenic source with high cross-reactivity with other fruits. J Allergy Clin Immunol. 2012;129:AB232. [Google Scholar]
- 133. Carnés J, De Larramendi CH, Ferrer A, et al. Recently introduced foods as new allergenic sources: sensitisation to Goji berries (Lycium barbarum). Food Chem. 2013;137:130–135. [DOI] [PubMed] [Google Scholar]
- 134. Rivera CA, Ferro CL, Bursua AJ, et al. Probable interaction between Lycium barbarum (Goji) and Warfarin. Pharmacother J Hum Pharmacol Drug Ther. 2012;32:e50–e53. [DOI] [PubMed] [Google Scholar]
- 135. Zhang J, Tian L, Xie B. Bleeding due to a probable interaction between warfarin and Gouqizi (Lycium barbarum L.). Toxicol Rep. 2015;2:1209–1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Henriksen BS, Chan G, Hoffman RO, et al. Interrelationships between maternal carotenoid status and newborn infant macular pigment optical density and carotenoid status. Invest Ophthalmol Vis Sci. 2013;54:5568–5578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Lai JS, Veetil VO, Lanca C, et al. Maternal lutein and zeaxanthin concentrations in relation to offspring visual acuity at 3 years of age: the GUSTO study. Nutrients. 2020;12:274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Hammond BR. Possible role for dietary lutein and zeaxanthin in visual development. Nutr Rev. 2008;66:695–702. [DOI] [PubMed] [Google Scholar]
- 139. Provis JM, Penfold PL, Cornish EE, et al. Anatomy and development of the macula: specialisation and the vulnerability to macular degeneration. Clin Exp Optom. 2005;88:269–281. [DOI] [PubMed] [Google Scholar]
- 140. Yuodelis C, Hendrickson A. A qualitative and quantitative analysis of the human fovea during development. Vision Res. 1986;26:847–855. [DOI] [PubMed] [Google Scholar]
- 141. Buonocore G, Tei M, Perrone S. Lutein as a protective agent against neonatal oxidative stress. J Pediatr Neonatal Individ Med. 2014;3:e030244. [Google Scholar]
- 142. Sukgen EA, Koçluk Y. Comparison of clinical outcomes of intravitreal ranibizumab and aflibercept treatment for retinopathy of prematurity. Graefes Arch Clin Exp Ophthalmol. 2019;257:49–55. [DOI] [PubMed] [Google Scholar]
- 143. Fu Z, Meng SS, Burnim SB, Smith LEH, et al. Lutein facilitates physiological revascularization in a mouse model of retinopathy of prematurity. Clin Exp Ophthalmol. 2017;45:529–538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Manzoni P, Guardione R, Bonetti P, et al. Lutein and zeaxanthin supplementation in preterm very low-birth-weight neonates in neonatal intensive care units: a multicenter randomized controlled trial. Am J Perinatol 2013;30:25–32. [DOI] [PubMed] [Google Scholar]
- 145. Romagnoli C, Giannantonio C, Cota F, et al. A prospective, randomized, double blind study comparing lutein to placebo for reducing occurrence and severity of retinopathy of prematurity. J Matern Neonatal Med. 2011;24:147–150. [DOI] [PubMed] [Google Scholar]
- 146. Cota F, Costa S, Giannantonio C, et al. Lutein supplementation and retinopathy of prematurity: a meta-analysis. J Matern Neonatal Med. 2022;35:175–180. [DOI] [PubMed] [Google Scholar]
- 147. Long AC, Kuchan M, Mackey AD. Lutein as an ingredient in pediatric nutritionals. J AOAC Int. 2019;102:1034–1043. [DOI] [PubMed] [Google Scholar]
- 148. Zielińska MA, Wesołowska A, Pawlus B, et al. Health effects of carotenoids during pregnancy and lactation. Nutrients. 2017;9:838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Zielinska MA, Hamulka J, Wesolowska A. Carotenoid content in breastmilk in the 3rd and 6th month of lactation and its associations with maternal dietary intake and anthropometric characteristics. Nutrients. 2019;11:193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150. Sherry CL, Oliver JS, Renzi LM, et al. Lutein supplementation increases breast milk and plasma lutein concentrations in lactating women and infant plasma concentrations but does not affect other carotenoids. J Nutr. 2014;144:1256–1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151. Lietz G, Mulokozi G, Henry JCK, et al. Xanthophyll and hydrocarbon carotenoid patterns differ in plasma and breast milk of women supplemented with red palm oil during pregnancy and lactation. J Nutr. 2006;136:1821–1827. [DOI] [PubMed] [Google Scholar]
- 152. Jewell VC, Northrop-Clewes CA, Tubman R, et al. Nutritional factors and visual function in premature infants. Proc Nutr Soc. 2001;60:171–178. [DOI] [PubMed] [Google Scholar]
- 153. Bernstein PS, Sharifzadeh M, Liu A, et al. Blue-light reflectance imaging of macular pigment in infants and children. Invest Ophthalmol Vis Sci. 2013;54:4034–4040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154. Rubin LP, Chan GM, Barrett-Reis BM, et al. Effect of carotenoid supplementation on plasma carotenoids, inflammation and visual development in preterm infants. J Perinatol. 2012;32:418–424. [DOI] [PubMed] [Google Scholar]
- 155. Costa S, Giannantonio C, Romagnoli C, et al. Lutein and zeaxanthin concentrations in formula and human milk samples from Italian mothers. Eur J Clin Nutr. 2015;69:531–532. [DOI] [PubMed] [Google Scholar]
- 156. Bettler J, Zimmer JP, Neuringer M, et al. Serum lutein concentrations in healthy term infants fed human milk or infant formula with lutein. Eur J Nutr. 2010;49:45–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157. Addo EK, Gorusupudi A, Allman S, et al. The Lutein and Zeaxanthin in Pregnancy (L-ZIP) study—carotenoid supplementation during pregnancy: ocular and systemic effects—study protocol for a randomized controlled trial. Trials. 2021;22:300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158. Bernstein PS, Arunkumar R. The emerging roles of the macular pigment carotenoids throughout the lifespan and in prenatal supplementation. J Lipid Res. 2021;62:100038.doi: 10.1194/jlr.TR120000956 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159. Kaarniranta K, Machalińska A, Veréb Z, et al. Estrogen signalling in the pathogenesis of age-related macular degeneration. Curr Eye Res. 2015;40:226–233. [DOI] [PubMed] [Google Scholar]
- 160. Heesterbeek TJ, Lorés-Motta L, Hoyng CB, et al. Risk factors for progression of age-related macular degeneration. Ophthalmic Physiol Opt. 2020;40:140–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161. Zetterberg M. Age-related eye disease and gender. Maturitas. 2016;83:19–26. [DOI] [PubMed] [Google Scholar]
- 162. Feskanich D, Cho E, Schaumberg DA, et al. Menopausal and reproductive factors and risk of age-related macular degeneration. Arch Ophthalmol. 2008;126:519–524. [DOI] [PubMed] [Google Scholar]
- 163. Hwang S, Kang SW, Han J, et al. Female reproductive factors and the risk of exudative age-related macular degeneration. Retina. 2021;41:2088–2097. [DOI] [PubMed] [Google Scholar]
- 164. Cho BJ, Heo JW, Shin JP, et al. Association between reproductive factors and age-related macular degeneration in postmenopausal women: the Korea National Health and Nutrition Examination Survey 2010–2012. PLoS One. 2014;9:e102816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165. Erke MG, Bertelsen G, Peto T, et al. Lactation, female hormones and age-related macular degeneration: the Tromsø study. Br J Ophthalmol. 2013;97:1036–1039. [DOI] [PubMed] [Google Scholar]
- 166. Alves-Rodrigues A, Shao A. The science behind lutein. Toxicol Lett. 2004;150:57–83. [DOI] [PubMed] [Google Scholar]
- 167. Johnson EJ. Role of lutein and zeaxanthin in visual and cognitive function throughout the lifespan. Nutr Rev. 2014;72:605–612. [DOI] [PubMed] [Google Scholar]
- 168. Lindbergh C, Terry D, Mewborn C, et al. Aging and dementia—1lutein and zeaxanthin supplementation improves neurocognitive function in older adults as measured using functional magnetic resonance imaging: a randomized controlled trial. Arch Clin Neuropsychol. 2016;31:574.2–583. [Google Scholar]
- 169. Buscemi S, Corleo D, Di Pace F, et al. The effect of lutein on eye and extra-eye health. Nutrients. 2018;10:1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170. Leermakers ETM, Darweesh SKL, Baena CP, et al. The effects of lutein on respiratory health across the life course: a systematic review. Am J Clin Nutr. 2016;103:481–494. [DOI] [PubMed] [Google Scholar]
- 171. Cooke MC, Coates AM, Buckley ES, et al. Lutein intake and blood lutein concentration are positively associated with physical activity in adults: a systematic review. Nutrients. 2018;10:1186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172. Hajizadeh-Sharafabad F, Ghoreishi Z, Maleki V, et al. Mechanistic insights into the effect of lutein on atherosclerosis, vascular dysfunction, and related risk factors: a systematic review of in vivo, ex vivo and in vitro studies. Pharmacol Res. 2019;149:104477. [DOI] [PubMed] [Google Scholar]
- 173. Nouchi R, Suiko T, Kimura E, et al. Effects of lutein and astaxanthin intake on the improvement of cognitive functions among healthy adults: a systematic review of randomized controlled trials. Nutrients. 2020;12:617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174. Yagi A, Nouchi R, Butler L, et al. Lutein has a positive impact on brain health in healthy older adults: a systematic review of randomized controlled trials and cohort studies. Nutrients. 2021;13:1746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175. Berendschot TTJM, Van Norren D. On the age dependency of the macular pigment optical density. Exp Eye Res. 2005;81:602–609. [DOI] [PubMed] [Google Scholar]
- 176. Olmedilla-Alonso B, Beltrán-de-Miguel B, Estévez-Santiago R, et al. Markers of lutein and zeaxanthin status in two age groups of men and women: dietary intake, serum concentrations, lipid profile and macular pigment optical density. Nutr J. 2014;13:52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177. McBurney MI, Blumberg JB, Costello RB, et al. Beyond nutrient deficiency—opportunities to improve nutritional status and promote health modernizing DRIs and supplementation recommendations. Nutrients. 2021;13:1844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178. Lupton JR, Atkinson SA, Chang N, et al. Exploring the benefits and challenges of establishing a DRI-like process for bioactives. Eur J Nutr. 2014;53(suppl 1):S1–S9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179. Ranard KM, Jeon S, Mohn ES, et al. Dietary guidance for lutein: consideration for intake recommendations is scientifically supported. Eur J Nutr. 2017;56:37–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180. Yates AA, Dwyer JT, Erdman JW, et al. ; serving as an ad hoc Working Group on a Framework for Developing Recommended Intakes for Dietary Bioactives. Perspective: framework for developing recommended intakes of bioactive dietary substances. Adv Nutr. 2021;12:1087–1099. doi: 10.1093/advances/nmab044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.U.S. Department of Health & Human Services. National Institutes of Health. Division of Program Coordination, Planning, and Strategic Initiatives. Dietary Supplements: What You Need to Know – Consumer. Available at: https://ods.od.nih.gov/factsheets/WYNTK-Consumer/. Accessed May 1, 2022.
- 182. Yong JJ, Scott IU, Greenberg PB. Ocular nutritional supplements: are their ingredients and manufacturers’ claims evidence-based? Ophthalmology. 2015;122:595–599. [DOI] [PubMed] [Google Scholar]
- 183. Kruger CL, Murphy M, DeFreitas Z, et al. An innovative approach to the determination of safety for a dietary ingredient derived from a new source: case study using a crystalline lutein product. Food Chem Toxicol. 2002;40:1535–1549. [DOI] [PubMed] [Google Scholar]
- 184. Wenzel AJ, Sheehan JP, Gerweck C, et al. Macular pigment optical density at four retinal loci during 120 days of lutein supplementation. Ophthalmic Physiol Opt. 2007;27:329–335. [DOI] [PubMed] [Google Scholar]
- 185. Dagnelie G, Zorge IS, McDonald TM. Lutein improves visual function in some patients with retinal degeneration: a pilot study via the Internet. Optometry. 2000;71:147–164. [PubMed] [Google Scholar]
- 186. Olmedilla B, Granado F, Southon S, et al. A European multicentre, placebo-controlled supplementation study with α-tocopherol, carotene-rich palm oil, lutein or lycopene: analysis of serum responses. Clin Sci (Lond). 2002;102:447–456. [PubMed] [Google Scholar]
- 187. Shao A, Hathcock JN. Risk assessment for the carotenoids lutein and lycopene. Regul Toxicol Pharmacol. 2006;45:289–298. [DOI] [PubMed] [Google Scholar]
- 188. EFSA Panel on Food Additives and Nutrient Sources added to food (ANS). Scientific Opinion on the re-evaluation of lutein (E 161b) as a food additive on request of the European Commission. EFSA J. 2010;8:1678. [Google Scholar]
- 189. EFSA Panel on Dietetic Products, Nutrition and Allergies (NDA). Statement on the safety of synthetic zeaxanthin as an ingredient in food supplements. EFSA J. 2012;10:2891. [Google Scholar]
- 190. Malinow MR, Feeney-Burns L, Peterson LH, et al. Diet-related macular anomalies in monkeys. Investig Ophthalmol Vis Sci. 1980;19:857–863. [PubMed] [Google Scholar]
- 191. Johnson EJ, Neuringer M, Russell RM, et al. Nutritional manipulation of primate retinas, III: effects of lutein or zeaxanthin supplementation on adipose tissue and retina of xanthophyll-free monkeys. Invest Ophthalmol Vis Sci. 2005;46:692–702. [DOI] [PubMed] [Google Scholar]
- 192. Stringham JM, Stringham NT. Serum and retinal responses to three different doses of macular carotenoids over 12 weeks of supplementation. Exp Eye Res. 2016;151:1–8. [DOI] [PubMed] [Google Scholar]
- 193. Semba RD, Dagnelie G. Are lutein and zeaxanthin conditionally essential nutrients for eye health? Med Hypotheses. 2003;61:465–472. [DOI] [PubMed] [Google Scholar]