Abstract
The association between circulating concentrations of C-reactive protein (CRP) and future atherothrombotic events has provoked speculation about a possible pathogenetic role of CRP. However, we show here that transgenic expression of human CRP had no effect on development, progression, or severity of spontaneous atherosclerosis, or on morbidity or mortality, in male apolipoprotein E (apoE)-deficient C57BL/6 mice up to 56 weeks, despite deposition of human CRP and mouse complement component 3 in the plaques. Although female apoE knockouts develop atherosclerosis more rapidly than males, the human CRP transgene is under sex hormone control and is expressed at human levels only in males. We therefore studied only male mice. The concentration of mouse serum amyloid P component, an extremely sensitive systemic marker of inflammation, remained normal throughout except for transient spikes in response to fighting in a few animals, indicating that atherogenesis in this model is not associated with an acute-phase response. However, among human CRP transgenic mice, the circulating CRP concentration was higher in apoE knockouts than in wild-type controls. The higher CRP values were associated with substantially lower estradiol concentrations in the apoE-deficient animals. Human CRP transgene expression is thus up-regulated in apoE-deficient mice, apparently reflecting altered estrogen levels, despite the absence of other systemic signs of inflammation. Extrapolation to human pathology from this xenogeneic combination of human CRP with apoE deficiency-mediated mouse atherosclerosis must be guarded. Nevertheless, the present results do not suggest that human CRP is either proatherogenic or atheroprotective in vivo.
Keywords: atherosclerosis, inflammation, transgenic
Atherosclerosis is caused by focal accumulation of low-density lipoprotein (LDL) in the arterial wall and is associated with local inflammation in the lesions (1). Atherothrombosis supervening on unstable atherosclerotic plaques causes most coronary events and strokes and is associated with increased intensity of this local inflammation. Furthermore, a substantial proportion of acute myocardial infarctions and strokes are preceded by clinical episodes of infection and/or inflammation elsewhere in the body (2, 3). In this context, considerable interest was aroused by the original reports of a prognostic association between nonspecific circulating markers of inflammation, in particular the classical acute-phase protein C-reactive protein (CRP), and future coronary events in patients with known coronary artery disease (4-6). Interest was further heightened by the demonstration that baseline measurements of CRP in ostensibly healthy individuals in the general population also significantly predicted future atherothrombotic events (7-9). Similar associations are found for diverse systemic markers of inflammation, including white blood cell count, erythrocyte sedimentation rate, various acute-phase plasma proteins, and even serum albumin as a negative acute-phase reactant (10), but the relationship with CRP usually shows the greatest statistical significance. This result may simply be because CRP is an exquisitely sensitive nonspecific marker of inflammation and also a robust and easily measured analyte. Although large-scale epidemiological studies of general populations published since 2000 [culminating in the recent Reykjavik Study (11)] demonstrate that the association of increased baseline CRP with coronary events is less marked than was suggested by the early smaller scale studies, it remains highly significant.
These clinical and epidemiological studies have evoked much speculation about a possible pathogenetic role for CRP in both atherogenesis and atherothrombosis (reviewed in refs. 12 and 13). Well established in vitro activities of CRP, especially its binding to LDL (14) and its capacity to activate complement (15, 16), suggest possible mechanisms, and a variety of other interactions have been reported lately in cell culture studies. However, few of the latter have used well characterized CRP preparations or included specificity controls, and their in vivo relevance has not yet been demonstrated (reviewed in ref. 12). Although CRP and complement proteins are present within human atherosclerotic plaques (17), plasma proteins are known to enter the arterial wall by transudation and to be detectable in plaques (18, 19), and there are no observations that distinguish between possible pathogenic effects of CRP in the lesions and neutral or even atheroprotective functions (20, 21).
To directly investigate the possible role of human CRP in atherogenesis in vivo, we have studied normal diet-fed apolipoprotein E (apoE) knockout mice with and without transgenic expression of human CRP as they spontaneously develop hypercholesterolemia and atherosclerosis.
Materials and Methods
Mice. All animal work was performed under United Kingdom Home Office Licence and in accordance with all applicable laws and regulations. Normal wild-type C57BL/6 mice, designated apoE+/+ (Charles River Breeding Laboratories), and C57BL/6 mice transgenic for human CRP, designated apoE+/+-hCRP+, created by Ciliberto et al. (22) (gift of B. Kyewski, Deutsches Krebsforschungszentrum, Heidelberg), were bred to yield progeny for use in the experiments, the human CRP transgene being identified by PCR precisely as described (23). To generate mice hemizygous for human CRP on an apoE-/- background, designated apoE-/--hCRP+, apoE-/-tm1Unc mice previously extensively backcrossed onto a C57BL/6J background (Iffa Credo) were crossed with apoE+/+-hCRP+ mice. The apoE genotype was determined by PCR screening by using The Jackson Laboratory protocol (http://jaxmice.jax.org). The circulating human CRP concentration in the human CRP transgenic mice on a wild-type C57BL/6 background is markedly higher in males than in females, both at baseline and in response to acute inflammation (23-25). We therefore used male mice for the present study of effects of human CRP on atherosclerosis in apoE-/- animals, even though female apoE-/- mice are known to develop atherosclerosis more rapidly (26).
Experimental Protocol. Male mice of the four different genotypes (apoE+/+, apoE-/-, apoE+/+-hCRP+, and apoE-/--hCRP+) were housed at four animals per cage under conventional conditions and received normal mouse diet (RM1, Special Diet Services, Witham, Essex, England) ad libitum throughout. Groups were closely age matched, and apoE-/- and apoE-/--hCRP+ mice were littermates. One cohort (A) was studied from age 8 weeks with serial tail bleeds at 4-week intervals until being killed for measurement of atherosclerotic lesions at 20 weeks. A second cohort (B) was killed at 12 weeks, and a third (C) was killed at 56 weeks. Group sizes were as shown in the results. All blood samples were taken at least 4 h after the start of the daily light phase when food consumption was minimal. Serum was separated after clotting overnight and was stored frozen at -80°C until analyzed, except for gel filtration studies that were performed on unfrozen samples that were briefly kept at 4°C until tested.
Analysis of Atherosclerotic Lesions. Mice were killed by carbon dioxide inhalation at the ages shown, and their hearts were perfused, fixed, and processed histologically as described (27). Alternate 10-μm sections through the aortic sinus were examined in a Leica DRM XA2 microscope (Leica Microsystems UK, Milton Keynes, U.K.) equipped with a ×2.5 objective and total magnification of ×40, and the whole area within the outer perimeter of the oil red O-stained zones was quantified. Color images were captured by analogue video camera under identical lighting, microscope, camera, and personal computer conditions and were analyzed by using Leica qwin software. The mean lesion area (μm2) for each animal was calculated from the sum of the areas in all sections in which the aortic valve leaflets were present, divided by the number of sections analyzed. Immunoperoxidase immunohistochemical staining was performed by using standard techniques on sections that were not used for oil red O staining. After blocking by incubation with 20% vol/vol normal rabbit serum and elimination of endogenous peroxidase activity by incubation with H2O2, optimal dilutions of primary antisera determined by prior titration were applied. Monospecific goat anti-human CRP, sheep anti-mouse complement component 3 (C3), and sheep anti-mouse serum amyloid P component (SAP) antisera were raised by immunization with the respective isolated pure antigens (28-30). Tissue-bound primary antibodies were detected by using rabbit anti-goat IgG antibody (DAKO), which crossreacts with sheep IgG, followed by peroxidase-goat anti-peroxidase complexes (DAKO), visualization with metal-enhanced diaminobenzidine (PerBio Science UK, Cramlington, U.K.), and hematoxylin counterstaining. The specificity of all immunostaining was established by its complete abolition when the primary antisera were absorbed before use with the respective pure antigens; human CRP and mouse SAP were immobilized covalently on CNBr-activated Sepharose (Amersham Pharmacia Biosciences), and mouse C3 was captured on plain Sepharose beads by complement fixation in fresh mouse serum, as described (31). The mouse macrophage differentiation antigen (Mac-3) was detected by using monoclonal rat anti-mouse macrophage glycoprotein antibodies (Santa Cruz Biotechnology, Autogen Bioclear Ltd, Wiltshire, U.K.), biotinylated rabbit anti rat-IgG (Vector Laboratories), and avidin DH-biotinylated horse radish peroxidase complexes (Vector Laboratories). Vascular cell adhesion molecule-1 (VCAM-1) was detected with polyclonal rabbit anti-mouse VCAM-1 (Santa Cruz Biotechnology), affinity-isolated goat anti-rabbit IgG and rabbit peroxidase-anti-peroxidase complexes (DakoCytomation). In sections processed without the primary antibody, no staining was observed for either Mac-3 or VCAM-1. All immunostained sections were examined and graded semiquantitatively by two independent expert observers who were blinded to the identities of the mice.
Serum Protein, Lipid, and Hormone Concentrations. Human CRP was measured by an automated microparticle-enhanced turbidimetric immunoassay (Roche Diagnostics), with a lower limit of detection of 0.2 mg/liter, run on the COBAS MIRA instrument (32). Serum cholesterol and triglyceride concentrations were determined by using colorimetric assays (Roche Diagnostics) on the same instrument. Matrix effects on CRP assay and recovery of isolated pure human CRP (28) spiked into apoE+/+ and apoE-/- sera were studied in parallel by using the Dade-Behring BNII microparticle-enhanced turbidimetric immunoassay (33) and the Roche Diagnostics method. The BNII and Roche Diagnostics MIRA assays are both specific for human CRP; neither detected anything in sera of mice that were not transgenic for human CRP. Murine SAP and C3 were measured by electroimmunoassay, calibrated with highly purified mouse SAP and C3, respectively, as described (29, 30). Acute-phase production of transgenic human CRP, and of mouse SAP and C3, was investigated after a single s.c. injection of 0.5 ml of 10% wt/vol casein solution (ICN) in 0.05 M NaHCO3 buffer (34). Size exclusion chromatography using an ÅKTA Explorer 100 (Amersham Pharmacia Biosciences) HPLC modular system was performed on a 24.7-ml Superdex 200 HR10/30 column with exclusion limit 1.3 × 106 Da and separation range 104 to 6 × 105 Da. Undiluted 100-μl serum samples were eluted at 0.25 ml/min with 10 mM Tris-buffered 140 mM NaCl containing 2 mM CaCl2, pH 8.0, monitored at 280 nm, and 250-μl fractions were collected for CRP and cholesterol assay. Serum concentrations of estradiol and testosterone were measured by electrochemiluminescence immunoassay (Roche Diagnostics) (35) in the bleed-out samples from the 56-week cohort.
Human CRP Turnover. The plasma clearance rates of 125I-human CRP, after i.v. injection into groups of age-matched male apoE-/--hCRP+ and apoE-/- mice, were measured exactly as described (36).
Statistical Analysis. Significance of differences between values in experimental and control groups were sought by the Wilcoxon sum of ranks (Mann-Whitney) test. The Kruskal-Wallis non-parametric test was used for ANOVA.
Results
Identical Hyperlipidemia in apoE Knockout Mice in the Presence and Absence of Transgenic Human CRP Expression. The apoE-deficient mice developed the same progressive hypercholesterolemia and hypertriglyceridemia, irrespective of transgenic human CRP expression (see Table 3, which is published as supporting information on the PNAS web site). The median (range) serum cholesterol concentration was ≈2.7 (1.9-3.5) mmol/liter in the apoE+/+ and apoE+/+-hCRP+ mice at all ages tested up to 56 weeks and was ≈11 (7-17) mmol/liter in both apoE-/- and apoE-/--hCRP+ groups at 8 weeks, rising to ≈17 (9-21) mmol/liter at 56 weeks. The median (range) serum triglyceride concentration was ≈1.0 (0.5-1.5) mmol/liter in the apoE+/+ and apoE+/+-hCRP+ mice at all ages tested up to 56 weeks and was ≈1.5 (0.7-5) mmol/liter in both apoE-/- and apoE-/--hCRP+ groups at 8 weeks, rising to ≈2.0 (1.2-5) mmol/liter at 56 weeks. There was no difference in weight between groups with and without transgenic human CRP; for example, at 1 yr, the mean (SD) weights were as follows: apoE-/--hCRP+, 35.2 (1.8) g and apoE-/-, 34.9 (3.0) g, n = 16 in each group, whereas all apoE+/+-hCRP+ (n = 3) and apoE+/+ mice (n = 6) weighed 33.0 g at this age. There was also no difference in morbidity between any of the groups, regardless of their apoE or human CRP status, and they all showed the same low mortality, <5 per 100 at 1 yr, as the normal wild-type apoE+/+ controls.
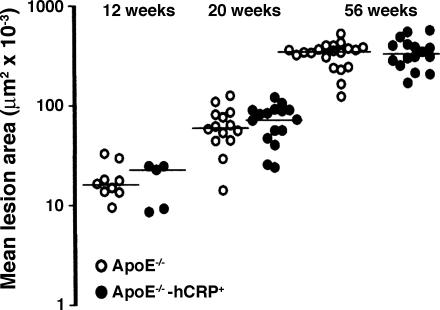
Identical Aortic Atherosclerotic Plaque Size in apoE Knockout Mice in the Presence and Absence of Transgenic Human CRP Expression. Three separate cohorts of mice were killed at 12, 20, and 56 weeks of age, respectively, for estimation of the extent of atherosclerosis in the aortic sinus. Wild-type animals had no atheroma, regardless of the presence or absence of transgenic human CRP (data not shown). All of the apoE-deficient mice had substantial atherosclerotic plaques that increased in size with time, but there was no difference at any time point between the groups with and without transgenic human CRP (Fig. 1). There were also no differences between the groups in histological appearances or morphology of the lesions. All animals in each group are included in this analysis, but exclusion of those that were mounting an acute-phase response of SAP, indicative of intercurrent pathology as discussed below, did not affect the result at any of the time points tested.
Fig. 1.
Atherosclerotic lesion area within the aortic sinus of apoE-/- and apoE-/--hCRP+ mice. Lesion size increased progressively with age in both groups, but there was no significant difference between the animals with and without human CRP at any time point. The horizontal lines are the medians of the values in each group at each time point. Shown at 12 weeks are apoE-/- (n = 9) and apoE-/--hCRP+ (n = 5); shown at 20 weeks are apoE-/- (n = 14) and apoE-/--hCRP+ (n = 16); and shown at 56 weeks are apoE-/- (n = 21) and apoE-/--hCRP+ (n = 16).
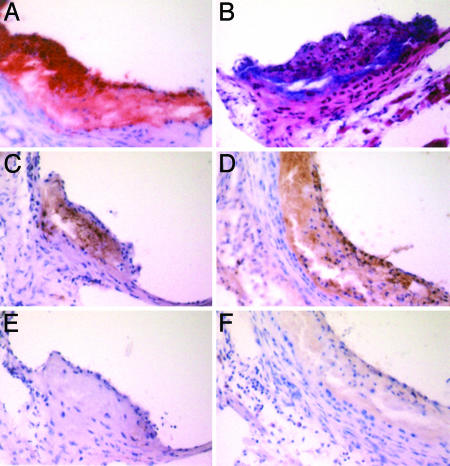
Human CRP, Mouse C3, Mac-3, and VCAM-1 Within Atherosclerotic Plaques. There was specific immunohistochemical staining for mouse C3 in all of the plaques, with the same intensity regardless of the presence or absence of human transgenic CRP expression. In apoE-/--hCRP+ mice, CRP was also demonstrable within the plaques (Fig. 2). No staining for mouse SAP was observed in tissues from any of the groups, nor did the anti-human CRP antiserum stain tissues from mice that were not transgenic for human CRP. Staining for both Mac-3 and VCAM-1 within the atherosclerotic lesions increased progressively in intensity from 12 to 20 to 56 weeks in both groups of apoE-/- mice, but was not different between apoE-/- and apoE-/--hCRP+ animals.
Fig. 2.
Typical atherosclerotic plaques in the aortic sinus of an apoE-/--hCRP+ mouse at 20 weeks. (A) Oil red O stain. (B) Hematoxylin/eosin stain (C) Immunostain with anti-human CRP antibody. (D) Immunostain with anti-mouse C3 antibody. (E) Immunostain with anti-human CRP antibody preabsorbed with immobilized pure human CRP, showing complete absence of staining observed in C.(F) Anti-mouse C3 antibody preabsorbed with immobilized mouse C3, showing complete absence of staining observed in D. (Original magnification: ×320.)
Normal Circulating Concentrations of Mouse SAP. Male C57BL/6 mice have an unavoidable tendency to fight with each other (37), leading to minor injuries and inflammation and thus to acute-phase responses that may complicate assessment of inflammation related to development of atherosclerosis in the apoE-/- mice. However, in contrast to its human homolog, which is a stable constitutive plasma protein, mouse SAP is an exquisitely sensitive acute-phase reactant, as responsive to bacterial lipo-polysaccharides as the pyrogen test (38). We therefore used mouse SAP to control for systemic evidence of inflammation. Baseline values in healthy normal C57BL/6 animals are 2-10 mg/liter (39), and mice with serum SAP values of >12 mg/liter all showed signs of fighting, with ruffled, nonglossy coats and occasionally frank wounds. These clinical signs and the raised SAP concentrations were all transient and affected only a minority of different mice at the different time points. No mice had sustained increased values of SAP, and samples with transiently increased SAP values were therefore excluded from the analysis of acute-phase reactants. With this proviso, mice of the four different genotypes (apoE+/+, apoE+/+-hCRP+, apoE-/-, and apoE-/--hCRP+) had circulating mouse SAP values within the normal range at all time points between 8 and 56 weeks (Table 1). None of the 16 apoE-/--hCRP+ animals that were killed at 56 weeks had SAP concentrations outside the normal range (Table 1), although the atherosclerosis was much more extensive at that time (Fig. 1). Mouse SAP assays thus provided no evidence of a systemic inflammatory response associated with either hyperlipidemia or the development and progression of atherosclerosis in apoE-/- mice, regardless of whether they expressed transgenic human CRP or not.
Table 1. Serum concentrations of mouse SAP and human CRP in apoE−/− and apoE+/+ mice with and without transgenic human CRP.
| ApoE+/+
|
ApoE+/+-hCRP+
|
ApoE−/−
|
ApoE−/−-hCRP+
|
|||||
|---|---|---|---|---|---|---|---|---|
| Age, wk | mSAP | hCRP | mSAP | hCRP | mSAP | hCRP | mSAP | hCRP |
| Cohort A | ||||||||
| 8 | 5 (4-7) n = 4 | NA | 9 (5-12) n = 8 | 11.1 (3.0-17.7) n = 8 | 9 (5-12) n = 21 | NA | 9 (5-12) n = 11 | 10.9 (2.0-22.8) n = 11 |
| 12 | 6 (3-10) n = 7 | NA | 6 (1-8) n = 18 | 7.8 (1.1-13.7) n = 18 | 6 (3-11) n = 17 | NA | 6 (4-11) n = 17 | 17.1 (3.8-36.1) n = 17, P < 0.005 |
| 16 | 6 (2-11) n = 7 | NA | 6 (3-12) n = 20 | 6.2 (1.2-14.2) n = 20 | 6.5 (3-11) n = 18 | NA | 6 (4-11) n = 13 | 24.0 (9.8-31.2) n = 13, P < 0.0001 |
| 20 | 6 (1-7) n = 5 | NA | 4 (1-11) n = 17 | 9.1 (1.3-12.6) n = 17 | 5 (3-12) n = 27 | NA | 4.5 (1-12) n = 28 | 20.9 (1.8-41.1) n = 28, P < 0.0001 |
| Cohort B | ||||||||
| 12 | NA | ND | 17.5 (2.9-21.9) n = 5 | ND | 22.0 (11.2-32.6) n = 5, P = 0.1508 | |||
| Cohort C | ||||||||
| 56 | NA | ND | 3.5 (2.4-6.3) n = 3 | 3 (2-7) n = 16 | 27.2 (12.6-73.7) n = 16, P < 0.0001 | |||
Concentrations (mg/liter) are presented as median (range). Cohort A was bled serially at the ages shown before atherosclerosis was measured at 20 weeks. The other cohorts were bled only when atherosclerosis was measured at 12 and 56 weeks, respectively. P values for difference between CRP concentrations in apoE−/−-hCRP+ and apoE+/+-hCRP+ groups by Wilcoxon test. Note group size in cohort B is too small to provide a robust result. NA, not applicable; ND, not determined.
Increased Circulating Concentrations of Human CRP in apoE-/- Mice. The known widely variable baseline circulating CRP concentration among male human CRP transgenic mice (23-25) was observed here in both the apoE-/- and apoE+/+ background, even after exclusion from the analysis of samples containing acute-phase values of mouse SAP. However, from 12 weeks onwards, the CRP values were consistently higher in the apoE-/--hCRP+ mice compared with the apoE+/+-hCRP+ controls, but did not change significantly after that time (Table 1). These differences in CRP values between the apoE-/- and apoE+/+ groups were not due to interference in the CRP immunoassay by the progressive hypercholesterolemia of the apoE-/- mice. Two different CRP assays, the MIRA and the BNII, gave consistently comparable results, and there was identical recovery of CRP when apoE-/--hCRP+ serum was diluted 1:4 in either lipemic apoE-/- serum or normal apoE+/+ mouse serum. Furthermore, both assays showed similar recovery when isolated pure human CRP was spiked into normal human serum, or typically hyperlipidemic apoE-/- mouse serum, or even Tris-buffered saline containing 4 g/liter BSA.
Circulating Human CRP Is Not Complexed and Is Cleared Normally in apoE-/- Mice. Although aggregated human CRP specifically binds to native human LDL and very low-density lipoprotein (VLDL), native soluble human CRP does not bind to native LDL or indeed any other macromolecular ligand in normal human serum (14). In contrast, native soluble human CRP does bind to β-VLDL, the abnormal lipoprotein in serum of patients with type III hyperlipo-proteinemia, and also to normal VLDL when this is added to normal serum in sufficient quantity (40). However, when sera from apoE-/--hCRP+ mice, from apoE+/+-hCRP+ mice, and from apoE-/- mice spiked with pure human CRP were analyzed by size-exclusion chromatography, the elution volume (12.65 ml) and profile of the human CRP were constant and completely distinct from the fractions containing cholesterol (elution volume 7.75 ml). There was thus no evidence for complex formation between human CRP and any lipoprotein or other macromolecular ligand in the serum. If such interactions occur, they must be very weak and are unlikely to explain the higher serum CRP concentrations observed in the apoE-/--hCRP+ mice. In addition, the plasma clearance of intravenously injected trace radiolabeled human CRP was identical in apoE-/--hCRP+ and apoE+/+-hCRP+ mice, indicating that the higher CRP concentrations in the apoE-/--hCRP+ mice must reflect increased CRP synthesis.
Acute-Phase Response to Inflammation in apoE-/- and apoE+/+ Mice. After s.c. injection of casein, the peak acute-phase concentration of transgenic human CRP at day 2 was significantly greater in apoE-/- than apoE+/+ mice: median (range) 42 (41-63) mg/liter, n = 5, compared with 19 (7-24) mg/liter, n = 9, (P < 0.01). Values fell thereafter, with no difference between the groups on days 4 and 7. In contrast, the acute-phase response of mouse SAP was the same in these two groups as well as in apoE-/- and apoE+/+ mice without transgenic human CRP. Median SAP values rose from baseline of 3-7 mg/liter to peak at 67-86 mg/liter on day 2, falling to 16-20 mg/liter on day 4 and 9-13 mg/liter on day 7. The mouse C3 response was also the same in all four groups, rising from 300 to 400 mg/liter before stimulation to a peak of ≈600 mg/liter at day 2-4, and falling back to only slightly above normal by day 7.
Decreased Serum Estradiol Concentration in apoE-/- Mice. At 50 weeks of age, the only time at which the measurement was made, the circulating estradiol concentration was substantially lower in apoE-/- than in apoE+/+ mice, regardless of the presence or absence of the human CRP transgene (Table 2). There were no differences in testosterone values between the groups (Table 2).
Table 2. Serum concentrations of estradiol and testosterone at age 50 weeks in apoE−/− and apoE+/+ mice with and without transgenic human CRP.
| Serum concentrations, median (range)
|
||||
|---|---|---|---|---|
| Genotype | n | Testosterone, nmol/liter | Estradiol*, pmol/liter | n (%) undetectable values |
| apoE+/+ | 13 | 1.6 (0.6-151.2) | 486 (324-664) | 0 |
| apoE+/+-hCRP | 18 | 2.4 (0.6-116.8) | 471 (344-720) | 0 |
| apoE−/− | 19 | 1.6 (0.2-79.2) | 102 (49-300) | 9 (47.4) |
| apoE−/−-hCRP | 15 | 2.6 (0.2-49.2) | 99 (99-268) | 11 (73.3) |
| p† | 0.34 | 0.0001 | 0.0001 | |
The estradiol assay had a sensitivity of 50 pmol/liter. Serum samples were of limited volume and had to be diluted 1:1 or 1:2 for measurement. Values for samples that were undetectable at these dilutions are replaced by the cut-off multiplied by the dilution minus 1 (e.g., <50 when diluted at 1:2 is replaced by 99; when diluted at 1:1, it is replaced by 49). The P values obtained by nonparametric analysis are not significantly changed by using a different replacement value.
Kruskal-Wallis test.
Discussion
Mouse CRP is a trace plasma protein, the concentration of which does not exceed 1-2 mg/liter even at the peak of the acute-phase response (30, 41), whereas the plasma concentration and acute-phase behavior of human CRP in human CRP transgenic male C57BL/6 mice are comparable with these features of CRP in humans. These mice are thus an appropriate model for investigation of the in vivo effects of human CRP. Female mice express the human CRP transgene only at very much lower levels (23-25) and were therefore not studied here. The mechanism underlying the sex differential in transgene expression in mice is not known, because the transgene includes the whole human promoter sequence (22), but there is no sex difference in CRP expression in humans.
In the present experiments, there was no difference in aortic sinus atherosclerotic plaque size between male apoE-/- and apoE-/--hCRP+ mice at any of the time points analyzed up to 56 weeks of age. In most mice, the initial serum concentrations of human CRP were in the range associated in epidemiological studies with higher risk of future coronary events [≈2.5 mg/liter or more (11, 42) (Table 1)], but lower baseline values in a few animals were not associated with any difference in eventual atherosclerotic plaque size.
Inflammation, stress and infection are all known to be proatherogenic in apoE-/- mice (43-48). In studying the possible specific actions of human CRP, it is therefore essential to be aware of, and if necessary exclude, intercurrent inflammatory pathology, such as the effects of occasional fighting (which is inevitable among male C57BL/6 mice) or subclinical intercurrent infections that may occur in mouse facilities. Measurements of mouse SAP, which is an exquisitely sensitive acute-phase reactant (38, 39), are uniquely valuable in this respect. However, the minor transient acute-phase responses caused by fighting in some mice in the present study had no effect on atherogenesis. Also, very notably, the development of atherosclerosis in the apoE-/- mice proceeded without sufficient inflammation to be reflected systemically, even by mouse SAP.
Nevertheless, at 12 weeks of age, the concentrations of human CRP were generally higher in apoE-/--hCRP+ than in apoE+/+-hCRP+ mice, although they did not change significantly thereafter. We excluded the possibility that the higher CRP values were an assay artifact related to measurement by optical techniques in lipemic serum. We also found no evidence for significant binding of human CRP to the abnormal murine lipoproteins in apoE-/- serum. The plasma clearance of intravenously injected human CRP was identical in apoE-/- and apoE+/+ animals, so the higher serum CRP values must reflect increased transgenic human CRP production in the apoE-/- mice. Expression of this human CRP transgene in the mouse is under strong sex hormone control (23-25), and it was therefore notable that there was a marked reduction in circulating estradiol concentration in apoE-/- mice compared with apoE+/+ controls at ≈50 weeks. Insufficient serum was available from earlier time points to assay these hormones, but estrogen/testosterone imbalance related to apoE deficiency and/or its metabolic consequences, such as hypercholesterolemia, is a likely explanation of the raised human CRP values. In addition, there may be different regulation of the human transgene compared with autologous murine SAP and C3, which showed no increased production. It is known, for example, that the transgene is aberrantly expressed in this model, with abundant CRP production in the kidney and other tissues, whereas CRP in humans is produced in significant amounts only by the liver (49). Baseline CRP concentration in humans shows a strong positive relationship with obesity (50-52), and, although at 1 yr the weights of the apoE-/- mice were only ≈6% greater than those of the apoE+/+ animals, a difference that was not statistically significant, there may perhaps be differences in their adipose tissue. In particular, macrophage accumulation and proinflammatory cytokine production are more marked in obese adipose tissue (53), which might be more extensive in apoE-/- mice, and may conceivably be reflected more sensitively by human CRP than mouse SAP.
The presence of human CRP within the atherosclerotic plaques of the human CRP transgenic apoE-/- mice, and of mouse C3 in the plaques in all apoE-/- animals, is not informative about whether these proteins are having either beneficial or harmful local effects. Although our findings provide no evidence for a proatherogenic role of human CRP in vivo, it is essential to recognize that the transgenic human CRP is in a murine rather than human environment of lipoproteins, complement, extracellular matrix, tissue and inflammatory cells, cytokines, mediators, and cellular receptors. For example, the lipoproteins that are pivotal in pathogenesis of atherosclerosis differ greatly between mouse and human, as do their respective complement systems. We have confirmed that human CRP complexed with its highest affinity ligand, C-polysaccharide, does activate C3 in mouse serum (results not shown), but the effects of human CRP binding to potentially important endogenous murine ligands (such as lipoproteins, apoptotic cells, and cellular debris) are not known. Also, mouse complement C5-9 has much lower activity than the human terminal sequence and is not required for atherogenesis in apoE knockout mice (54). Therefore, any extrapolation to human pathophysiology from this or similar models must be guarded.
A recent study using essentially the same model as here concluded that human CRP accelerated progression of atherosclerosis in apoE-/- mice (55). Female and male animals were used, and some received turpentine injections to induce inflammation and acute-phase responses. Differences in lesion size were observed only in males, and only at the end of the study, at 29 weeks. In the unstimulated mice, the difference was of a marginal statistical significance that could be abolished by elimination of a single outlier (55). The difference in the turpentine-treated group cannot be ascribed specifically to human CRP because turpentine is a major nonspecific inflammatory stimulus. Also, the baseline and acute-phase human CRP concentrations reported in male mice by Paul et al. (55) are extraordinarily high (100-500 mg/liter), suggesting, if the values are correct, the presence of active intercurrent inflammatory pathology in the animals studied, for which no controls were reported. This result is important because, as cited above, inflammation, stress, and infection are all proatherogenic in apoE-/- mice. Paul et al. also reported increased collagen deposition and increased abundance of VCAM-1 and angiotensin type 1 receptor in the plaques of human CRP transgenic mice, and decreased plasma C3 concentration. In contrast, we detected no differences in plaque histology or morphology in our cohorts and no difference in C3 values; nor was there any difference in presence, distribution, or abundance of either VCAM-1 or of the macrophage marker Mac-3 in the lesions at any time point.
Clinical evidence about an association between CRP values and the necessarily indirect surrogate indices of overall burden of atherosclerosis in humans is conflicting. The significant epidemiological association of increased baseline circulating CRP concentration is with atherothrombotic events, rather than with the underlying atherosclerosis. Also, CRP values are not increased, even in patients with severe coronary atherosclerosis, when they experience variant angina caused by coronary spasm rather than acute coronary syndromes that presage occlusive atherothrombotic events (56). Thus, because normal diet-fed apoE-/- mice do not develop unstable coronary plaques or suffer from spontaneous atherothrombotic events, despite their severe atherosclerosis, the convincing negative result of the present study is not surprising. More complex plaques have been reported in the innominate artery of aged fat-fed apoE-/- mice, with evidence of intra-plaque hemorrhage and atherothrombosis (57, 58). These lesions merit further investigation in the human CRP transgenic model, especially in view of the report that arterial thrombosis is increased after vascular injury in human CRP transgenic mice (59).
Supplementary Material
Acknowledgments
We thank Duncan Moore and the staff of the Central Biological Unit, Royal Free and University College Medical School, for their invaluable contribution to these studies; Dorothy Harris and Kuljit Singh of GlaxoSmithKline for technical advice; Dr. S. J. Whiting and Dr. G. L. Jones, Royal Free Hospital, for the hormone assays; Janet Gilbertson and Wai-Lin Tsang for technical assistance; and Beth Jones for preparation of the manuscript. This work was supported in part by Medical Research Council (MRC) (U.K.) Program Grant G97900510 (to M.B.P.) and an MRC Research Training Fellowship (to G.M.H.).
Author contributions: G.M.H., J.R.G., M.C.K., W.L.H., A.P.D., G.A.T., and M.B.P. performed research; G.M.H., C.A.S., and M.B.P. analyzed data; M.B.P. designed research; and M.B.P. wrote the paper.
Abbreviations: apoE, apolipoprotein E; C3, complement component 3; CRP, C-reactive protein; LDL, low-density lipoprotein; Mac-3, mouse macrophage differentiation antigen; SAP, serum amyloid P component; VCAM-1, vascular cell adhesion molecule 1.
References
- 1.Ross, R. (1999) N. Engl. J. Med. 340, 115-126. [DOI] [PubMed] [Google Scholar]
- 2.Spodick, D. H. (1985) Ann. Intern. Med. 102, 699-702. [DOI] [PubMed] [Google Scholar]
- 3.Smeeth, L., Thomas, S. L., Hall, A. J., Hubbard, R., Farringdon, P. & Vallance, P. (2004) New Engl. J. Med. 351, 2611-2618. [DOI] [PubMed] [Google Scholar]
- 4.Liuzzo, G., Biasucci, L. M., Gallimore, J. R., Grillo, R. L., Rebuzzi, A. G., Pepys, M. B. & Maseri, A. (1994) N. Engl. J. Med. 331, 417-424. [DOI] [PubMed] [Google Scholar]
- 5.Thompson, S. G., Kienast, J., Pyke, S. D. M., Haverkate, F. & van de Loo, J. C. W. (1995) N. Engl. J. Med. 332, 635-641. [DOI] [PubMed] [Google Scholar]
- 6.Haverkate, F., Thompson, S. G., Pyke, S. D. M., Gallimore, J. R. & Pepys, M. B. (1997) Lancet 349, 462-466. [DOI] [PubMed] [Google Scholar]
- 7.Kuller, L. H., Tracy, R. P., Shaten, J. & Meilahn, E. N., for the MRFIT Research Group (1996) Am. J. Epidemiol. 144, 537-547. [DOI] [PubMed] [Google Scholar]
- 8.Ridker, P. M., Cushman, M., Stampfer, M. J., Tracy, R. P. & Hennekens, C. H. (1997) N. Engl. J. Med. 336, 973-979. [DOI] [PubMed] [Google Scholar]
- 9.Koenig, W., Sund, M., Fröhlich, M., Fischer, H.-G., Löwel, H., Döring, A., Hutchinson, W. L. & Pepys, M. B. (1999) Circulation 99, 237-242. [DOI] [PubMed] [Google Scholar]
- 10.Danesh, J., Collins, R., Appleby, P. & Peto, R. (1998) J. Am. Coll. Cardiol. 279, 1477-1482. [DOI] [PubMed] [Google Scholar]
- 11.Danesh, J., Wheeler, J. G., Hirschfield, G. M., Eda, S., Eiriksdottir, G., Rumley, A., Lowe, G. D. O., Pepys, M. B. & Gudnason, V. (2004) N. Engl. J. Med. 350, 1387-1397. [DOI] [PubMed] [Google Scholar]
- 12.Pepys, M. B. & Hirschfield, G. M. (2003) J. Clin. Invest. 111, 1805-1812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hirschfield, G. M. & Pepys, M. B. (2003) Q. J. Med. 96, 793-807. [DOI] [PubMed] [Google Scholar]
- 14.de Beer, F. C., Soutar, A. K., Baltz, M. L., Trayner, I., Feinstein, A. & Pepys, M. B. (1982) J. Exp. Med. 156, 230-242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kaplan, M. H. & Volanakis, J. E. (1974) J. Immunol. 112, 2135-2147. [PubMed] [Google Scholar]
- 16.Siegel, J., Rent, R. & Gewurz, H. (1974) J. Exp. Med. 140, 631-647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Torzewski, J., Torzewski, M., Bowyer, D. E., Fröhlich, M., Koenig, W., Waltenberger, J., Fitzsimmons, C. & Hombach, V. (1998) Arterioscler. Thromb. Vasc. Biol. 18, 1386-1392. [DOI] [PubMed] [Google Scholar]
- 18.Stender, S. & Zilversmit, D. B. (1981) Arteriosclerosis 1, 38-49. [DOI] [PubMed] [Google Scholar]
- 19.Zhang, Y., Cliff, W. J., Schoefl, G. I. & Higgins, G. (1993) Am. J. Pathol. 143, 496-506. [PMC free article] [PubMed] [Google Scholar]
- 20.Bhakdi, S., Torzewski, M., Paprotka, K., Schmitt, S., Barsoom, H., Suriyaphol, P., Han, S.-R., Lackner, K. J. & Husmann, M. (2004) Circulation 109, 1870-1876. [DOI] [PubMed] [Google Scholar]
- 21.Bhakdi, S., Lackner, K. J., Han, S.-R., Torzewski, M. & Husmann, M. (2004) Thromb. Haemostasis 91, 639-645. [DOI] [PubMed] [Google Scholar]
- 22.Ciliberto, G., Arcone, R., Wagner, E. F. & Ruther, U. (1987) EMBO J. 6, 4017-4022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hirschfield, G. M., Herbert, J., Kahan, M. C. & Pepys, M. B. (2003) J. Immunol. 171, 6046-6051. [DOI] [PubMed] [Google Scholar]
- 24.Szalai, A. J., Briles, D. E. & Volanakis, J. E. (1995) J. Immunol. 155, 2557-2563. [PubMed] [Google Scholar]
- 25.Szalai, A. J., van Ginkel, F. W., Dalrymple, S. A., Murray, R., McGhee, J. R. & Volanakis, J. E. (1998) J. Immunol. 160, 5294-5299. [PubMed] [Google Scholar]
- 26.Caligiuri, G., Nicoletti, A., Zhou, X., Tornberg, I. & Hansson, G. K. (1999) Atherosclerosis 145, 301-308. [DOI] [PubMed] [Google Scholar]
- 27.Groot, P. H. E., van Vlijmen, B. J. M., Benson, G. M., Hofker, M. H., Schiffelers, R., Vidgeon-Hart, M. & Havekes, L. M. (1996) Arterioscler. Thromb. Vasc. Biol. 16, 926-933. [DOI] [PubMed] [Google Scholar]
- 28.de Beer, F. C. & Pepys, M. B. (1982) J. Immunol. Methods 50, 17-31. [DOI] [PubMed] [Google Scholar]
- 29.Pepys, M. B., Dash, A. C., Fielder, A. H. L. & Mirjah, D. D. (1977) Immunology 33, 491-499. [PMC free article] [PubMed] [Google Scholar]
- 30.Pepys, M. B. (1979) Immunology 37, 637-641. [PMC free article] [PubMed] [Google Scholar]
- 31.Pepys, M. B., Bell, A. J. & Rowe, I. F. (1975) Scand. J. Immunol. 5, 75-78. [Google Scholar]
- 32.Eda, S., Kaufmann, J., Roos, W. & Pohl, S. (1998) J. Clin. Lab. Anal. 12, 137-144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rifai, N., Tracy, R. P. & Ridker, P. M. (1999) Clin. Chem. 45, 2136-2141. [PubMed] [Google Scholar]
- 34.Baltz, M. L., Gomer, K., Davies, A. J. S., Evans, D. J., Klaus, G. G. B. & Pepys, M. B. (1980) Clin. Exp. Immunol. 39, 355-360. [PMC free article] [PubMed] [Google Scholar]
- 35.Bieglmayer, C., Chan, D. W., Sokoll, L., Imdahl, R., Kobayashi, M., Yamada, E., Lilje, D. J., Luthe, H., Meissner, J., Messeri, G., et al. (2004) Clin. Chem. Lab. Med. 42, 1186-1202. [DOI] [PubMed] [Google Scholar]
- 36.Hutchinson, W. L., Noble, G. E., Hawkins, P. N. & Pepys, M. B. (1994) J. Clin. Invest. 94, 1390-1396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Van Loo, P. L., Van Zutphen, L. F. & Baumans, V. (2003) Lab. Anim. 37, 300-313. [DOI] [PubMed] [Google Scholar]
- 38.Poole, S., Gordon, A. H., Baltz, M. & Stenning, B. E. (1984) Br. J. Exp. Pathol. 65, 431-439. [PMC free article] [PubMed] [Google Scholar]
- 39.Pepys, M. B., Baltz, M., Gomer, K., Davies, A. J. S. & Doenhoff, M. (1979) Nature 278, 259-261. [DOI] [PubMed] [Google Scholar]
- 40.Rowe, I. F., Soutar, A. K., Trayner, I. M., Thompson, G. R. & Pepys, M. B. (1984) Clin. Exp. Immunol. 58, 237-244. [PMC free article] [PubMed] [Google Scholar]
- 41.Siboo, R. & Kulisek, E. (1978) J. Immunol. Methods 23, 59-67. [DOI] [PubMed] [Google Scholar]
- 42.Danesh, J., Whincup, P., Walker, M., Lennon, L., Thomson, A., Appleby, P., Gallimore, J. R. & Pepys, M. B. (2000) BMJ 321, 199-204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Huber, S. A., Sakkinen, P., Conze, D., Hardin, N. & Tracy, R. (1999) Arterioscler. Thromb. Vasc. Biol. 19, 2364-2367. [DOI] [PubMed] [Google Scholar]
- 44.Alber, D. G., Powell, K. L., Vallance, P., Goodwin, D. A. & Grahame-Clarke, C. (2000) Circulation 102, 779-785. [DOI] [PubMed] [Google Scholar]
- 45.Hsich, E., Zhou, Y. F., Paigen, B., Johnson, T. M., Burnett, M. S. & Epstein, S. E. (2001) Atherosclerosis 156, 23-28. [DOI] [PubMed] [Google Scholar]
- 46.Lalla, E., Lamster, I. B., Hofmann, M. A., Bucciarelli, L., Jerud, A. P., Tucker, S., Lu, Y., Papapanou, P. N. & Schmidt, A. M. (2003) Arterioscler. Thromb. Vasc. Biol. 23, 1405-1411. [DOI] [PubMed] [Google Scholar]
- 47.Kumari, M., Grahame-Clarke, C., Shanks, N., Marmot, M., Lightman, S. & Vallance, P. (2003) Stress 6, 297-299. [DOI] [PubMed] [Google Scholar]
- 48.Naghavi, M., Wyde, P., Litovsky, S., Madjid, M., Akhtar, A., Naguib, S., Siadaty, M. S., Sanati, S. & Casscells, W. (2003) Circulation 107, 762-768. [DOI] [PubMed] [Google Scholar]
- 49.Szalai, A. J., Weaver, C. T., McCrory, M. A., van Ginkel, F. W., Reiman, R. M., Kearney, J. F., Marion, T. N. & Volanakis, J. E. (2003) Arthritis Rheum. 48, 1602-1611. [DOI] [PubMed] [Google Scholar]
- 50.Tracy, R. P., Psaty, B. M., Macy, E., Bovill, E. G., Cushman, M., Cornell, E. S. & Kuller, L. H. (1997) Arterioscler. Thromb. Vasc. Biol. 17, 2167-2176. [DOI] [PubMed] [Google Scholar]
- 51.Danesh, J., Muir, J., Wong, Y.-K., Ward, M., Gallimore, J. R. & Pepys, M. B. (1999) Eur. Heart J. 20, 954-959. [DOI] [PubMed] [Google Scholar]
- 52.Greenfield, J. R., Samaras, K., Jenkins, A. B., Kelly, P. J., Spector, T. D., Gallimore, J. R., Pepys, M. B. & Campbell, L. V. (2004) Circulation 109, 3022-3028. [DOI] [PubMed] [Google Scholar]
- 53.Weisberg, S. P., McCann, D., Desai, M., Rosenbaum, M., Leibel, R. L. & Ferrante, A. W., Jr. (2003) J. Clin. Invest. 112, 1796-1808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Patel, S., Thelander, E. M., Hernandez, M., Montenegro, J., Hassing, H., Burton, C., Mundt, S., Hermanowski-Vosatka, A., Wright, S. D., Chao, Y.-S., et al. (2001) Biochem. Biophys. Res. Commun. 286, 164-170. [DOI] [PubMed] [Google Scholar]
- 55.Paul, A., Ko, K. W. S., Li, L., Yechoor, V., McCrory, M. A., Szalai, A. J. & Chan, L. (2004) Circulation 109, 647-655. [DOI] [PubMed] [Google Scholar]
- 56.Liuzzo, G., Biasucci, L. M., Rebuzzi, A. G., Gallimore, J. R., Caligiuri, G., Lanza, G. A., Quaranta, G., Monaco, C., Pepys, M. B. & Maseri, A. (1996) Circulation 94, 2373-2380. [DOI] [PubMed] [Google Scholar]
- 57.Johnson, J. L. & Jackson, C. L. (2001) Atherosclerosis 154, 399-406. [DOI] [PubMed] [Google Scholar]
- 58.Rosenfeld, M. E., Polinsky, P., Virmani, R., Kauser, K., Rubanyi, G. & Schwartz, S. M. (2000) Arterioscler. Thromb. Vasc. Biol. 20, 2587-2592. [DOI] [PubMed] [Google Scholar]
- 59.Danenberg, H. D., Szalai, A. J., Swaminathan, R. V., Peng, L., Chen, Z., Seifert, P., Fay, W. P., Simon, D. I. & Edelman, E. R. (2003) Circulation 108, 512-515. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.