Abstract
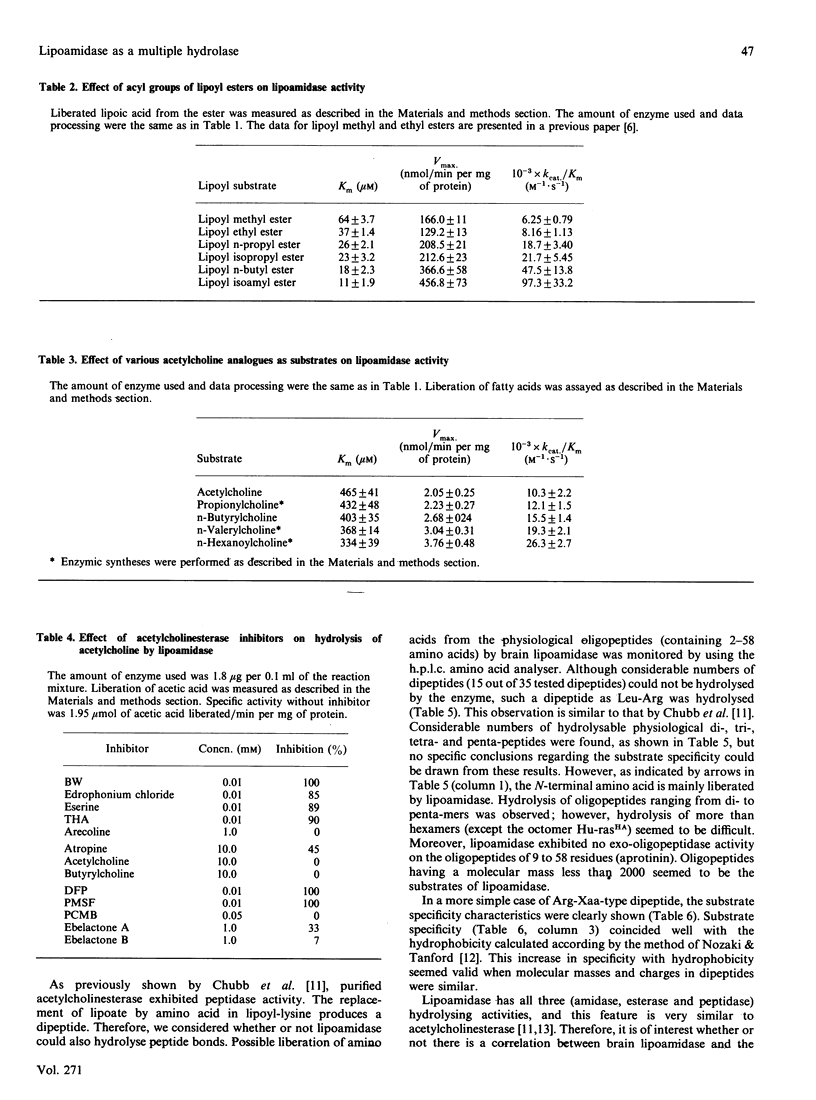
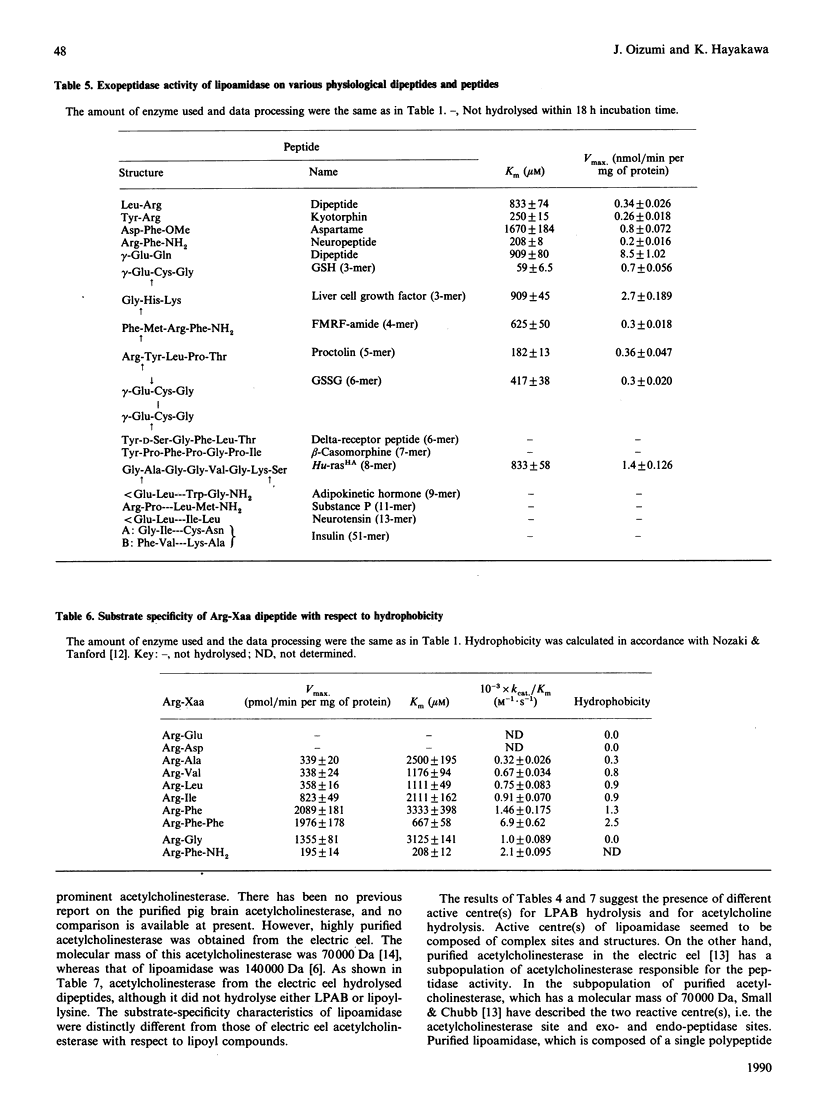
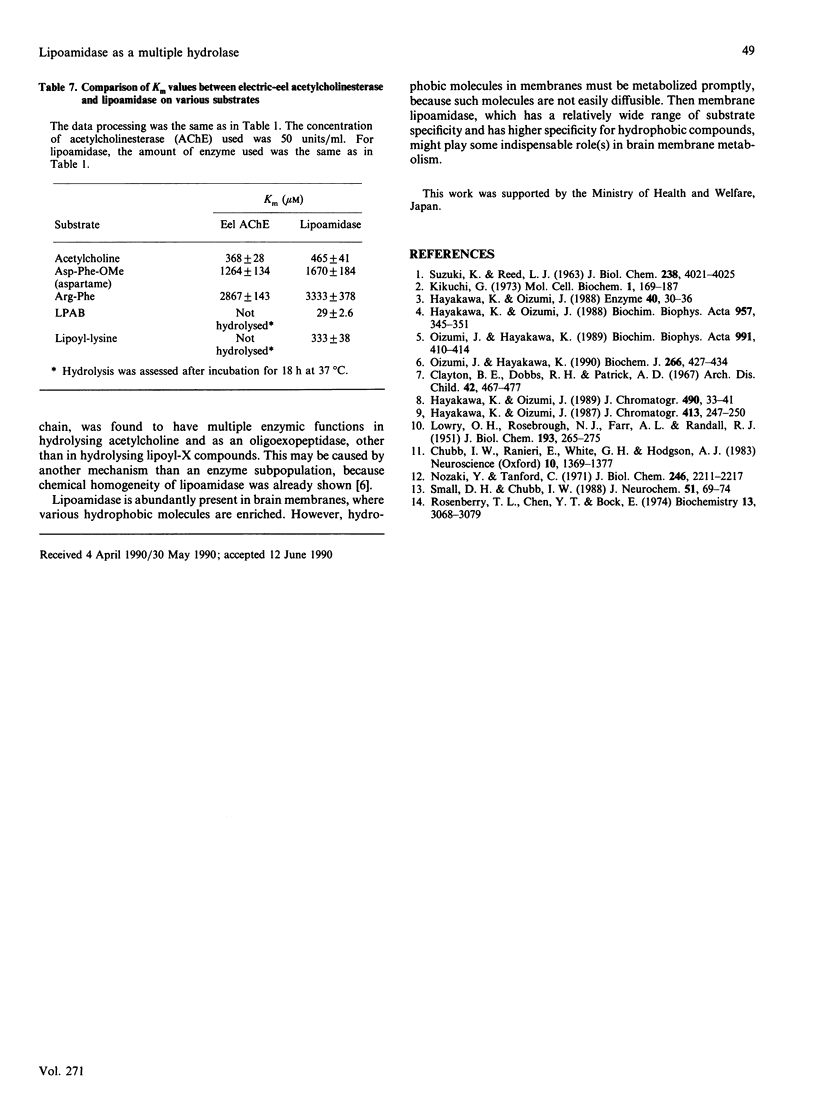
The substrate specificity of lipoamidase, purified from the pig brain membrane with lipoyl 4-aminobenzoate (LPAB) as a substrate, was extensively studied. This single polypeptide was found to hydrolyse the bonding between amide, ester and peptide compounds. However, stringent structural requirements were found in the substrates, e.g. LPAB was hydrolysed, whereas biotinyl 4-aminobenzoate was not, as stated in our previous paper [Oizmui & Hayakawa (1990) Biochem. J. 266, 427-434]. The enzyme specifically recognized the whole molecular structure of the substrate, whereas it loosely recognized the bond structure of the substrate; e.g. the dipeptide Asp-Phe was not hydrolysed, whereas the methyl ester of Asp-Phe (aspartame) was. The exopeptidase activity was demonstrated by lipoamidase; however, longer peptides than the hexamer seemed not to be substrates. Lipoyl esters, which were electrically neutral, exhibited higher specificity with longer acyl groups. Molecular mass and molecular hydrophobicity (hydropathy) seemed to determine the substrate specificity. Lipoyl-lysine, acetylcholine and oligopeptides were hydrolysed at similar Km values; however, acetylcholine was hydrolysed at a velocity 100 times higher. Although many similar specificities were found between electric eel acetylcholinesterase and lipoamidase, distinctly different specificity was demonstrated with lipoyl compounds. The role of lipoamidase, which resides on the brain membrane and possesses higher specificity for hydrophobic molecules, remains to be elucidated.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chubb I. W., Ranieri E., White G. H., Hodgson A. J. The enkephalins are amongst the peptides hydrolyzed by purified acetylcholinesterase. Neuroscience. 1983 Dec;10(4):1369–1377. doi: 10.1016/0306-4522(83)90118-5. [DOI] [PubMed] [Google Scholar]
- Clayton B. E., Dobbs R. H., Patrick A. D. Leigh's subacute necrotizing encephalopathy: clinical and biochemical study, with special reference to therapy with lipoate. Arch Dis Child. 1967 Oct;42(225):467–478. doi: 10.1136/adc.42.225.467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayakawa K., Oizumi J. Determination of free biotin in plasma by liquid chromatography with fluorimetric detection. J Chromatogr. 1987 Jan 23;413:247–250. doi: 10.1016/0378-4347(87)80234-7. [DOI] [PubMed] [Google Scholar]
- Hayakawa K., Oizumi J. Determination of lipoyllysine derived from enzymes by liquid chromatography. J Chromatogr. 1989 May 5;490(1):33–41. doi: 10.1016/s0378-4347(00)82758-9. [DOI] [PubMed] [Google Scholar]
- Hayakawa K., Oizumi J. Human serum lipoamidase. Enzyme. 1988;40(1):30–36. doi: 10.1159/000469138. [DOI] [PubMed] [Google Scholar]
- Hayakawa K., Oizumi J. Isolation and characterization of human breast milk lipoamidase. Biochim Biophys Acta. 1988 Dec 2;957(3):345–351. doi: 10.1016/0167-4838(88)90224-5. [DOI] [PubMed] [Google Scholar]
- Kikuchi G. The glycine cleavage system: composition, reaction mechanism, and physiological significance. Mol Cell Biochem. 1973 Jun 27;1(2):169–187. doi: 10.1007/BF01659328. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Nozaki Y., Tanford C. The solubility of amino acids and two glycine peptides in aqueous ethanol and dioxane solutions. Establishment of a hydrophobicity scale. J Biol Chem. 1971 Apr 10;246(7):2211–2217. [PubMed] [Google Scholar]
- Oizumi J., Hayakawa K. Biotinidase and lipoamidase in guinea pig livers. Biochim Biophys Acta. 1989 Jun 27;991(3):410–414. doi: 10.1016/0304-4165(89)90066-4. [DOI] [PubMed] [Google Scholar]
- Oizumi J., Hayakawa K. Lipoamidase (lipoyl-X hydrolase) from pig brain. Biochem J. 1990 Mar 1;266(2):427–434. doi: 10.1042/bj2660427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberry T. L., Chen Y. T., Bock E. Structure of 11S acetylcholinesterase. Subunit composition. Biochemistry. 1974 Jul 16;13(15):3068–3079. doi: 10.1021/bi00712a012. [DOI] [PubMed] [Google Scholar]
- SUZUKI K., REED L. J. LIPOAMIDASE. J Biol Chem. 1963 Dec;238:4021–4025. [PubMed] [Google Scholar]
- Small D. H., Chubb I. W. Identification of a trypsin-like site associated with acetylcholinesterase by affinity labelling with [3H]diisopropyl fluorophosphate. J Neurochem. 1988 Jul;51(1):69–74. doi: 10.1111/j.1471-4159.1988.tb04836.x. [DOI] [PubMed] [Google Scholar]


